Office-Based Structural Autologous Fat Injection Laryngoplasty for Unilateral Vocal Fold Paralysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
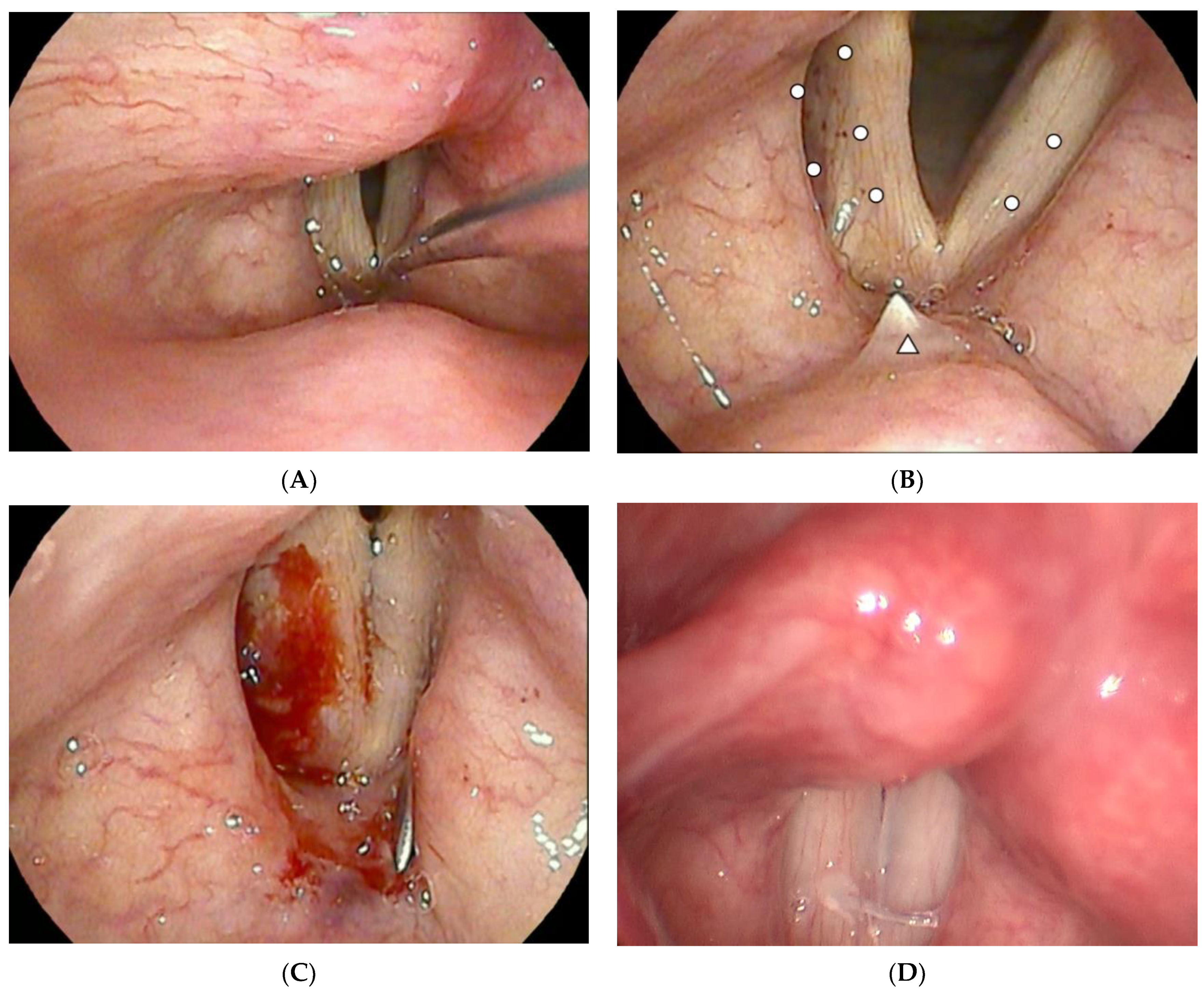
2.3. Operation Methods
2.4. Laryngeal and Voice Assessment
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cates, D.J.; Venkatesan, N.N.; Strong, B.; Kuhn, M.A.; Belafsky, P.C. Effect of Vocal Fold Medialization on Dysphagia in Patients with Unilateral Vocal Fold Immobility. Otolaryngol. Head Neck Surg. 2016, 155, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Spataro, E.A.; Grindler, D.J.; Paniello, R.C. Etiology and Time to Presentation of Unilateral Vocal Fold Paralysis. Otolaryngol. Head Neck Surg. 2014, 151, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Al-Khtoum, N.; Shawakfeh, N.; Al-Safadi, E.; Al-Momani, O.; Hamasha, K. Acquired unilateral vocal fold paralysis: Retrospective analysis of a single institutional experience. N. Am. J. Med. Sci. 2013, 5, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.O.; Sherman, A.E.; Hovis, K.L.; Bonnet, K.; Schlundt, D.; Garrett, C.G.; Davies, L. Life Experience of Patients With Unilateral Vocal Fold Paralysis. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Spector, B.C.; Netterville, J.L.; Billante, C.; Clary, J.; Reinisch, L.; Smith, T.L. Quality-of-life assessment in patients with unilateral vocal cord paralysis. Otolaryngol. Head Neck Surg. 2001, 125, 176–182. [Google Scholar] [CrossRef]
- Cohen, S.M.; Dupont, W.D.; Courey, M.S. Quality-of-life impact of non-neoplastic voice disorders: A meta-analysis. Ann. Otol. Rhinol. Laryngol. 2006, 115, 128–134. [Google Scholar] [CrossRef]
- Cohen, S.M.; Kim, J.; Roy, N.; Asche, C.; Courey, M. The impact of laryngeal disorders on work-related dysfunction. Laryngoscope 2012, 122, 1589–1594. [Google Scholar] [CrossRef]
- Lorenz, R.R.; Esclamado, R.M.; Teker, A.M.; Strome, M.; Scharpf, J.; Hicks, D.; Milstein, C.; Lee, W.T. Ansa cervicalis-to-recurrent laryngeal nerve anastomosis for unilateral vocal fold paralysis: Experience of a single institution. Ann. Otol. Rhinol. Laryngol. 2008, 117, 40–45. [Google Scholar] [CrossRef]
- Junlapan, A.; Sung, C.K.; Damrose, E.J. Type I thyroplasty: A safe outpatient procedure. Laryngoscope 2019, 129, 1640–1646. [Google Scholar] [CrossRef]
- Tsai, M.-S.; Yang, M.-Y.; Chang, G.-H.; Tsai, Y.-T.; Lin, M.-H.; Hsu, C.-M. Autologous thyroid cartilage graft implantation in medialization laryngoplasty: A modified approach for treating unilateral vocal fold paralysis. Sci. Rep. 2017, 7, 4790. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wu, S.-H.; Tu, Y.-K.; Lin, W.-J.; Liu, S.-A. Hyaluronic Acid Injection Laryngoplasty for Unilateral Vocal Fold Paralysis—A Systematic Review and Meta-Analysis. Cells 2020, 9, 2417. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.