Postoperative Outcomes and Reinterventions Following Fenestrated/Branched Endovascular Aortic Repair in Post-Dissection and Complex Degenerative Abdominal and Thoraco-Abdominal Aortic Aneurysms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Type of Study and Setting
2.2. Patient Population
2.3. Preoperative Management and Planning
2.4. Surgical Procedure and Follow-Up
2.5. Outcome Measures and Definition of Variables
2.6. Statistical Analysis
3. Results
3.1. Demographics and Baseline Characteristics
3.2. Procedural Details
3.3. Early Postoperative Outcomes
3.4. Short-Term Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fattori, R.; Montgomery, D.; Lovato, L.; Kische, S.; Di Eusanio, M.; Ince, H.; Eagle, K.A.; Isselbacher, E.M.; Nienaber, C.A. Survival after endovascular therapy in patients with type B aortic dissection: A report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc. Interv. 2013, 6, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, G.R.; Perry, R.J. Thoracic and Thoracoabdominal Aortic Aneurysm: Etiology, Epidemiology, Natural History, Medical Management, and Decision Making. In Rutherford’s Vascular Surgery and Endovascular Therapy, 9th ed.; Elsevier: Philadelphia, PA, USA, 2019; Volume 1, pp. 3266–3324. [Google Scholar]
- Tsilimparis, N.; Perez, S.; Dayama, A.; Ricotta, J.J. Endovascular repair with fenestrated-branched stent grafts improves 30-day outcomes for complex aortic aneurysms compared with open repair. Ann. Vasc. Surg. 2013, 27, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.P.; Crawford, A.S.; Flanagan, C.P.; Aiello, F.A.; Arous, E.J.; Judelson, D.R.; Messina, L.M.; Robichaud, D.I.; Valliere, S.A.; Schanzer, A. Evolution of fenestrated/branched endovascular aortic aneurysm repair complexity and outcomes at an organized center for the treatment of complex aortic disease. J. Vasc. Surg. 2021, 73, 1148–1155.e2. [Google Scholar] [CrossRef]
- Oderich, G.S.; Ribeiro, M.; Hofer, J.; Wigham, J.; Cha, S.; Chini, J.; Macedo, T.A.; Gloviczki, P. Prospective, nonrandomized study to evaluate endovascular repair of pararenal and thoracoabdominal aortic aneurysms using fenestrated-branched endografts based on supraceliac sealing zones. J. Vasc. Surg. 2017, 65, 1249–1259.e10. [Google Scholar] [CrossRef]
- Oderich, G.S.; Forbes, T.L.; Chaer, R.; Davies, M.G.; Lindsay, T.F.; Mastracci, T.; Singh, M.J.; Timaran, C.; Woo, E.Y. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J. Vasc. Surg. 2021, 73, 4S–52S. [Google Scholar] [CrossRef] [PubMed]
