Robot-Assisted Radiofrequency Ablation Combined with Thermodynamic Simulation for Epilepsy Reoperations
Abstract
:1. Introduction
2. Materials and Methods
3. Results
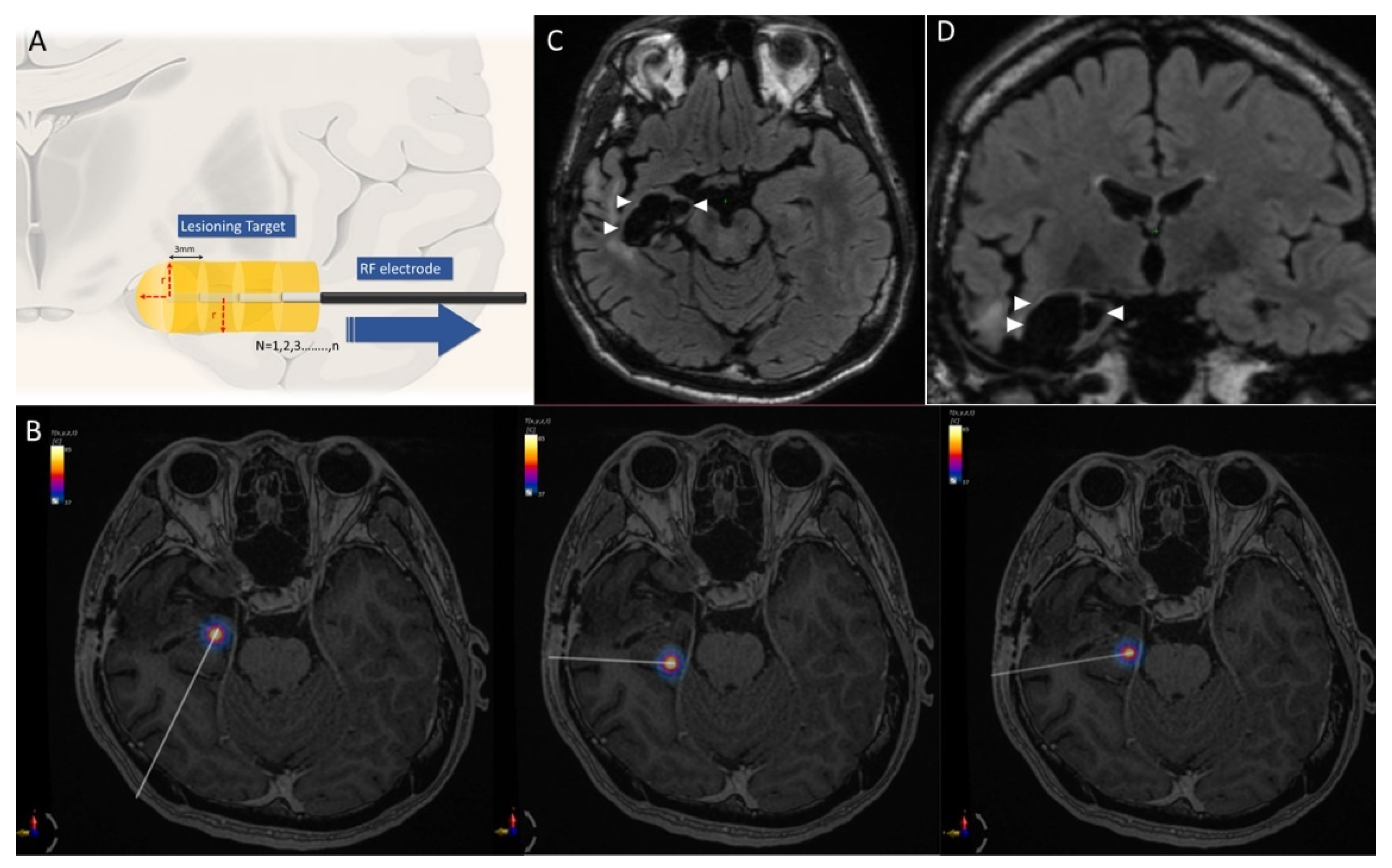
3.1. Ablation Data
3.2. Case 1. Right Temporal Seizure after ATL
3.3. Case 2. LGS Treated with Anterior CC
3.4. Case 3. Intractable Seizure Secondary to Arteriovenous Malformation (AVM,)
3.5. Surgical Complication and Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshe, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Tellez-Zenteno, J.F.; Hernandez-Ronquillo, L.; Buckley, S.; Zahagun, R.; Rizvi, S. A validation of the new definition of drug-resistant epilepsy by the International League Against Epilepsy. Epilepsia 2014, 55, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Jobst, B.C.; Cascino, G.D. Resective epilepsy surgery for drug-resistant focal epilepsy: A review. JAMA 2015, 313, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Douglass, L.M.; Salpekar, J. Surgical options for patients with Lennox-Gastaut syndrome. Epilepsia 2014, 55 (Suppl. S4), 21–28. [Google Scholar] [CrossRef] [PubMed]
- Cukiert, A.; Burattini, J.A.; Mariani, P.P.; Camara, R.B.; Seda, L.; Baldauf, C.M.; Argentoni, M.; Baise-Zung, C.; Forster, C.R.; Mello, V.A. Extended, one-stage callosal section for treatment of refractory secondarily generalized epilepsy in patients with Lennox-Gastaut and Lennox-like syndromes. Epilepsia 2006, 47, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Grote, A.; Witt, J.A.; Surges, R.; von Lehe, M.; Pieper, M.; Elger, C.E.; Helmstaedter, C.; Ormond, D.R.; Schramm, J.; Delev, D. A second chance—Reoperation in patients with failed surgery for intractable epilepsy: Long-term outcome, neuropsychology and complications. J. Neurol. Neurosurg. Psychiatry 2016, 87, 379–385. [Google Scholar] [CrossRef]
- Quigg, M.; Harden, C. Minimally invasive techniques for epilepsy surgery: Stereotactic radiosurgery and other technologies. J. Neurosurg. 2014, 121 (Suppl. S2), 232–240. [Google Scholar] [CrossRef]
- Bourdillon, P.; Isnard, J.; Catenoix, H.; Montavont, A.; Rheims, S.; Ryvlin, P.; Ostrowsky-Coste, K.; Mauguiere, F.; Guenot, M. Stereo-electro-encephalography-Guided Radiofrequency Thermocoagulation: From In Vitro and In Vivo Data to Technical Guidelines. World Neurosurg. 2016, 94, 73–79. [Google Scholar] [CrossRef]
- Cossu, M.; Fuschillo, D.; Casaceli, G.; Pelliccia, V.; Castana, L.; Mai, R.; Francione, S.; Sartori, I.; Gozzo, F.; Nobili, L.; et al. Stereoelectroencephalography-guided radiofrequency thermocoagulation in the epileptogenic zone: A retrospective study on 89 cases. J. Neurosurg. 2015, 123, 1358–1367. [Google Scholar] [CrossRef]
- Kang, J.Y.; Wu, C.; Tracy, J.; Lorenzo, M.; Evans, J.; Nei, M.; Skidmore, C.; Mintzer, S.; Sharan, A.D.; Sperling, M.R. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia 2016, 57, 325–334. [Google Scholar] [CrossRef]