-C.; Fang, T.-J.; Hsin, L.-J.; Li, H.-Y.; Wong, A.M.K. Early hyaluronate injection improves quality of life but not neural recovery in unilateral vocal fold paralysis: An open-label randomized controlled study. Restor. Neurol. Neurosci. 2015, 33, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Lodder, W.L.; Dikkers, F.G. Comparison of voice outcome after vocal fold augmentation with fat or calcium hydroxylapatite. Laryngoscope 2015, 125, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Damrose, E.J.; Morzaria, S. A meta-analysis of voice outcome comparing calcium hydroxylapatite injection laryngoplasty to silicone thyroplasty. Otolaryngol. Head Neck Surg. 2013, 148, 197–208. [Google Scholar] [CrossRef]
- Siu, J.; Tam, S.; Fung, K. A comparison of outcomes in interventions for unilateral vocal fold paralysis: A systematic review. Laryngoscope 2016, 126, 1616–1624. [Google Scholar] [CrossRef]
- Aviv, J.E.; Takoudes, T.G.; Ma, G.; Close, L.G. Office-based esophagoscopy: A preliminary report. Otolaryngol. Head Neck Surg. 2001, 125, 170–175. [Google Scholar] [CrossRef]
- Mallur, P.S.; Rosen, C.A. Office-based laryngeal injections. Otolaryngol. Clin. N. Am. 2013, 46, 85–100. [Google Scholar] [CrossRef]
- Hu, H.-C.; Lin, S.-Y.; Hung, Y.-T.; Chang, S.-Y. Feasibility and Associated Limitations of Office-Based Laryngeal Surgery Using Carbon Dioxide Lasers. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 485–491. [Google Scholar] [CrossRef]
- Shindo, M.L.; Zaretsky, L.S.; Rice, D.H. Autologous fat injection for unilateral vocal fold paralysis. Ann. Otol. Rhinol. Laryngol. 1996, 105, 602–606. [Google Scholar] [CrossRef]
- Havas, T.E.; Priestley, K.J. Autologous fat injection laryngoplasty for unilateral vocal fold paralysis. ANZ J. Surg. 2003, 73, 938–943. [Google Scholar] [CrossRef]
- Pagano, R.; Morsomme, D.; Camby, S.; Lejeune, L.; Finck, C. Long-term Results of 18 Fat Injections in Unilateral Vocal Fold Paralysis. J. Voice Off. J. Voice Found. 2017, 31, 505.e501–505.e509. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-D.; Chen, S.-H.; Tsai, M.-H.; Tsou, Y.-A. Autologous Fat Injection Laryngoplasty for Unilateral Vocal Fold Paralysis. J. Clin. Med. 2021, 10, 5034. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. Hand rejuvenation with structural fat grafting. Plast. Reconstr. Surg. 2002, 110, 1731–1744. [Google Scholar] [CrossRef]
- Coleman, S.R. Structural fat grafting: More than a permanent filler. Plast. Reconstr. Surg. 2006, 118, 108s–120s. [Google Scholar] [CrossRef]
