Characteristics and Risk Factors of Myocardial Injury after Traumatic Hemorrhagic Shock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Data Collection
2.4. Potential Confounders and Risk Factors
2.5. Outcome
2.6. Statistical Analysis
3. Results
3.1. Studies Included
3.2. Risk Factors for Myocardial Injury after Traumatic Hemorrhagic Shock
3.3. Relevant Factors for Death in Patients with Myocardial Injury after Traumatic Hemorrhagic Shock
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, M.; Wang, H.; Zeng, X.; Yin, P.; Zhu, J.; Chen, W.; Li, X.; Wang, L.; Wang, L.; Liu, Y.; et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 394, 1145–1158. [Google Scholar] [CrossRef]
- Trauma Trunkey, D. Accidental and intentional injuries account for more years of life lost in the U.S. than cancer and heart disease. Among the prescribed remedies are improved preventive efforts, speedier surgery and further research. Sci. Am. 1983, 249, 28–35. [Google Scholar] [PubMed]
- Probst, C.; Zelle, B.A.; Sittaro, N.A.; Lohse, R.; Krettek, C.; Pape, H.C. Late death after multiple severe trauma: When does it occur and what are the causes? J. Trauma 2009, 66, 1212. [Google Scholar] [CrossRef]
- De’Ath, H.D.; Rourke, C.; Davenport, R.; Manson, J.; Renfrew, I.; Uppal, R.; Davies, L.C.; Brohi, K. Clinical and biomarker profile of trauma-induced secondary cardiac injury. Br. J. Surg. 2012, 99, 789–797. [Google Scholar] [CrossRef]
- Ismailov, R.M.; Ness, R.B.; Weiss, H.B.; Lawrence, B.A.; Miller, T.R. Trauma associated with acute myocardial infarction in a multi-state hospitalized population. Int. J. Cardiol. 2005, 105, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.; Naganathar, S.; Praditsuktavorn, B.; Bugg, O.F.; McArthur, S.; Thiemermann, C.; Tremoleda, J.L.; Brohi, K. Modeling Cardiac Dysfunction Following Traumatic Hemorrhage Injury: Impact on Myocardial Integrity. Front. Immunol. 2019, 10, 2774. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.; Ferguson, N.