- Mallela, A.N.; Hect, J.L.; Abou-Al-Shaar, H.; Akwayena, E.; Abel, T.J. Stereotactic laser interstitial thermal therapy corpus callosotomy for the treatment of pediatric drug-resistant epilepsy. Epilepsia Open 2022, 7, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, P.; Rheims, S.; Catenoix, H.; Montavont, A.; Ostrowsky-Coste, K.; Isnard, J.; Guenot, M. Surgical techniques: Stereoelectroencephalography-guided radiofrequency-thermocoagulation (SEEG-guided RF-TC). Seizure 2020, 77, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041.e16. [Google Scholar] [CrossRef] [PubMed]
- Dagro, A.M.; Wilkerson, J.W.; Thomas, T.P.; Kalinosky, B.T.; Payne, J.A. Computational modeling investigation of pulsed high peak power microwaves and the potential for traumatic brain injury. Sci. Adv. 2021, 7, eabd8405. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Cross, J.H.; D’Souza, C.; French, J.A.; Haut, S.R.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshe, S.L.; et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia 2017, 58, 531–542. [Google Scholar] [CrossRef]
- Vojtech, Z.; Kramska, L.; Malikova, H.; Seltenreichova, K.; Prochazka, T.; Kalina, M.; Liscak, R. Cognitive outcome after stereotactic amygdalohippocampectomy. Seizure 2012, 21, 327–333. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. 1948. J. Appl. Physiol. (1985) 1998, 85, 5–34. [Google Scholar] [CrossRef]
- Avants, B.B.; Epstein, C.L.; Grossman, M.; Gee, J.C. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008, 12, 26–41. [Google Scholar] [CrossRef]
- Bourdillon, P.; Cucherat, M.; Isnard, J.; Ostrowsky-Coste, K.; Catenoix, H.; Guenot, M.; Rheims, S. Stereo-electroencephalography-guided radiofrequency thermocoagulation in patients with focal epilepsy: A systematic review and meta-analysis. Epilepsia 2018, 59, 2296–2304. [Google Scholar] [CrossRef]
- Lee, C.Y.; Li, H.T.; Wu, T.; Cheng, M.Y.; Lim, S.N.; Lee, S.T. Efficacy of limited hippocampal radiofrequency thermocoagulation for mesial temporal lobe epilepsy. J. Neurosurg. 2018, 131, 781–789. [Google Scholar] [CrossRef]
- Bourdillon, P.; Rheims, S.; Isnard, J.; Guenot, M. Letter to the Editor. Temporal lobe epilepsy: Open or stereotactic surgery? J. Neurosurg. 2019, 131, 989. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jermakowicz, W.J.; Chakravorti, S.; Cajigas, I.; Sharan, A.D.; Jagid, J.R.; Matias, C.M.; Sperling, M.R.; Buckley, R.; Ko, A.; et al. Effects of surgical targeting in laser interstitial thermal therapy for mesial temporal lobe epilepsy: A multicenter study of 234 patients. Epilepsia 2019, 60, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Jermakowicz, W.J.; Kanner, A.M.; Sur, S.; Bermudez, C.; D’Haese, P.F.; Kolcun, J.P.G.; Cajigas, I.; Li, R.; Millan, C.; Ribot, R.; et al. Laser thermal ablation for mesiotemporal epilepsy: Analysis of ablation volumes and trajectories. Epilepsia 2017, 58, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Usami, K.; Kubota, M.; Kawai, K.; Kunii, N.; Matsuo, T.; Ibayashi, K.; Takahashi, M.; Kamada, K.; Momose, T.; Aoki, S.; et al. Long-term outcome and neuroradiologic changes after multiple hippocampal transection combined with multiple subpial transection or lesionectomy for temporal lobe epilepsy. Epilepsia 2016, 57, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Soldozy, S.; Norat, P.; Yagmurlu, K.; Sokolowski, J.D.; Sharifi, K.A.; Tvrdik, P.; Park, M.S.; Kalani, M.Y.S. Arteriovenous malformation presenting with epilepsy: A multimodal approach to diagnosis and treatment. Neurosurg. Focus 2020, 48, E17. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lee, C.C.; Takami, H.; Shen, S.; Chen, K.T.; Wei, K.C.; Wu, M.H.; Worrell, G.; Chen, P.Y. Awake craniotomies for epileptic gliomas: Intraoperative and postoperative seizure control and prognostic factors. J. Neurooncol 2019, 142, 577–586. [Google Scholar] [CrossRef]
- Barbaro, N.M.; Quigg, M.; Ward, M.M.; Chang, E.F.; Broshek, D.K.; Langfitt, J.T.; Yan, G.; Laxer, K.D.; Cole, A.J.; Sneed, P.K.; et al. Radiosurgery versus open surgery for mesial temporal lobe epilepsy: The randomized, controlled ROSE trial. Epilepsia 2018, 59, 1198–1207. [Google Scholar] [CrossRef]


| Patient | Gender | Age | Underlying Etiology | Previous Surgery | RARFA Target | RARFA Trajectories | Ablation Volume (mm3) | Post-OP Pain Score | Post-OP Length of Stay | Post-OP Acute Seizure | ILAE Classification | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Simulation and Calculation | Post-OP Image Measurement (Percentage Error of with Calculation) | |||||||||||
| 1 | Male | 28 | Right hippocampal sclerosis | Partial anterior temporal lobectomy | Right remanent hippocampus | 3 | 9949.69 | 9727 (2.3%) | 1 | 2 | none | 1a |
| 2 | Male | 9 | Lennox Gastaut syndrome | Anterior callosotomy | Corpus callosum | 2 | 1867.87 | 1841 (1.4%) | 1 | 4 | none | 4 |
| 3 | Female | 45 | Left mesial temporal AVM | Stereotactic radiosurgery | Left mesial temporal (around residual vein) | 2 | 3941.89 | 4073 (3.2%) | 1 | 3 | none | 1a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-C.; Cheng, M.-Y.; Hung, P.-C.; Kuo, C.-Y.; Hsieh, H.-Y.; Lin, K.-L.; Tu, P.-H.; Wu, C.-T.; Hsu, P.-W.; Wei, K.-C.; et al. Robot-Assisted Radiofrequency Ablation Combined with Thermodynamic Simulation for Epilepsy Reoperations. J. Clin. Med. 2022, 11, 4804. https://doi.org/10.3390/jcm11164804
Wang Y-C, Cheng M-Y, Hung P-C, Kuo C-Y, Hsieh H-Y, Lin K-L, Tu P-H, Wu C-T, Hsu P-W, Wei K-C, et al. Robot-Assisted Radiofrequency Ablation Combined with Thermodynamic Simulation for Epilepsy Reoperations. Journal of Clinical Medicine. 2022; 11(16):4804. https://doi.org/10.3390/jcm11164804
Chicago/Turabian StyleWang, Yu-Chi, Mei-Yun Cheng, Po-Cheng Hung, Cheng-Yen Kuo, Hsiang-Yao Hsieh, Kuang-Lin Lin, Po-Hsun Tu, Chieh-Tsai Wu, Peng-Wei Hsu, Kuo-Chen Wei, and et al. 2022. "Robot-Assisted Radiofrequency Ablation Combined with Thermodynamic Simulation for Epilepsy Reoperations" Journal of Clinical Medicine 11, no. 16: 4804. https://doi.org/10.3390/jcm11164804
APA StyleWang, Y.-C., Cheng, M.-Y., Hung, P.-C., Kuo, C.-Y., Hsieh, H.-Y., Lin, K.-L., Tu, P.-H., Wu, C.-T., Hsu, P.-W., Wei, K.-C., & Chuang, C.-C. (2022). Robot-Assisted Radiofrequency Ablation Combined with Thermodynamic Simulation for Epilepsy Reoperations. Journal of Clinical Medicine, 11(16), 4804. https://doi.org/10.3390/jcm11164804