- Sanderson, J.D.; Simpson, C.B. Laryngeal complications after lipoinjection for vocal fold augmentation. Laryngoscope 2009, 119, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.C.; Hung, Y.T.; Lin, S.Y.; Chang, S.Y. Office-based Autologous Fat Injection Laryngoplasty for Vocal Process Granuloma. J. Voice Off. J. Voice Found. 2016, 30, 758.e7–758.e11. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.C.; Hung, Y.T.; Lin, S.Y.; Tung, T.H.; Chang, S.Y. Office-Based Autologous Fat Injection Laryngoplasty for Glottic Insufficiency in Patients Under 50 Years Old. J. Voice Off. J. Voice Found. 2019, 33, 747–750. [Google Scholar] [CrossRef]
- Chou, C.; Lin, T.; Chou, C. Influential factors in autologous fat transplantation—Focusing on the lumen size of injection needle and the injecting volume. J. IPRAS 2013, 9, 25–27. [Google Scholar]
- Cantarella, G.; Mazzola, R.F.; Gaffuri, M.; Iofrida, E.; Biondetti, P.; Forzenigo, L.V.; Pignataro, L.; Torretta, S. Structural Fat Grafting to Improve Outcomes of Vocal Folds’ Fat Augmentation: Long-term Results. Otolaryngol. Head Neck Surg. 2018, 158, 135–143. [Google Scholar] [CrossRef]
- Rosen, C.A.; Lee, A.S.; Osborne, J.; Zullo, T.; Murry, T. Development and validation of the voice handicap index-10. Laryngoscope 2004, 114, 1549–1556. [Google Scholar] [CrossRef]
- Arffa, R.E.; Krishna, P.; Gartner-Schmidt, J.; Rosen, C.A. Normative values for the Voice Handicap Index-10. J. Voice Off. J. Voice Found. 2012, 26, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M. “GRBAS” scale for evaluating the hoarse voice & frequency range of phonation. Clin. Exam. Voice 1981, 5, 83–84. [Google Scholar]
- Jeong, G.-E.; Lee, D.H.; Lee, Y.S.; Ahn, D.S.; Lee, D.K.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Treatment Efficacy of Voice Therapy Following Injection Laryngoplasty for Unilateral Vocal Fold Paralysis. J. Voice 2022, 36, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Eto, H.; Kato, H.; Suga, H.; Aoi, N.; Doi, K.; Kuno, S.; Yoshimura, K. The fate of adipocytes after nonvascularized fat grafting: Evidence of early death and replacement of adipocytes. Plast. Reconstr. Surg. 2012, 129, 1081–1092. [Google Scholar] [CrossRef]
- Strong, A.L.; Cederna, P.S.; Rubin, J.P.; Coleman, S.R.; Levi, B. The Current State of Fat Grafting: A Review of Harvesting, Processing, and Injection Techniques. Plast. Reconstr. Surg. 2015, 136, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Iyyanki, T.; Hubenak, J.; Liu, J.; Chang, E.I.; Beahm, E.K.; Zhang, Q. Harvesting technique affects adipose-derived stem cell yield. Aesthetic Surg. J. 2015, 35, 467–476. [Google Scholar] [CrossRef]
- Titze, I.; Abbott, K. Vocology: The Science and Practice of Voice Habilitation; National Center for Voice and Speech: Salt Lake City, UT, USA, 2012. [Google Scholar]
- Verdolini Abbott, K.; Li, N.Y.K.; Branski, R.C.; Rosen, C.A.; Grillo, E.; Steinhauer, K.; Hebda, P.A. Vocal exercise may attenuate acute vocal fold inflammation. J. Voice 2012, 26, 814.e11–814.e13. [Google Scholar] [CrossRef]