- De Marino, P.M.; Ibraheem, A.; Gafur, N.; Verhoeven, E.L.; Katsargyris, A. Outcomes of fenestrated and branched endovascular aortic repair for chronic post-dissection thoracoabdominal aortic aneurysms. J. Cardiovasc. Surg. 2020, 61, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, E.; Oderich, G.S.; Farber, M.A.; Schneider, D.B.; Timaran, C.H.; Schanzer, A.; Beck, A.W.; Motta, F.; Sweet, M.P. Outcomes of endovascular repair of chronic postdissection compared with degenerative thoracoabdominal aortic aneurysms using fenestrated-branched stent grafts. J. Vasc. Surg. 2020, 72, 822–836.e9. [Google Scholar] [CrossRef]
- Oikonomou, K.; Kasprzak, P.; Katsargyris, A.; De Marino, P.M.; Pfister, K.; Verhoeven, E.L. Mid-Term Results of Fenestrated/Branched Stent Grafting to Treat Post-Dissection Thoraco-Abdominal Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 102–109. [Google Scholar] [CrossRef]
- Spear, R.; Hertault, A.; Van Calster, K.; Settembre, N.; Delloye, M.; Azzaoui, R.; Sobocinski, J.; Fabre, D.; Tyrrell, M.; Haulon, S. Complex endovascular repair of postdissection arch and thoracoabdominal aneurysms. J. Vasc. Surg. 2018, 67, 685–693. [Google Scholar] [CrossRef]
- Troisi, N.; Donas, K.P.; Austermann, M.; Tessarek, J.; Umscheid, T.; Torsello, G. Secondary procedures after aortic aneurysm repair with fenestrated and branched endografts. J. Endovasc. Ther. 2011, 18, 146–153. [Google Scholar] [CrossRef]
- Silverberg, D.; Aburamileh, A.; Rimon, U.; Raskin, D.; Khaitovich, B.; Halak, M. Secondary interventions after fenestrated and branched endovascular repair of complex aortic aneurysms. J. Vasc. Surg. 2020, 72, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Kärkkäinen, J.M.; Tenorio, E.R.; Jain, A.; Mendes, B.C.; Macedo, T.A.; Pather, K.; Gloviczki, P.; Oderich, G.S. Outcomes of target vessel endoleaks after fenestrated-branched endovascular aortic repair. J. Vasc. Surg. 2020, 72, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Oderich, G.S.; Ribeiro, M.; de Souza, L.R.; Hofer, J.; Wigham, J.; Cha, S. Endovascular repair of thoracoabdominal aortic aneurysms using fenestrated and branched endografts. J. Thorac. Cardiovasc. Surg. 2017, 153, S32–S41.e7. [Google Scholar] [CrossRef]
- Law, Y.; Tsilimparis, N.; Rohlffs, F.; Makaloski, V.; Behrendt, C.-A.; Heidemann, F.; Wipper, S.H.; Debus, E.S.; Kölbel, T. Fenestrated or branched endovascular aortic repair for postdissection thoracoabdominal aortic aneurysm. J. Vasc. Surg. 2019, 70, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Eleshra, A.; Panuccio, G.; Spanos, K.; Rohlffs, F.; Tsilimparis, N.; Kölbel, T. Fenestrated and Branched Endovascular Aortic Repair of Thoracoabdominal Aortic Aneurysm with More than 4 Target Visceral Vessels Due to Renovisceral Arterial Anatomical Variations: Feasibility and Early Results. J. Endovasc. Ther. 2021, 28, 692–699. [Google Scholar] [CrossRef]