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Jalan, R.; Gines, P.; Olson, J.C.; Mookerjee, R.P.; Moreau, R.; Garcia-Tsao, G.; Arroyo, V.; Kamath, P.S. Acute-on chronic liver failure. J. Hepatol. 2012, 57, 1336–1348. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Mickley, H.; Crea, F.; Van de Werf, F.; et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- O’gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef] [PubMed]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernández-Mondéjar, E.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Nardi, G.; et al. Management of bleeding and coagulopathy following major trauma: An updated European guideline. Crit. Care 2013, 17, R76. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Mathew, R.O.; Kuo, Y.H.; Asif, A. Risk of severe acute kidney injury in multiple trauma patients: Risk estimation based on a national trauma dataset. Injury 2020, 51, 45–50. [Google Scholar] [CrossRef]
- Ruetzler, K.; Smilowitz, N.R.; Berger, J.S.; Devereaux, P.J.; Maron, B.A.; Newby, L.K.; de Jesus Perez, V.; Sessler, D.I.; Wijeysundera, D.N.; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; et al. Diagnosis and Management of Patients With Myocardial Injury after Noncardiac Surgery: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e287–e305. [Google Scholar] [CrossRef] [PubMed]
- Rixen, D.; Siegel, J.H. Bench-to-bedside review: Oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post-traumatic shock. Crit. Care 2005, 9, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Pickkers, P.; Darmon, M.; Hoste, E.; Joannidis, M.; Legrand, M.; Ostermann, M.; Prowle, J.R.; Schneider, A.; Schetz, M. Acute kidney injury in the critically ill: An updated review on pathophysiology and management. Intens. Care Med. 2021, 47, 835–850. [Google Scholar] [CrossRef]
- Botto, F.; Alonso-Coello, P.; Chan, M.T.; Villar, J.C.; Xavier, D.; Srinathan, S.; Guyatt, G.; Cruz, P.; Graham, M.; Wang, C.Y.; et al. Myocardial injury after noncardiac surgery: A large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014, 120, 564–578. [Google Scholar]