- Kaneko, M.; Shiromoto, O.; Fujiu-Kurachi, M.; Kishimoto, Y.; Tateya, I.; Hirano, S. Optimal Duration for Voice Rest After Vocal Fold Surgery: Randomized Controlled Clinical Study. J. Voice 2017, 31, 97–103. [Google Scholar] [CrossRef]
- Berry, D.A.; Verdolini, K.; Montequin, D.W.; Hess, M.M.; Chan, R.W.; Titze, I.R. A quantitative output-cost ratio in voice production. J. Speech Lang. Hear. Res. JSLHR 2001, 44, 29–37. [Google Scholar] [CrossRef]
- Young, V.N.; Jeong, K.; Rothenberger, S.D.; Gillespie, A.I.; Smith, L.J.; Gartner-Schmidt, J.L. Minimal clinically important difference of voice handicap index-10 in vocal fold paralysis. Laryngoscope 2018, 128, 1419–1424. [Google Scholar] [CrossRef]
- Pijls-Johannesma, M.; Houben, R.; Boersma, L.; Grutters, J.; Seghers, K.; Lambin, P.; Wanders, R.; De Ruysscher, D. High-dose radiotherapy or concurrent chemo-radiation in lung cancer patients only induces a temporary, reversible decline in QoL. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2009, 91, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Yang, Y.H.; Liu, C.Y.; Lin, M.H.; Chang, G.H.; Tsai, Y.T.; Li, H.Y.; Tsai, Y.H.; Hsu, C.M. Unilateral Vocal Fold Paralysis and Risk of Pneumonia: A Nationwide Population-Based Cohort Study. Otolaryngol. Head Neck Surg. 2018, 158, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Steurer, M.; Passler, C.; Denk, D.M.; Schneider, B.; Niederle, B.; Bigenzahn, W. Advantages of recurrent laryngeal nerve identification in thyroidectomy and parathyroidectomy and the importance of preoperative and postoperative laryngoscopic examination in more than 1000 nerves at risk. Laryngoscope 2002, 112, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Shindo, M.; Chheda, N.N. Incidence of Vocal Cord Paralysis With and Without Recurrent Laryngeal Nerve Monitoring During Thyroidectomy. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 481–485. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, J.W.; Chung, C.H.; Mok, J.O.; Shim, S.S.; Koh, Y.W.; Choi, E.C. Utility of injection laryngoplasty in the management of post-thyroidectomy vocal cord paralysis. Thyroid 2010, 20, 513–517. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, G.; Ahn, J.; Son, Y.-I. Early voice rehabilitation with injection laryngoplasty in patients with unilateral vocal cord palsy after thyroidectomy. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 3745–3750. [Google Scholar] [CrossRef]
- Miar, S.; Walters, B.; Gonzales, G.; Malka, R.; Baker, A.; Guda, T.; Dion, G.R. Augmentation and vocal fold biomechanics in a recurrent laryngeal nerve injury model. Laryngoscope Investig. Otolaryngol. 2022, 7. [Google Scholar] [CrossRef]
- McCulloch, T.M.; Andrews, B.T.; Hoffman, H.T.; Graham, S.M.; Karnell, M.P.; Minnick, C. Long-term follow-up of fat injection laryngoplasty for unilateral vocal cord paralysis. Laryngoscope 2002, 112, 1235–1238. [Google Scholar] [CrossRef]