- Schneider, D.B.; Oderich, G.S.; Farber, M.A.; Schanzer, A.; Beck, A.W.; Timaran, C.H.; Sweet, M.P.; Tenorio, E. SS03. Target Artery Outcomes after Branched and Fenestrated Endovascular Repair of Pararenal and Thoracoabdominal Aortic Aneurysms in the U.S. Investigational Device Exemption Experience. J. Vasc. Surg. 2018, 67, e83. [Google Scholar] [CrossRef]
- Baker, A.C.; Oderich, G.S. Principles of Side Branch Incorporation and‘Bail Out’ Maneuvers. In Endovascular Aortic Repair: Current Techniques with Fenestrated, Branched and Parallel Stent-Grafts, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 277–294. [Google Scholar] [CrossRef]
- Konstantinou, N.; Kölbel, T.; Dias, N.V.; Verhoeven, E.; Wanhainen, A.; Gargiulo, M.; Oikonomou, K.; Verzini, F.; Heidemann, F.; Sonesson, B.; et al. Revascularization of occluded renal artery stent grafts after complex endovascular aortic repair and its impact on renal function. J. Vasc. Surg. 2021, 73, 1566–1572. [Google Scholar] [CrossRef]
- Mastracci, T.M.; Greenberg, R.K.; Eagleton, M.J.; Hernandez, A.V. Durability of branches in branched and fenestrated endografts. J. Vasc. Surg. 2013, 57, 926–933. [Google Scholar] [CrossRef]
- Oderich, G.S.; Greenberg, R.K.; Farber, M.; Lyden, S.; Sanchez, L.; Fairman, R.; Jia, F.; Bharadwaj, P. Results of the United States multicenter prospective study evaluating the Zenith fenestrated endovascular graft for treatment of juxtarenal abdominal aortic aneurysms. J. Vasc. Surg. 2014, 60, 1420–1428.e5. [Google Scholar] [CrossRef]
- Mirza, A.K.; Tenorio, E.; Kärkkäinen, J.M.; Ozbek, P.; Oderich, G.S. Technical video of endovascular repair of chronic postdissection thoracoabdominal aortic aneurysm using a five-vessel preloaded fenestrated-branched stent graft. J. Vasc. Surg. 2019, 69, 296–302.e1. [Google Scholar] [CrossRef]
- Tenorio, E.R.; Oderich, G.S.; Sandri, G.A.; Ozbek, P.; Kärkkäinen, J.M.; Vrtiska, T.; Macedo, T.A.; Gloviczki, P. Prospective nonrandomized study to evaluate cone beam computed tomography for technical assessment of standard and complex endovascular aortic repair. J. Vasc. Surg. 2020, 71, 1982–1993.e5. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, E.R.; Oderich, G.S.; Sandri, G.A.; Ozbek, P.; Kärkkäinen, J.M.; Macedo, T.A.; Vrtiska, T.; Cha, S. Impact of onlay fusion and cone beam computed tomography on radiation exposure and technical assessment of fenestrated-branched endovascular aortic repair. J. Vasc. Surg. 2019, 69, 1045–1058.e3. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, F.; Panuccio, G.; Tsilimparis, N.; Rohlffs, F.; Ahmed, E.M.; Debus, E.S.; Kölbel, T. Balloon-Anchoring Technique to Stabilize Target Vessel Catheterization in Complex Endovascular Aortic Repair. J. Endovasc. Ther. 2020, 27, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Erben, Y.; Oderich, G.S.; Gloviczki, P. The Loop Technique: Addressing Celiac Artery Dissection in a Branched and Fenestrated Endograft for the Treatment of a Type III Thoracoab-dominal Aneurysm. J. Endovasc. Ther. 2016, 23, 614–617. [Google Scholar] [CrossRef]
- Ferreira, M.; Katsargyris, A.; Rodrigues, E.; Ferreira, D.; Cunha, R.; Bicalho, G.; Oderich, G.; Verhoeven, E.L.G. “Snare-Ride”: A Bailout Technique to Catheterize Target Vessels with Unfriendly Anatomy in Branched Endovascular Aortic Repair. J. Endovasc. Ther. 2017, 24, 556–558. [Google Scholar] [CrossRef]
- Makaloski, V.; Tsilimparis, N.; Rohlffs, F.; Spanos, K.; Debus, E.S.; Kölbel, T. Use of a Steerable Sheath for Retrograde Access to Antegrade Branches in Branched Stent-Graft Repair of Complex Aortic Aneurysms. J. Endovasc. Ther. 2018, 25, 566–570. [Google Scholar] [CrossRef]
- Mezzetto, L.; Scorsone, L.; Silingardi, R.; Gennai, S.; Piffaretti, G.; Mantovani, A.; Bush, R.L.; Haulon, S.; Veraldi, G.F. Bridging stents in fenestrated and branched endovascular aneurysm repair: A systematic review. Ann. Vasc. Surg. 2021, 73, 454–462. [Google Scholar] [CrossRef]
- Torsello, P.G. Physician-Initiated Trial Investigating the BeGraft Peripheral Plus Stent Graft System as Bridging Stent in BEVAR for Complex Aortic Aneurysms. clinicaltrials.gov, Clinical Trial Registration NCT03982940; August 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03982940 (accessed on 3 March 2022).
- Torsello, P.G. Physician-Initiated Trial Investigating the BeGraft Peripheral Stent Graft System as Bridging Stent in FEVAR for Complex Aortic Aneurysms. clinicaltrials.gov, Clinical Trial Registration study/NCT03987035; August 2021. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03987035 (accessed on 3 March 2022).
- W. L. Gore & Associates. Real-World Data Collection of the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis When Used as a Bridging Stent with Branched and Fenestrated Endografts in the Treatment of Aortic Aneurysms Involving the Renal-Mesenteric Arteries. clinicaltrials.gov, Clinical Trial Registration NCT05143138; November 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT05143138 (accessed on 3 March 2022).
- Motta, F.; Parodi, F.E.; Knowles, M.; Crowner, J.R.; Pascarella, L.; McGinigle, K.L.; Marston, W.A.; Kibbe, M.R.; Ohana, E.; Farber, M.A. Performance of Viabahn balloon-expandable stent compared with self-expandable covered stents for branched endovascular aortic repair. J. Vasc. Surg. 2021, 73, 410–416.e2. [Google Scholar] [CrossRef]