- Puelacher, C.; Pinto, B.B.; Mills, N.L.; Duceppe, E.; Popova, E.; Duma, A.; Nagele, P.; Omland, T.; Hammerer-Lercher, A.; Buse, G.L. Expert consensus on peri-operative myocardial injury screening in noncardiac surgery: A literature review. Eur. J. Anaesthesiol. 2021, 38, 600–608. [Google Scholar] [CrossRef]
- Anker, S.D.; Voors, A.; Okonko, D.; Clark, A.L.; James, M.K.; Von Haehling, S.; Kjekshus, J.; Ponikowski, P.; Dickstein, K. Prevalence, incidence, and prognostic value of anaemia in patients after an acute myocardial infarction: Data from the OPTIMAAL trial. Eur. Heart J. 2009, 30, 1331–1339. [Google Scholar] [CrossRef]
- Wischmann, P.; Kuhn, V.; Suvorava, T.; Muessig, J.M.; Fischer, J.W.; Isakson, B.E.; Kelm, M. Anaemia is associated with severe RBC dysfunction and a reduced circulating NO pool: Vascular and cardiac eNOS are crucial for the adaptation to anaemia. Basic Res. Cardiol. 2020, 115, 43. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, A.; Cortese-Krott, M.M.; Kelm, M.; Li, N.; Pernow, J. Novel perspectives on redox signaling in red blood cells and platelets in cardiovascular disease. Free Radic. Biol. Med. 2021, 168, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Combes, A.; Aissaoui, N. Cardiac injury in COVID-19. Intens. Care Med. 2021, 48, 111–113. [Google Scholar] [CrossRef]
- De’Ath, H.D.; Manson, J.; Davenport, R.; Glasgow, S.; Renfrew, I.; Davies, L.C.; Brohi, K. Trauma-induced secondary cardiac injury is associated with hyperacute elevations in inflammatory cytokines. Shock 2013, 39, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Naganathar, S.; De’Ath, H.D.; Wall, J.; Brohi, K. Admission biomarkers of trauma-induced secondary cardiac injury predict adverse cardiac events and are associated with plasma catecholamine levels. J. Trauma Acute Care Surg. 2015, 79, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Herwald, H.; Theopold, U. Hemostasis in invertebrates and vertebrates: An evolutionary excursion. J. Innate Immun. 2011, 3, 1–2. [Google Scholar] [CrossRef]