- Lin, T.-M.; Lin, T.-Y.; Chou, C.-K.; Lai, C.-S.; Lin, S.-D. Application of microautologous fat transplantation in the correction of sunken upper eyelid. Plast. Reconstr. Surg. Glob. Open 2014, 2, e259. [Google Scholar] [CrossRef]
- Hirano, M. Cover-body theory of vocal cord vibration. Speech Sci. 1985, 1–46. [Google Scholar]

| N = 23 | |
|---|---|
| Age (years) | |
| Mean ± SD | 58.65 ± 13.77 |
| Range | 30 to 82 |
| Sex | |
| Male | 9 (39.1%) |
| Female | 14 (60.9%) |
| BMI (kg/m2) | |
| Mean ± SD | 26.75 ± 4.33 |
| Range | 20.86 to 34.48 |
| Palsy side | |
| Left | 13 (56.5%) |
| Right | 10 (43.5%) |
| Etiology | |
| Thyroid related | 11 (47.8%) |
| Pulmonary and mediastinum related | 6 (26.1%) |
| Cardiovascular surgery | 1 (4.3%) |
| Cervical spine surgery | 1 (4.3%) |
| Brain surgery | 1 (4.3%) |
| Idiopathic | 3 (13.0%) |
| Ipsilateral Mean ± SD | Contralateral Mean ± SD | |
|---|---|---|
| Injection points | ||
| Medial injection | 3.22 ± 0.80 | 2.00 ± 0.74 |
| Lateral injection | 2.13 ± 0.46 | 0 |
| Injected fat volume (mL) | 0.79 ± 0.17 | 0.19 ± 0.11 |
| Mean ± SD | p Value | |||||
|---|---|---|---|---|---|---|
| PreOP | 2 Weeks | 3 Months | PreOP vs. 2 Wk | 2 Wk vs. 3 Mo | PreOP vs. 3 Mo | |
| Grade | 2.17 ± 0.76 | 1.74 ± 0.69 | 1.05 ± 0.65 | p = 0.002 * | p < 0.0001 * | p < 0.0001 * |
| Roughness | 1.35 ± 0.93 | 1.26 ± 0.54 | 0.91 ± 0.53 | p = 0.604 | p = 0.017 * | p = 0.056 |
| Breathiness | 1.65 ± 1.02 | 0.78 ± 0.90 | 0.45 ± 0.67 | p = 0.001 * | p = 0.016 * | p < 0.0001 * |
| Asthenia | 0.70 ± 0.93 | 0.22 ± 0.52 | 0.09 ± 0.29 | p = 0.013 * | p = 0.162 | p = 0.004 * |
| Strain | 0.13 ± 0.46 | 0.04 ± 0.21 | 0.09 ± 0.43 | p = 0.426 | p = 0.665 | p = 0.747 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.W.-G.; Chen, C.-H.; Lin, T.-M.; Chang, A.C.-H.; Tsai, T.-P.; Chang, S.-Y. Office-Based Structural Autologous Fat Injection Laryngoplasty for Unilateral Vocal Fold Paralysis. J. Clin. Med. 2022, 11, 4806. https://doi.org/10.3390/jcm11164806
Chen AW-G, Chen C-H, Lin T-M, Chang AC-H, Tsai T-P, Chang S-Y. Office-Based Structural Autologous Fat Injection Laryngoplasty for Unilateral Vocal Fold Paralysis. Journal of Clinical Medicine. 2022; 11(16):4806. https://doi.org/10.3390/jcm11164806
Chicago/Turabian StyleChen, Andy Wei-Ge, Chih-Hua Chen, Tsai-Ming Lin, Angela Chih-Hui Chang, Tzu-Pei Tsai, and Shyue-Yih Chang. 2022. "Office-Based Structural Autologous Fat Injection Laryngoplasty for Unilateral Vocal Fold Paralysis" Journal of Clinical Medicine 11, no. 16: 4806. https://doi.org/10.3390/jcm11164806
APA StyleChen, A. W.-G., Chen, C.-H., Lin, T.-M., Chang, A. C.-H., Tsai, T.-P., & Chang, S.-Y. (2022). Office-Based Structural Autologous Fat Injection Laryngoplasty for Unilateral Vocal Fold Paralysis. Journal of Clinical Medicine, 11(16), 4806. https://doi.org/10.3390/jcm11164806