| Variable * | All Patients (n = 137) | Degenerative Aneurysms (n = 107) | Post-Dissection Aneurysms (n = 30) | p-Value |
|---|---|---|---|---|
| Age, years | 70.4 ± 9.9 | 73.3 ± 7.3 | 59.9 ± 10.9 | 0.000 |
| Gender, Female | 31(22.6) | 21 (19.6) | 10 (33.3) | 0.113 |
| BMI, kg/m2 | 26.8 ± 4.9 | 26.6 ± 4.9 | 27.4 ± 4.9 | 0.416 |
| ASA score | 0.443 | |||
| 2 | 3 (2.2) | 3 (2.8) | 0 (0.0) | |
| 3 | 101 (73.7) | 80 (74.7) | 21 (70.0) | |
| 4 | 32 (23.4) | 23 (21.5) | 9 (30.0) | |
| Ejection fraction, % | 62.7 ± 11.7 | 61.4 ± 12.6 | 66.9 ± 6.3 | 0.099 |
| Cardiovascular Comorbidities | ||||
| CAD | 35 (25.5) | 33 (30.8) | 2 (6.7) | 0.007 |
| MI | 9 (6.6) | 8 (7.5) | 1(3.3) | 0.683 |
| Arrhythmia | 35 (25.5) | 29 (27.1) | 6 (20.0) | 0.416 |
| CHF | 17 (12.4) | 16 (15.0) | 1 (3.3) | 0.086 |
| Hypertension | 112 (81.7) | 85 (79.4) | 27 (90.0) | 0.213 |
| Ischemic stroke or TIA | 17 (12.4) | 16 (15.0) | 1 (3.3) | 0.119 |
| PAD | 17 (12.4) | 14 (13.1) | 3 (10.0) | 0.764 |
| Diabetes mellitus | 18 (13.1) | 17 (15.9) | 1(3.3) | 0.122 |
| Active smoker | 43 (31.4) | 40 (37.4) | 3 (10.0) | 0.005 |
| COPD | 24 (17.5) | 21 (19.6) | 3 (10.0) | 0.283 |
| GFR, ml/min | 67.9 ± 23.0 | 67.0 ± 21.2 | 71.1 ± 28.3 | 0.381 |
| Connective tissue disorder | 2(1.5) | 1 (0.9) | 1 (3.3) | 0.394 |
| Antiplatelet therapy | 120 (87.6) | 94 (87.9) | 26 (86.7) | 0.999 |
| Statin use | 92 (67.2) | 75 (70.1) | 17 (56.7) | 0.145 |
| ACE inhibitors | 95 (69.3) | 74 (69.2) | 21 (70.0) | 0.984 |
| Betablockers | 92 (67.2) | 68 (63.6) | 24 (80.0) | 0.101 |
| Hostile abdomen | 18 (13.2) | 16 (15.0) | 2 (6.7) | 0.299 |
| Preoperative debranching | 7 (5.1) | 1 (0.9) | 6 (20.0) | 0.000 |
| Preoperative MISACE | 54 (39.4) | 31 (29.0) | 23 (76.7) | 0.000 |
| Prior aortic surgery | 49 (35.7) | 24 (22.4) | 25 (83.3) | 0.000 |
| Endovascular | 28 (20.4) | 18 (16.8) | 10 (33.3) | |
| Open | 10 (7.3) | 4 (3.8) | 6 (20.0) | |
| Both | 11 (8.0) | 2 (1.9) | 9 (30.0) | |
| Aneurysm size | 61.9± 15.4 | 63.2± 16.1 | 57.4± 11.9 | 0.067 |
| Status of aneurysm | 0.133 | |||
| Asymptomatic | 117 (85.4) | 89 (83.2) | 29(96.7) | |
| Symptomatic non-ruptured | 14(10.2) | 13(12.1) | 1(3.3) | |
| Ruptured | 6(4.4) | 6(5.6) | 0(0) | |
| Extent of aneurysm | 0.000 | |||
| Extensive TAAA (I-III) | 42 (30.7) | 21 (19.6) | 21 (70.0) | |
| AAA/Crawford IV | 95 (69.3) | 86 (80.4) | 9 (30.0) | |
| Significant iliac calcification | 45 (32.8) | 41 (38.3) | 4 (13.3) | 0.010 |
| Iliac tortuosity index (n = 135) | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.1 | 0.098 |
