Is Alcohol Consumption Associated with a Lower Risk of Cardiovascular Events in Patients Treated with Statins? An Observational Real-World Experience
Abstract
:1. Introduction
2. Materials and Methods
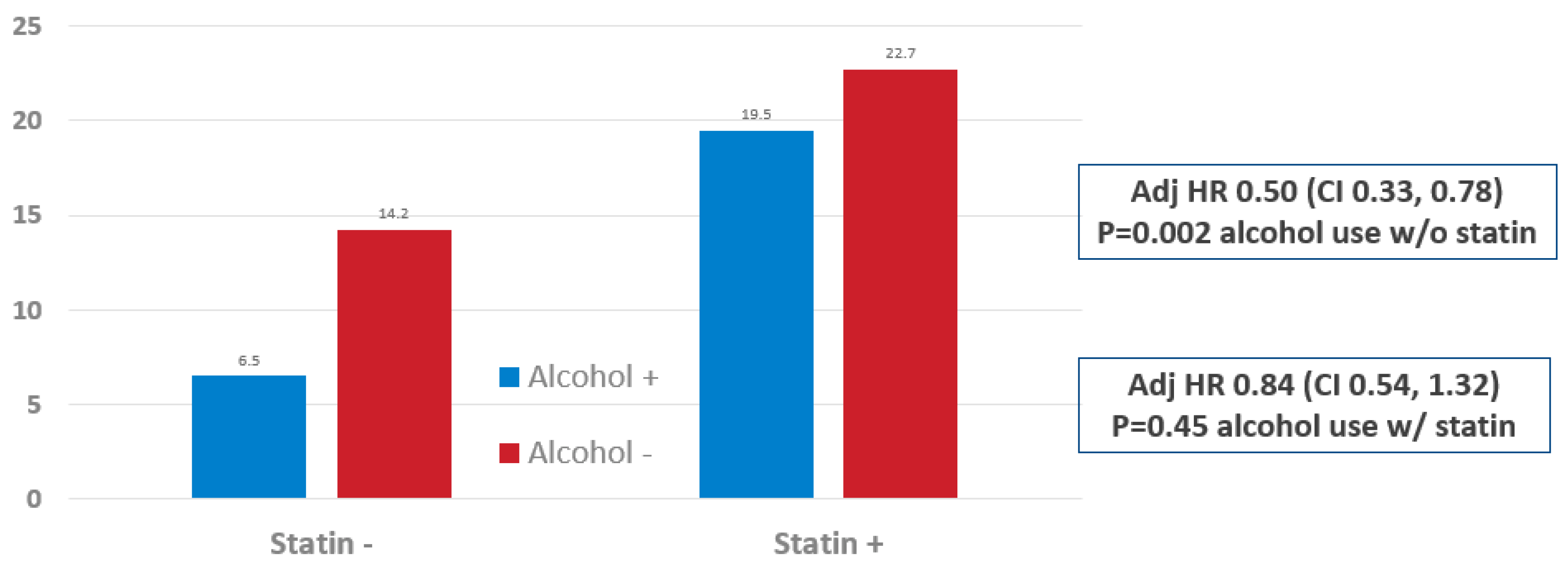
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Borschmann, R. GBD 2017 Causes of Death CO\ollaborators. Global, regional, and national age sex specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T. Alcohol and heart disease. Circulation 1996, 94, 3023–3025. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Bybee, K.A.; Lavie, C. Alcohol and cardiovascular health: The razor-sharp double-edged sword. J. Am. Coll. Cardol. 2007, 50, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health Advisory Committee to the Director. ACD Working Group for Review of the Moderate Alcohol and Cardiovascular Health Trial. Available online: https://acd.od.nih.gov>documents>presentations>PDF (accessed on 14 June 2018).