- Jackson, S.P.; Darbousset, R.; Schoenwaelder, S.M. Thromboinflammation: Challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood 2019, 133, 906–918. [Google Scholar] [CrossRef]
- Darlington, D.N.; Gonzales, M.D.; Craig, T.; Dubick, M.A.; Cap, A.P.; Schwacha, M.G. Trauma-Induced Coagulopathy Is Associated with a Complex Inflammatory Response in the Rat. Shock 2015, 44 (Suppl. S1), 129–137. [Google Scholar] [CrossRef]
- Kornblith, L.Z.; Moore, H.B.; Cohen, M.J. Trauma-induced coagulopathy: The past, present, and future. J. Thromb. Haemost. 2019, 17, 852–862. [Google Scholar] [CrossRef]
- Johansson, P.I.; Stensballe, J.; Ostrowski, S.R. Shock induced endotheliopathy (SHINE) in acute critical illness—A unifying pathophysiologic mechanism. Crit. Care 2017, 21, 25. [Google Scholar] [CrossRef]
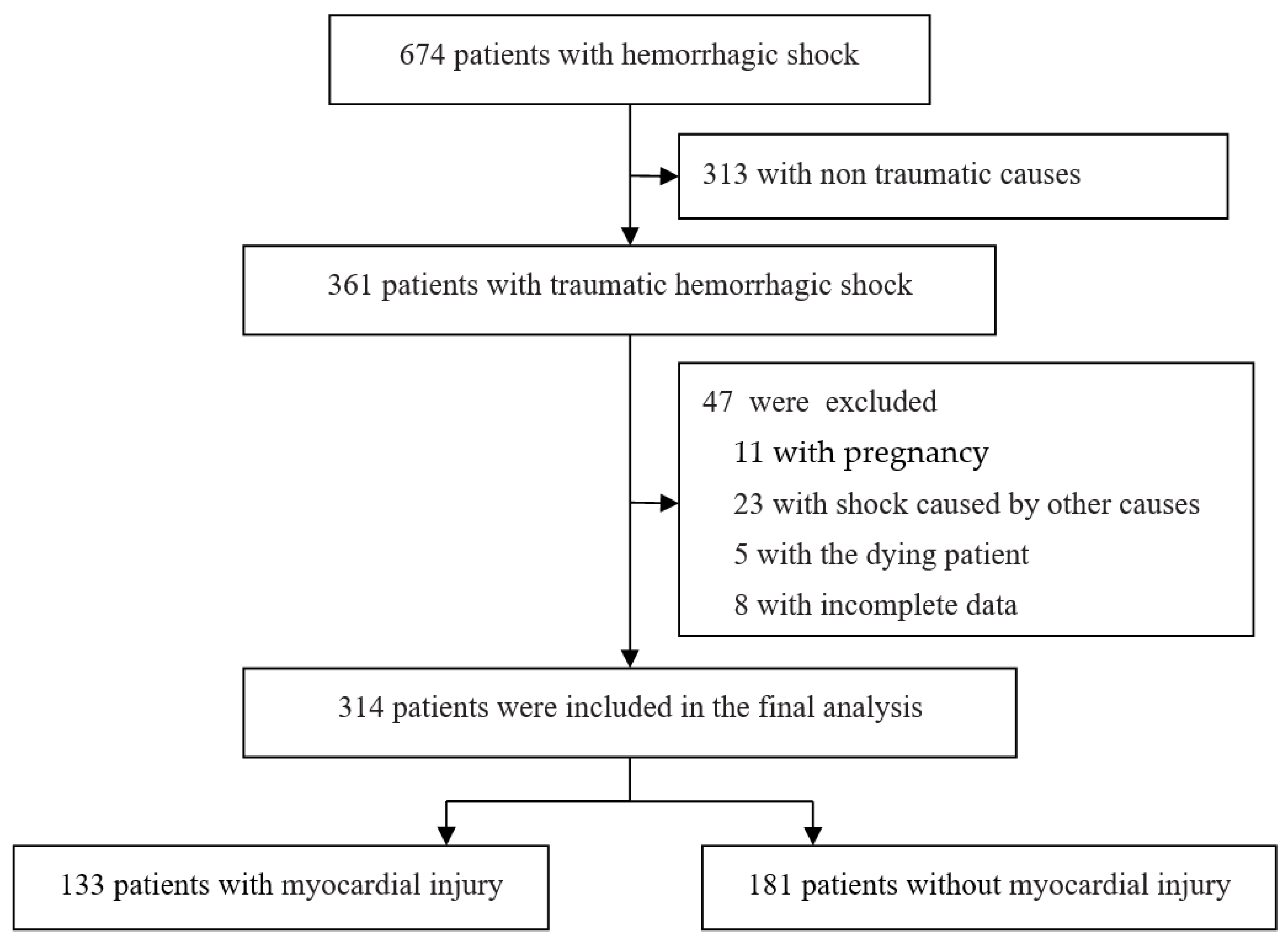
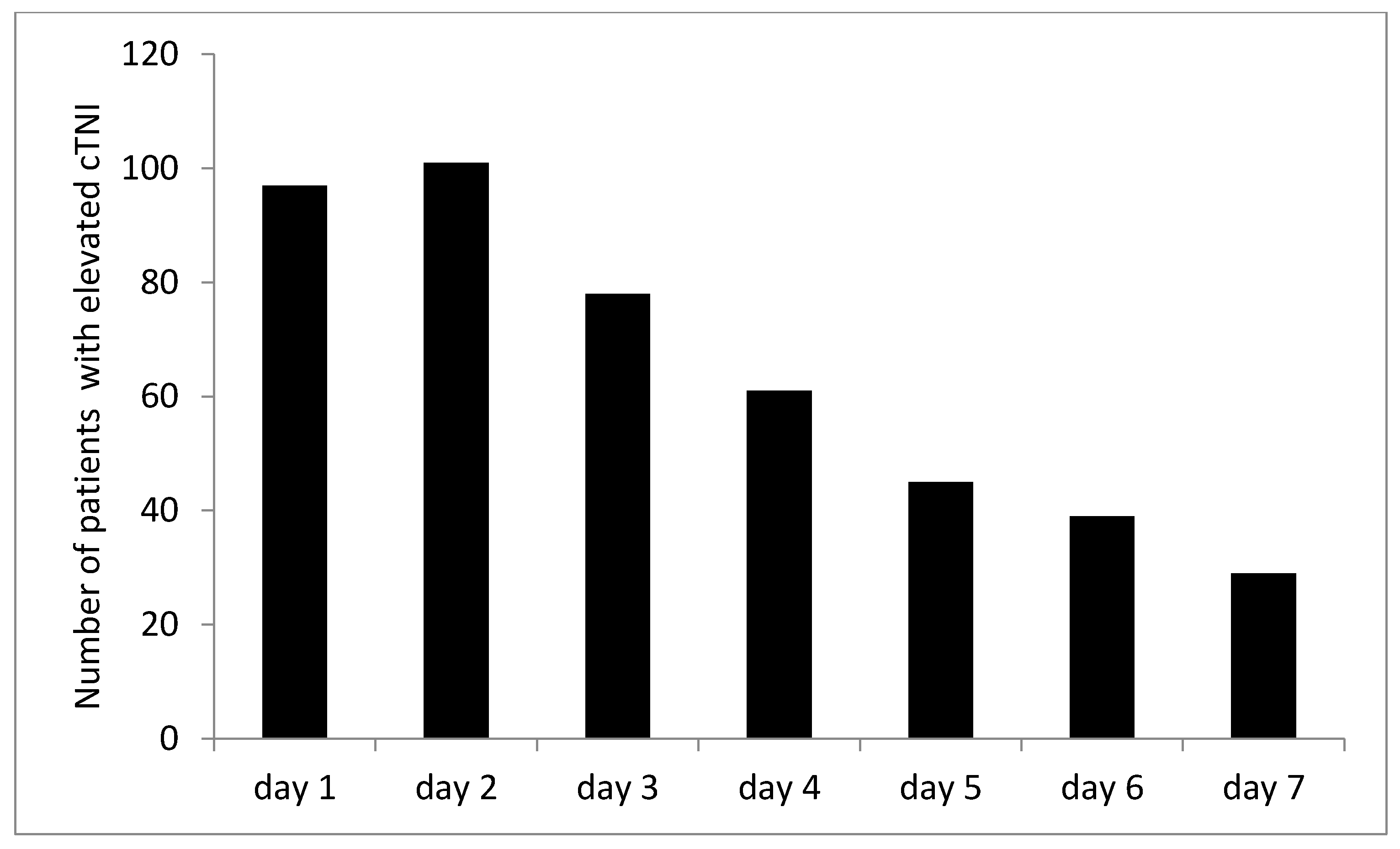
| Characteristics | n = 314 |
|---|---|
| Male (n, %) | 152 (48.4) |
| Age [M (P25–P75), years] | 63.0 (49.8–82.0) |
| Preadmission conditions (n, %) | |
| Coronary heart disease | 14 (4.5) |
| Hypertension | 102 (32.5) |
| Diabetes mellitus | 45 (14.3) |
| Cerebral hemorrhage | 26 (8.3) |
| Gastrointestinal hemorrhage | 5 (1.6) |
| Antiplatelet drugs | 48 (15.3) |
| Anticoagulant drugs | 7 (2.2) |
| Nonsteroidal anti-inflammatory drugs | 20 (6.4) |
| Causes of trauma (n, %) | |
| Falling from height | 47 (15.0) |
| Road traffic accident | 95 (30.3) |
| Falling from a standing position | 146 (46.5) |
| Others (crush, stab, animal bite) | 26 (8.3) |
| Main bleeding site (n, %) | |
| Thoracic | 55 (17.5) |
| Abdominal | 34 (10.8) |
| Pelvic | 31 (9.9) |
| Limbs | 145 (46.2) |
| Others (blood vessels, skin, and soft tissue) | 50 (15.9) |
| ISS [M (P25–P75)] | 25.0 (16.0–34.0) |
| APACHE Ⅱ score [M (P25–P75)] | 18.0 (14.0–23.0) |
| Acute myocardial injury (n, %) | 133 (42.4) |
| Acute myocardial infarction (n, %) | 2 (0.6) |
| Acute kidney injury (n, %) | 88 (28.0) |
| In hospital mortality (n, %) | 30 (9.6%) |