| Variable * | All Patients | Degenerative Aneurysms | Post-Dissection Aneurysms | p-Value |
|---|---|---|---|---|
| (n = 137) | (n = 107) | (n = 30) | ||
| Surgical setting | 0.048 | |||
| Elective | 117 (85.4) | 88 (82.2) | 29 (96.7) | |
| Urgent/Emergency | 20 (14.6) | 19 (17.8) | 1 (3.3) | |
| Type of endograft | 0.052 | |||
| Custom-made | 111 (81.0) | 83 (77.6) | 28(93.3) | |
| Off-the-shelf | 16 (11.7) | 14 (13.1) | 2 (6.7) | |
| Surgeon-modified | 10 (7.3) | 10 (9.3) | 0(0.0) | |
| Inverted limb | 17 (12.41) | 16 (15.0) | 1 (3.3) | 0.088 |
| Preloaded catheter | 13 (9.5) | 11 (10.3) | 2 (6.7) | 0.55 |
| Percutaneous femoral access | 123 (89.8) | 96 (89.7) | 27 (90.0) | 0.99 |
| N° of Target vessels | 3.6 ± 0.8 | 3.6 ± 0.8 | 3.9 ± 0.3 | 0.095 |
| CT | 106 (77) | 77 (72) | 29(96.7) | 0.004 |
| SMA | 125 (91) | 95 (88.8) | 30 (100) | 0.068 |
| RRA | 130 (94.9) | 103 (96) | 27 (90) | 0.177 |
| LRA | 134 (97.8) | 107 (100) | 30 (100) | 0.999 |
| ARA | 7 (5) | 7 (6) | 0 (0) | 0.347 |
| N° of Target vessels ≥4 | 100 (73.0) | 74 (69.0) | 26 (86.7) | 0.056 |
| Type of incorporation | ||||
| n | 505 | 388 | 117 | |
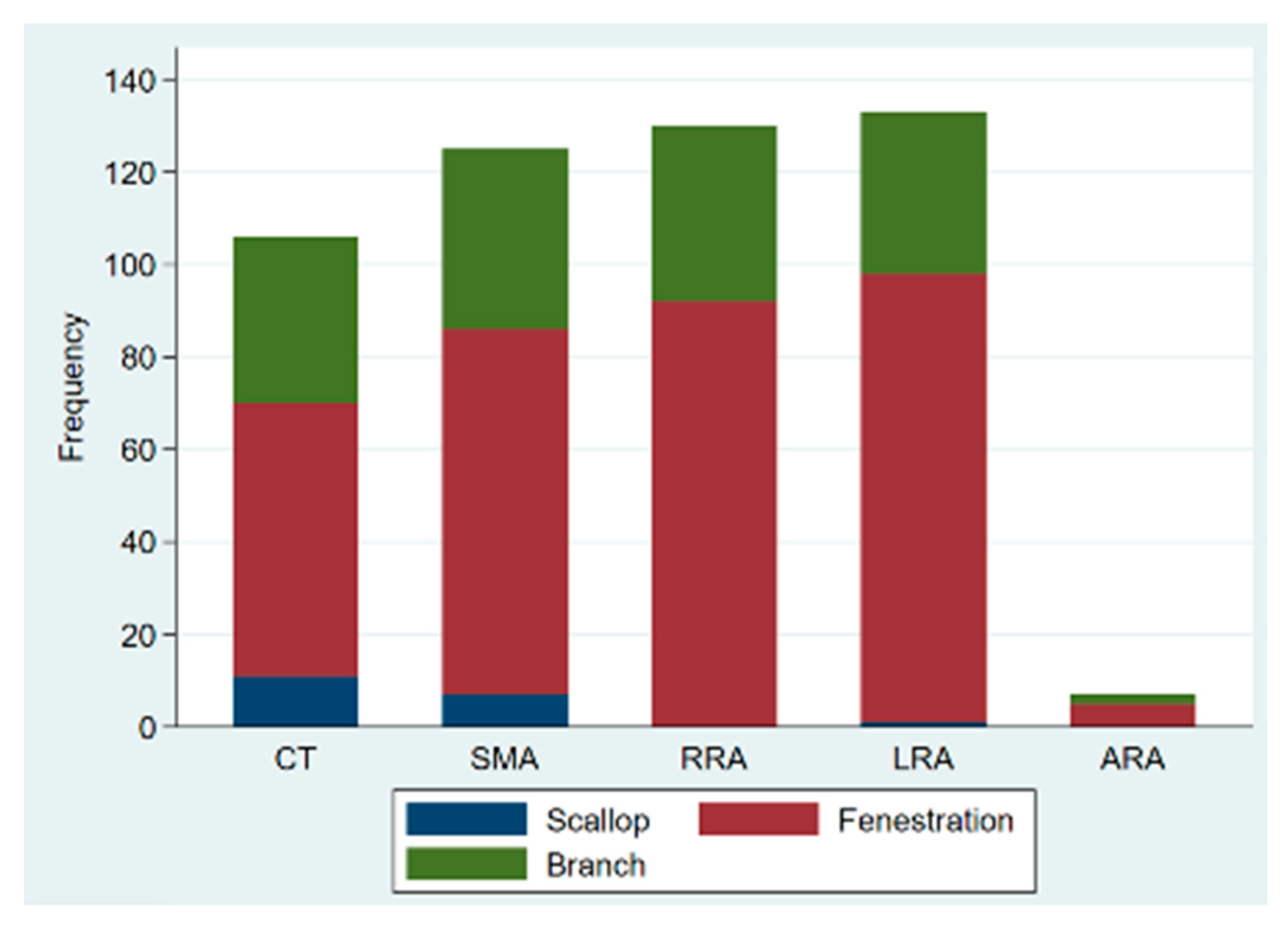
| Scallop | 22(4.4) | 22(5.7) | 0(0) | 0.008 |
| Fenestrations | 333 (65.9) | 241 (62) | 92 (78.6) | 0.001 |
| Branches | 150 (29.7) | 125 (32) | 25 (21.4) | 0.024 |
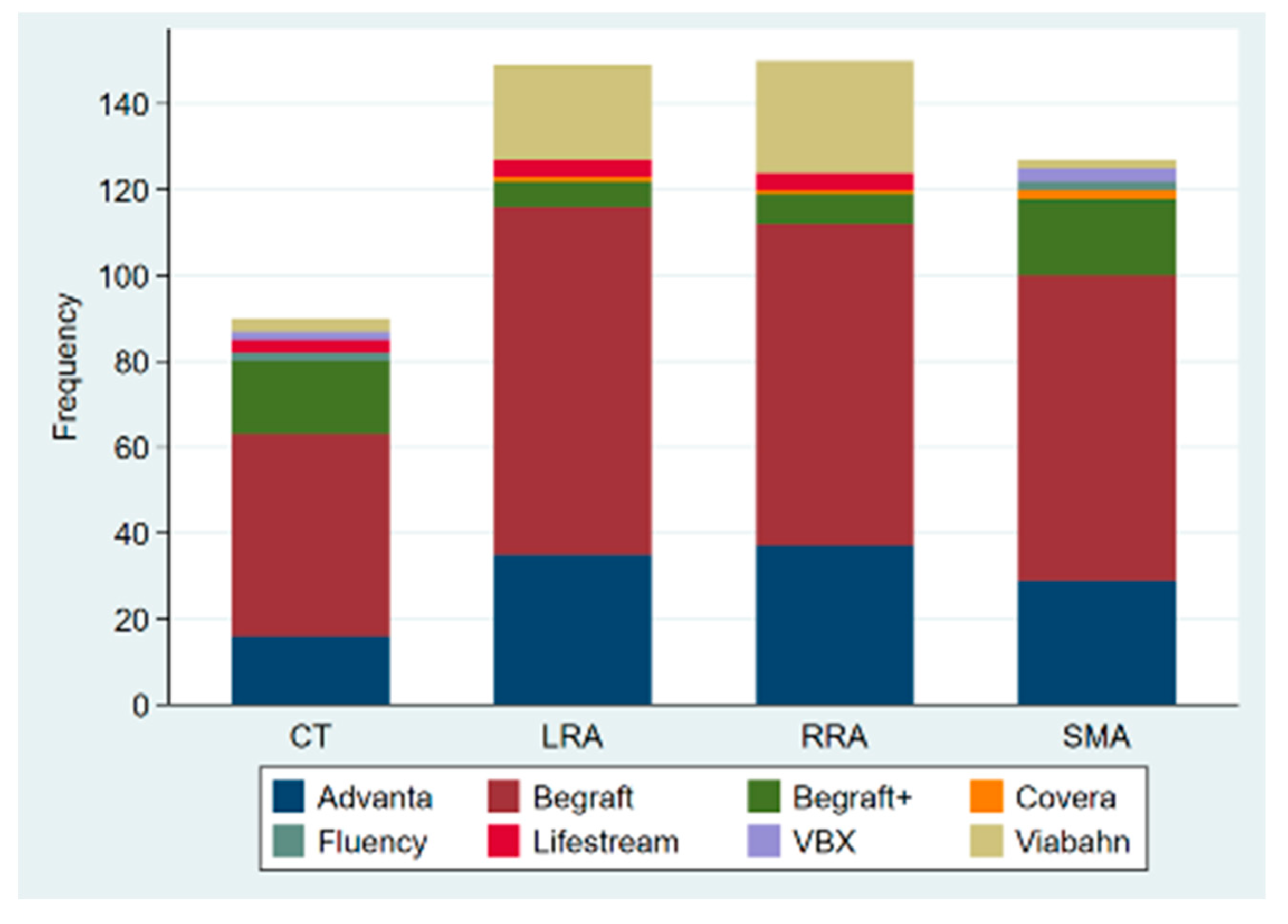
| Bridging stent diameter, mm | ||||
| CT | 8 ± 2 | 8 ± 2 | 9 ± 1 | 0.008 |
| SMA | 8 ± 1 | 8 ± 1 | 8 ± 1 | 0.17 |
| RRA | 6 ± 1 | 6 ± 1 | 7 ± 1 | 0.040 |
| LRA | 7 ± 1 | 6 ± 1 | 7 ± 1 | 0.011 |
| Bridging stent sealing length, mm ± SD | ||||
| CT | 43 ± 26 | 45 ± 28 | 38 ± 18 | 0.26 |
| SMA | 46 ± 24 | 47 ± 23 | 45 ± 26 | 0.74 |
| RRA | 40 ± 24 | 40 ± 25 | 40 ± 23 | 0.97 |
| LRA | 40 ± 23 | 39 ± 23 | 42 ± 24 | 0.54 |
| Adjunctive procedures | 41 (29.9) | 24 (22.4) | 17 (56.7) | <0.001 |
| Bifurcation relining | 14 (10.2) | 7 (6.5) | 7 (23.3) | 0.014 |
| Iliac side branch | 31 (22.6) | 18 (16.8) | 13(43.3) | 0.002 |
| Fusion | 51 (37.3) | 41 (38.3) | 10 (33.3) | 0.62 |
| CSF drainage | 69 (50.4) | 45 (42.1) | 24 (80.0) | <0.001 |
| Contrast volume, mL | 300 (200–400) | 300 (200–400) | 400 (300–500) | 0.009 |
| Fluoroscopy time, min | 83 (61–115) | 81 (60–116) | 89 (71–115) | 0.74 |
| Radiation dose, mGy | 3255 (2035–5889) | 3134 (2035–5909) | 4624 (1849–5736) | 0.75 |
| Total procedural time, min | 261 (203–333) | 255 (196–334) | 272 (232–315) | 0.73 |
| Technical success | 133 (97.1) | 105 (98.1) | 28 (97.1) | 0.21 |
| Endoleak at final angio | 22 (16.0) | 17 (15.9) | 5 (16.7) | 0.99 |
| Type I | 4 (2.9) | 1 (0.9) | 3 (10.0) | 0.033 |