- Raber, I.; McCarthy, C.P.; Vaduganathan, M.; Bhatt, D.L.; A Wood, D.; Cleland, J.G.F.; Blumenthal, R.S.; McEvoy, J.W. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet 2019, 393, 2155–2167. [Google Scholar] [CrossRef]
- Bangalore, S.; Steg, G.; Deedwania, P.; Crowley, K.; Eagle, K.A.; Goto, S. Beta-blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA 2012, 308, 1340–1349. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Glynn, R.J. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Yeboah, J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar]
- Bartholomew, C.L.; Muhlestein, J.B.; Anderson, J.L.; May, H.T.; Knowlton, K.U.; Bair, T.L.; Horne, B.D. Association of periodic fasting lifestyles with survival and incident major adverse cardiovascular events in patients undergoing cardiac catheterization. Eur. J. Prev. Cardiol. 2022, 28, 1774–1781. [Google Scholar] [CrossRef]
- Thompson, P. J-curve revisited: Cardiovascular benefits of moderate alcohol use cannot be dismissed. Med. J. Aust. 2013, 198, 419–422. [Google Scholar] [CrossRef]
- Bell, S.; Daskalopoulou, M.; Rapsomaniki, E.; George, J.; Britton, A.; Bobak, M.; Hemingway, H. Association between clinically recorded alcohol consumption and clinical presentation of 12 cardiovascular diseases: Population based study using linked health records. BMJ 2017, 356, j909. [Google Scholar] [CrossRef]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.S.; Willeit, P.; Warnakula, S.; Bolton, T.; Thompson, S. Emerging risk factors collaboration/EPIC-CVD/UK Biobank Alcohol Study Group. Risk thresholds for alcohol consumption: Combined analysis of individual participant data for 599,912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef]
- Viscontay, R.; Sunderland, M.; Slade, T.; Wilson, J.L. Are there non-linear relationships between alcohol consumption and long-term health? BMJ Open 2021, 11, e043985. [Google Scholar] [CrossRef]
- Naimi, T.S.; Brown, D.W.; Brewer, R.D.; Giles, W.H.; Mensah, G.; Serdula, M.K.; Mokdad, A.H.; Hungerford, D.W.; Lando, J.; Naimi, S.; et al. Cardiovascular risk factors and confounders among nondrinking and moderate-drinking US adults. Am. J. Prev. Med. 2005, 28, 369–373. [Google Scholar] [CrossRef]
- Davey Smith, G.; Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef]
- Millwood, I.Y.; Walters, R.G.; Mei, X.W.; Guo, Y.; Yang, L.; Bian, Z.; A Bennett, D.; Chen, Y.; Dong, C.; Hu, R.; et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: A prospective study of 500,000 men and women in China. Lancet 2019, 393, 1831–1842. [Google Scholar] [CrossRef]
- Larsson, S.C.; Burgess, S.; Mason, A.M.; Michaëlsson, K. Alcohol consumption and cardiovascular disease: A mendelian randomization study. Circ. Genomic Precis. Med. 2020, 13, e002814. [Google Scholar] [CrossRef] [PubMed]
- Biddinger, K.J.; Emdin, C.A.; Haas, M.E.; Wang, M.; Hindy, G.; Ellinor, P.T.; Aragam, K.G. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw. Open 2021, 5, e223849. [Google Scholar] [CrossRef]
- Arora, M.; ElSayed, A.; Beger, B.; Naidoo, P.; Shilton, T.; Jain, N.; Champagne, B.M. The Impact of Alcohol Consumption on Cardio. The impact of alcohol consumption on cardiovascular health: Myths and measures. Glob. Heart 2022, 17, 1. [Google Scholar] [CrossRef]
- Griswold, M.G.; Fullman, N.; Hawley, C.; Arian, N.; Zimsen, S.R.; Tymeson, H.D.; Farioli, A. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Blobal Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef]
- USDA. Dietary Guidelines for Americans. Scientific Report of the 2020 Dietary Guidelines Advisory Committee; USDA: Washington, DC, USA, 2020.