| Length of stay in hospital (days) | 16.0 (10.0–24.0) |
| Length of stay in ICU (days) | 11.0 (5.0–18.0) |
| Characters | Non-Myocardial Injury n = 181 | Myocardial Injury n = 133 | Test Value | p Value |
|---|---|---|---|---|
| Male (n, %) | 78 (43.1) | 74 (55.6) | 4.831 | 0.028 |
| Age [M (P25–P75), years] | 64.0 (50.5–81.5) | 62.0 (48.5–82.0) | 0.211 | 0.833 |
| Preadmission conditions (n, %) | ||||
| Coronary heart disease | 10 (5.5) | 4 (3.0) | 1.141 | 0.286 |
| Hypertension | 57 (31.5) | 45 (33.8) | 0.192 | 0.661 |
| Diabetes mellitus | 27 (14.9) | 18 (13.5) | 0.119 | 0.730 |
| Cerebral hemorrhage | 17 (9.4) | 9 (6.8) | 0.696 | 0.404 |
| Gastrointestinal hemorrhage | 4 (2.2) | 1 (0.8) | 1.040 | 0.308 |
| Antiplatelet drugs | 29 (16.0) | 19 (14.3) | 0.178 | 0.673 |
| Anticoagulant drugs | 5 (2.8) | 2 (1.5) | 0.557 | 0.455 |
| Nonsteroidal anti-inflammatory drugs | 13 (7.2) | 7 (5.3) | 0.473 | 0.491 |
| Causes of trauma (n, %) | ||||
| Falling from height | 19 (10.5) | 28 (21.1) | 6.711 | 0.01 |
| Road traffic accident | 49 (27.1) | 46 (34.6) | 2.052 | 0.152 |
| Falling from a standing position | 99 (54.7) | 47 (35.3) | 0.028 | 0.867 |
| Others (crush, stab, animal bite) | 14 (7.7) | 12 (9.0) | 0.167 | 0.682 |
| Main bleeding site (n, %) | ||||
| Thoracic | 26 (14.4) | 29 (21.8) | 2.937 | 0.087 |
| Abdominal | 12 (6.6) | 22 (16.5) | 7.800 | 0.005 |
| Pelvic | 13 (7.2) | 18 (13.5) | 3.476 | 0.062 |
| Limbs | 105 (58.0) | 40 (30.1) | 24.073 | <0.001 |
| Others (blood vessels, skin, and soft tissue) | 25 (13.8) | 25 (18.8) | 1.423 | 0.233 |
| Mean arterial pressure [M (P25–P75), mmHg] | 70.3 (63.3–80.8) | 67.3 (53.3–77.2) | 2.977 | 0.003 |
| Heart rate [M (P25–P75), beats/minutes] | 99.0 (84.8–114.0) | 116.0 (102.5–130.5) | 6.164 | <0.001 |
| Laboratory test | ||||
| Leukocyte count [M (P25–P75), ×109/L] | 9.7 (7.0–12.1) | 10.2 (8.1–14.8) | 2.195 | 0.028 |
| Neutrophil count [M (P25–P75), ×109/L] | 8.3 (5.9–11.1) | 9.3 (6.6–13.7) | 1.567 | 0.117 |