| Type II | 16 (11.7) | 14 (13.1) | 2 (6.7) | 0.52 |
| Type IIIc | 2 (1.5) | 2 (1.9) | 0 (0.0) | 0.45 |
| Outcome * | All (n = 137) | Degenerative Aneurysms n = 107 | Post-Dissection Aneurysms n = 30 | p-Value | |||
|---|---|---|---|---|---|---|---|
| MACE | 16 | (11.8) | 15 | (14) | 1 | (3.5) | 0.19 |
| Myocardial infarction | 8 | (5.8) | 8 | (7.5) | 0 | (0.0) | 0.20 |
| Acute heart failure | 11 | (8.0) | 10 | (9.9) | 1 | (3.3) | 0.46 |
| Ischemic stroke | 4 | (2.9) | 4 | (3.7) | 0 | (0.0) | 0.57 |
| Respiratory complications | 10 | (7.3) | 9 | (8.4) | 1 | (3.3) | 0.69 |
| Acute kidney injury | 16 | (11.7) | 12 | (11.2) | 4 | (13.0) | 0.75 |
| No dialysis | 12 | (8.8) | 10 | (9.4) | 2 | (6.7) | - |
| Temporary dialysis | 3 | (2.2) | 1 | (0.9) | 2 | (6.7) | - |
| Permanent dialysis | 1 | (0.7) | 1 | (0.9) | 0 | (0.0) | - |
| Spinal cord injury | 10 | (7.5) | 9 | (8.7) | 1 | (3.3) | 0.45 |
| Paraplegia | 5 | (3.8) | 5 | (4.9) | 0 | (0.0) | - |
| Paresis | 5 | (3.8) | 4 | (3.8) | 1 | (3.3) | - |
| Ischemic colitis | 2 | (1.5) | 2 | (1.9) | 0 | (0.0) | 0.99 |
| Access vessel complication | |||||||
| Femoral access | 10 | (7.3) | 8 | (7.5) | 2 | (6.7) | 0.999 |
| Upper access | 2 | (1.5) | 2 | (1.90 | 0 | (0.0) | 0.999 |
| Surgical wound complication | 4 | (2.9) | 4 | (3.7) | 0 | (0.0) | 0.576 |
| Endoleak | |||||||
| Type I & III | 21 | (15.3) | 11 | (10.3) | 10 | (33.0) | 0.004 |
| Ia | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | - |
| Ib | 7 | (4.7) | 1 | (0.9) | 6 | (20.0) | <0.001 |
| Ic | 1 | (0.7) | 0 | (0.0) | 1 | (0.8) | - |
| IIIa | 6 | (4.4) | 6 | (5.6) | 0 | (0.0) | 0.33 |
| IIIc | 7 | (5.1) | 4 | (3.7) | 3 | (10.0) | 0.18 |
| Type II | 10 | (7.3) | 8 | (7.5) | 2 | (6.7) | 0.99 |
| Postoperative LOS, days | 9 | (7–16) | 9 | (7–20) | 8.5 | (7–11) | 0.50 |
| ICU length of stay, days | 2 | (1–4) | 2 | (1–4) | 3 | (2–4) | 0.89 |
| Reintervention | 8 | (5.9) | 7 | (6.5) | 1 | (3.5) | 0.99 |
| Mortality | 4 | (2.9) | 4 | (3.7) | 0 | (0.0) | 0.58 |
| Outcome * | All (n = 133) † | Degenerative Aneurysms n = 103 * | Post-Dissection Aneurysms n = 30 | p-Value | |||
|---|---|---|---|---|---|---|---|
| Follow-up, months, m (sd) | 15.2 | (13.1) | 15.2 | (13.3) | 15.3 | (12.5) | 0.82 |
| Late Endoleak, n (%) | 37 | (27.8) | 23 | (22.3) | 14 | (46.7) | 0.009 |
| Type I | 11 | (8.8) | 3 | (2.8) | 8 | (30.0) | <0.001 |
| Ia | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | - |
| Ib | 8 | (6.0) | 3 | (2.9) | 5 | (16.7) | 0.015 |
| Ic | 3 | (2.3) | 0 | (0.0) | 3 | (10.0) | 0.011 |
| Type II | 11 | (8.3) | 8 | (7.8) | 3 | (10.0) | 0.71 |
| Type III | 15 | (11.0) | 12 | (11.2) | 3 | (10.0) | 0.85 |