| Statin—Yes | Statin—No | |
|---|---|---|
| Alcohol—Yes | ||
| N patients | 164 | 416 |
| Age, mean y (SD) | 62 (11) | 54 (16) |
| Sex, % male | 67.7% | 57.2% |
| Alcohol—No | ||
| N patients | 313 | 808 |
| Age, mean y (SD) | 64 (13) | 57 (17) |
| Sex, % male | 53.7% | 46.4% |
| Statin—Yes | Statin—No | |
|---|---|---|
| Alcohol—Yes | ||
| N patients | 599 | 292 |
| Age, mean y (SD) | 64 (12) | 63 (14) |
| Sex, % male | 75.9% | 67.8% |
| Alcohol—No | ||
| N patients | 1509 | 866 |
| Age, mean y (SD) | 67 (11) | 67 (13) |
| Sex, % male | 69.6% | 58.9% |
| Characteristic or | Primary Prevention | Secondary Prevention | ||||
|---|---|---|---|---|---|---|
| Outcome | Overall | Alcohol+ | Alcohol− | Overall | Alcohol+ | Alcohol− |
| Baseline Characteristics | ||||||
| Hyperlipidemia | 19.8% | 16.7% | 21.3% * | 54.4% | 55.0% | 54.2% |
| Hypertension | 25.9% | 23.4% | 27.2% | 56.8% | 54.7% | 57.5% |
| Diabetes | 21.8% | 14.1% | 25.8% † | 45.0% | 35.6% | 48.5% † |
| Smoking | 2.3% | 3.8% | 1.5% * | 8.6% | 14.4% | 6.4% † |
| Family History | 4.5% | 5.2% | 4.1% | 19.4% | 19.1% | 19.5% |
| Prior CAD | 0% | 0% | 0% | 76.2% | 70.3% | 78.4% † |
| Prior MI | 0% | 0% | 0% | 12.4% | 10.0% | 13.3% * |
| Prior Stroke | 4.5% | 4.3% | 4.6% | 7.1% | 4.7% | 8.0% † |
| HF History | 9.8% | 9.5% | 9.9% | 13.8% | 11.0% | 14.9% * |
| Atrial Fibrillation | 43.7% | 44.8% | 43.2% | 40.8% | 35.7% | 42.7% † |
| Renal Failure | 0.5% | 0.0% | 0.7% * | 2.2% | 2.1% | 2.3% |
| PVD | 19.9% | 16.9% | 21.5% * | 39.2% | 34.0% | 41.1% † |
| Angiography: Obstructive CAD | ||||||
| None | 100% | 100% | 100% | 29.5% | 30.0% | 29.3% |
| Mild | 0% | 0% | 0% | 13.4% | 14.4% | 13.1% |
| Significant | 0% | 0% | 0% | 57.1% | 55.6% | 57.6% |
| Hospital Treatment Modality | ||||||
| Medical Only | 100% | 100% | 100% | 71.4% | 70.3% | 71.9% |
| PCI | 0% | 0% | 0% | 25.3% | 26.1% | 25.0% |
| CABG | 0% | 0% | 0% | 3.2% | 3.5% | 3.1% |
| Longitudinal MACE Outcomes | ||||||
| MI | 0.9% | 0.5% | 1.0% | 6.2% | 5.7% | 6.4% |
| Stroke | 5.4% | 4.0% | 6.1% | 6.2% | 6.3% | 6.1% |
| HF Hospitalization | 3.4% | 2.3% | 4.0% | 5.7% | 4.8% | 6.1% |
| All-Cause Mortality | 5.3% | 3.4% | 6.3% * | 9.7% | 8.2% | 10.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, J.L.; Le, V.T.; Bair, T.L.; Muhlestein, J.B.; Knowlton, K.U.; Horne, B.D. Is Alcohol Consumption Associated with a Lower Risk of Cardiovascular Events in Patients Treated with Statins? An Observational Real-World Experience. J. Clin. Med. 2022, 11, 4797. https://doi.org/10.3390/jcm11164797
Anderson JL, Le VT, Bair TL, Muhlestein JB, Knowlton KU, Horne BD. Is Alcohol Consumption Associated with a Lower Risk of Cardiovascular Events in Patients Treated with Statins? An Observational Real-World Experience. Journal of Clinical Medicine. 2022; 11(16):4797. https://doi.org/10.3390/jcm11164797
Chicago/Turabian StyleAnderson, Jeffrey L., Viet T. Le, Tami L. Bair, Joseph B. Muhlestein, Kirk U. Knowlton, and Benjamin D. Horne. 2022. "Is Alcohol Consumption Associated with a Lower Risk of Cardiovascular Events in Patients Treated with Statins? An Observational Real-World Experience" Journal of Clinical Medicine 11, no. 16: 4797. https://doi.org/10.3390/jcm11164797
APA StyleAnderson, J. L., Le, V. T., Bair, T. L., Muhlestein, J. B., Knowlton, K. U., & Horne, B. D. (2022). Is Alcohol Consumption Associated with a Lower Risk of Cardiovascular Events in Patients Treated with Statins? An Observational Real-World Experience. Journal of Clinical Medicine, 11(16), 4797. https://doi.org/10.3390/jcm11164797