| Hemoglobin (Mean ± SD, g/L) | 101.3 ± 19.2 | 90.9 ± 25.4 | 4.167 | <0.001 |
| Platelet count [M (P25–P75), ×109/L] | 159.5 (121.0–206.0) | 135.0 (85.0–168.5) | 3.840 | <0.001 |
| Serum creatinine [M (P25–P75), μmol/L] | 65.5 (56.8–89.3) | 80.0 (60.3–113.5) | 3.223 | 0.001 |
| Total bilirubin [M (P25–P75), μmol/L] | 15.1 (11.6–21.1) | 17.0 (8.6–23.0) | 0.102 | 0.919 |
| Prothrombin time [M (P25–P75), s] | 13.8 (12.8–14.9) | 14.8 (13.3–16.6) | 6.561 | <0.001 |
| Fibrinogen [M (P25–P75), mg/dL] | 249.0 (160.5–278.8) | 154.0 (119.8–182.0) | 5.572 | <0.001 |
| D-dimer [M (P25–P75), ng/mL] | 3205.0 (1212.3–5354.8) | 7462.0 (3294.0–16,961.3) | 5.356 | <0.001 |
| pO2/FiO2 ratio [M (P25–P75), mmHg] | 333.7 (255.6–387.5) | 291.9 (207.2–362.4) | 2.514 | 0.012 |
| Serum lactate [M (P25–P75), mmol/L] | 2.2 (1.7–3.3) | 3.4 (1.9–6.7) | 4.920 | <0.001 |
| Serum chloride [M (P25–P75), mmol/L] | 106.7 (103.8–109.5) | 111.8 (107.9–113.6) | 3.192 | 0.001 |
| Uric acid [M (P25–P75), μmol/L] | 301.0 (186.8–453.0) | 291.0(230.0–378.3) | 2.563 | 0.01 |
| C-reactive protein [M (P25–P75), mg/L] | 47.0 (11.6–82.3) | 57.8 (22.2–90.7) | 0.280 | 0.779 |
| Serum Procalcitonin [M (P25–P75), ng/mL] | 0.7 (0.2–2.4) | 3.3 (0.4–8.6) | 2.245 | 0.025 |
| cTNI [M (P25–P75), ng/mL] | 0.007 (0.003–0.02) | 0.4 (0.2–0.7) | 12.411 | <0.001 |
| B-type Natriuretic Peptide [M (P25–P75), pg/mL] | 47.0 (25.0–94.8) | 68.0 (28.0–230.0) | 0.932 | 0.351 |
| LVEF [M (P25–P75), %] | 66.4 (61.0–70.0) | 64.0 (60.0–68.0) | 1.642 | 0.101 |
| Organ dysfunction (n, %) | ||||
| ARDS | 40 (22.1) | 49 (36.8) | 8.204 | 0.004 |
| AKI | 33 (18.2) | 55 (41.4) | 20.319 | <0.001 |
| Acute liver injury | 29 (16.0) | 54 (40.6) | 23.818 | <0.001 |
| ISS [M (P25–P75)] | 14.0 (14.0–25.0) | 22.0 (14.0–34.0) | 4.780 | <0.001 |
| APACHE Ⅱ score [M (P25–P75)] | 16.0 (12.0–19.0) | 19.0 (16.0–23.0) | 5.758 | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Characters | OR (95% CI) | p | OR (95% CI) | p |
| Male | 1.66 (1.06–2.60) | 0.028 | ||
| Falling from height | 2.27 (1.21–4.28) | 0.011 | ||