| IIIa | 13 | (9.5) | 10 | (9.4) | 3 | (10.0) | 0.7 |
| IIIb | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | - |
| IIIc | 2 | (1.5) | 2 | (1.9) | 0 | (0.0) | 0.99 |
| Graft limb occlusion | 7 | (5.3) | 4 | (3.9) | 3 | (10.0) | 0.19 |
| Aneurysm rupture | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Aneurysm sac change, mm/year (n = 107) | −0.1 | (−7.0; 3.0) | −0.95 | (−7.0; 2.2) | 1.6 | (−2.8; 7.3) | 0.40 |
| Sac shrinkage | 28 | (26.2) | 22 | (26.8) | 6 | (26.2) | 0.78 |
| Sac progression | 22 | (20.6) | 13 | (15.9) | 9 | (36.0) | 0.029 |
| Unchanged diameter | 57 | (53.3) | 47 | (57.3) | 10 | (40.0) | 0.13 |
| Late reintervention | 27 | (20.3) | 18 | (17.5) | 9 | (20.3) | 0.13 |
| Target vessel relining/recanalization | 11 | (8.3) | 5 | (4.9) | 6 | (20.0) | 0.016 |
| CT | 2 | (1.5) | 1 | (1.0) | 1 | (3.3) | 0.40 |
| SMA | 3 | (2.3) | 1 | (1.0) | 2 | (6.7) | 0.13 |
| RRA | 4 | (3.0) | 2 | (1.9) | 2 | (6.7) | 0.21 |
| LRA | 6 | (4.5) | 2 | (1.9) | 4 | (13.3) | 0.023 |
| Death | 9 | (6.8) | 9 | (8.7) | 0 | 0 | 0.094 |
| Variable | OR | CI95 | p-Value |
|---|---|---|---|
| Total procedure time > 240 min | 5.3 | (1.7–16.2) | 0.002 |
| Adjunctive procedures | 3.0 | (1.3–7.1) | 0.009 |
| Age < 60 years | 4.7 | (1.7–13.0) | 0.002 |
| Female gender | 1.9 | (0.8–4.8) | 0.177 |
| Extensive TAAA | 2.0 | (0.5–4.5) | 0.116 |
| Post-dissection aneurysm | 3.1 | (1.2–7.6) | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benfor, B.; Högl, J.; Gouveia e Melo, R.; Stana, J.; Prendes, C.F.; Pichlmaier, M.; Rantner, B.; Tsilimparis, N. Postoperative Outcomes and Reinterventions Following Fenestrated/Branched Endovascular Aortic Repair in Post-Dissection and Complex Degenerative Abdominal and Thoraco-Abdominal Aortic Aneurysms. J. Clin. Med. 2022, 11, 4768. https://doi.org/10.3390/jcm11164768
Benfor B, Högl J, Gouveia e Melo R, Stana J, Prendes CF, Pichlmaier M, Rantner B, Tsilimparis N. Postoperative Outcomes and Reinterventions Following Fenestrated/Branched Endovascular Aortic Repair in Post-Dissection and Complex Degenerative Abdominal and Thoraco-Abdominal Aortic Aneurysms. Journal of Clinical Medicine. 2022; 11(16):4768. https://doi.org/10.3390/jcm11164768
Chicago/Turabian StyleBenfor, Bright, Julia Högl, Ryan Gouveia e Melo, Jan Stana, Carlota Fernandez Prendes, Maximilian Pichlmaier, Barbara Rantner, and Nikolaos Tsilimparis. 2022. "Postoperative Outcomes and Reinterventions Following Fenestrated/Branched Endovascular Aortic Repair in Post-Dissection and Complex Degenerative Abdominal and Thoraco-Abdominal Aortic Aneurysms" Journal of Clinical Medicine 11, no. 16: 4768. https://doi.org/10.3390/jcm11164768