| Bleeding of abdominal | 2.79 (1.33–5.87) | 0.007 | ||
| Bleeding of limbs | 0.31 (0.19–0.50) | <0.001 | ||
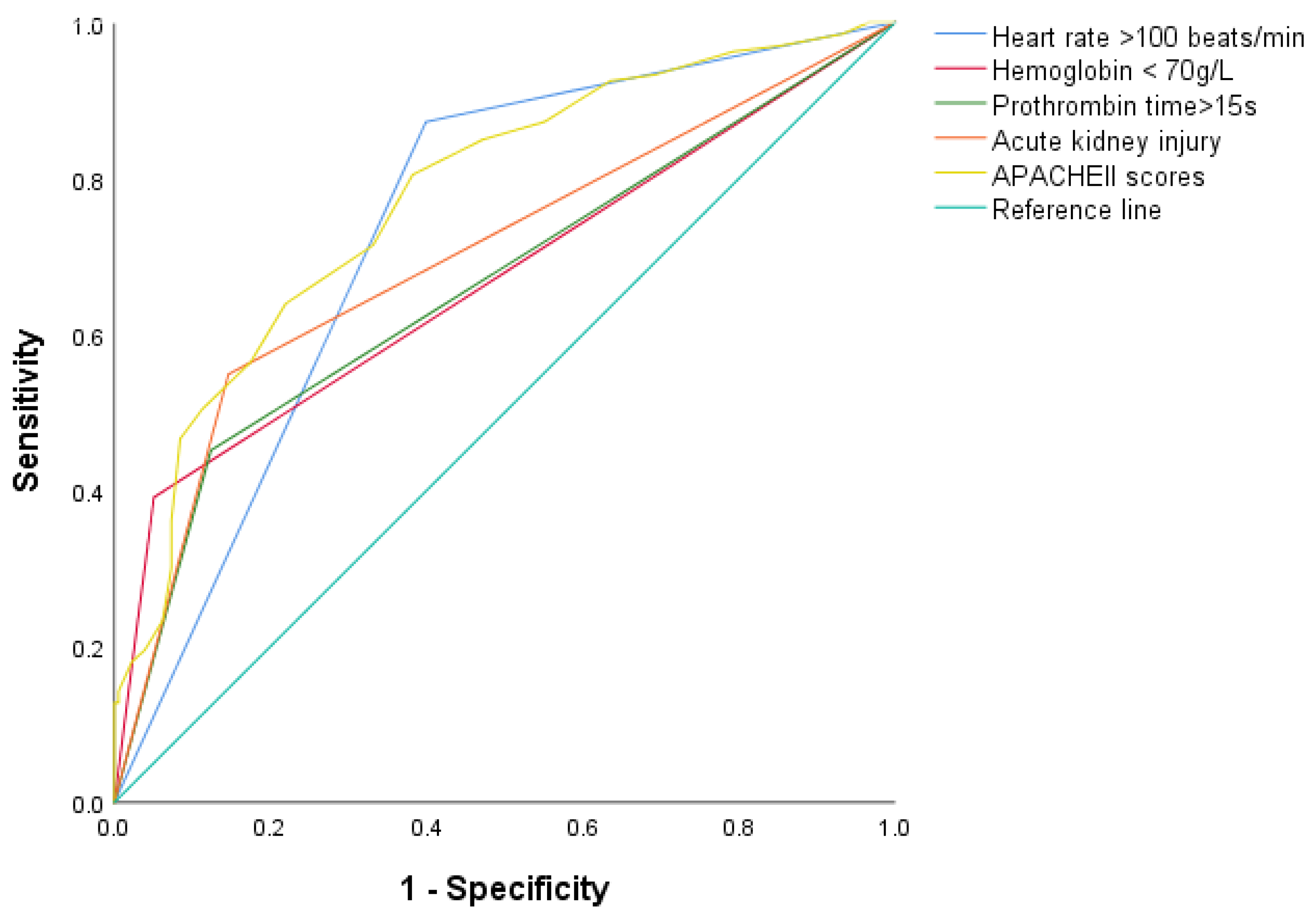
| Heart rate > 100 beats/min | 4.35 (2.59–7.30) | <0.001 | 3.33 (1.56–7.09) | 0.002 |
| Mean arterial pressure | 0.98 (0.97–0.99) | 0.006 | ||
| Leukocyte count | 1.05 (1.00–1.09) | 0.026 | ||
| Hemoglobin < 70 g/L | 5.74 (2.63–12.54) | <0.001 | 3.50 (1.15–10.6) | 0.027 |
| Platelet count | 0.995 (0.991–0.998) | 0.001 | ||
| Prothrombin time > 15 s | 4.61 (2.61–8.13) | <0.001 | 2.39 (1.12–5.10) | 0.024 |
| Fibrinogen | 0.995 (0.993–0.997) | <0.001 | ||
| D-dimer | 1.00 (1.00–1.00) | <0.001 | ||
| Total bilirubin | 0.99 (0.98–1.02) | 0.823 | ||
| Uric acid | 1.002 (1.001–1.004) | 0.013 | ||
| Serum chloride | 1.07 (1.02–1.12) | 0.003 | ||
| pO2/FiO2 ratio | 0.99 (0.98–0.99) | 0.009 | ||
| Serum lactate | 1.28 (1.14–1.45) | <0.001 | ||
| Serum Procalcitonin | 1.06 (0.97–1.16 | 0.184 | ||
| ARDS | 2.06 (1.25–3.38) | 0.005 | ||
| AKI | 3.16 (1.90–5.27) | <0.001 | 2.75 (1.27–5.93) | 0.010 |
| Acute liver injury | 3.58 (2.12–6.07) | <0.001 | ||
| ISS | 1.06 (1.03–1.08) | <0.001 | ||
| APACHE II score | 1.14 (1.09–1.19) | <0.001 | 1.08 (1.01–1.15) | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Guo, F.; Wang, C.; Wang, Z.; Chang, P.; Xue, H.; Wang, T.; Zhu, F. Characteristics and Risk Factors of Myocardial Injury after Traumatic Hemorrhagic Shock. J. Clin. Med. 2022, 11, 4799. https://doi.org/10.3390/jcm11164799
Zhao X, Guo F, Wang C, Wang Z, Chang P, Xue H, Wang T, Zhu F. Characteristics and Risk Factors of Myocardial Injury after Traumatic Hemorrhagic Shock. Journal of Clinical Medicine. 2022; 11(16):4799. https://doi.org/10.3390/jcm11164799
Chicago/Turabian StyleZhao, Xiujuan, Fuzheng Guo, Chu Wang, Zhenzhou Wang, Panpan Chang, Haiyan Xue, Tianbing Wang, and Fengxue Zhu. 2022. "Characteristics and Risk Factors of Myocardial Injury after Traumatic Hemorrhagic Shock" Journal of Clinical Medicine 11, no. 16: 4799. https://doi.org/10.3390/jcm11164799





