Clinical Outcomes of Second- versus First-Generation Carotid Stents: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Endpoints of Interest Identification
2.2. Data Search and Initial Screening
2.3. Study Eligibility and Quality Assessment
2.4. Data Extraction
2.5. Data Synthesis
2.6. Statistical Analysis
3. Results
3.1. Eligible Trials and Results Display
3.2. Quality Assessment and Risk of Bias
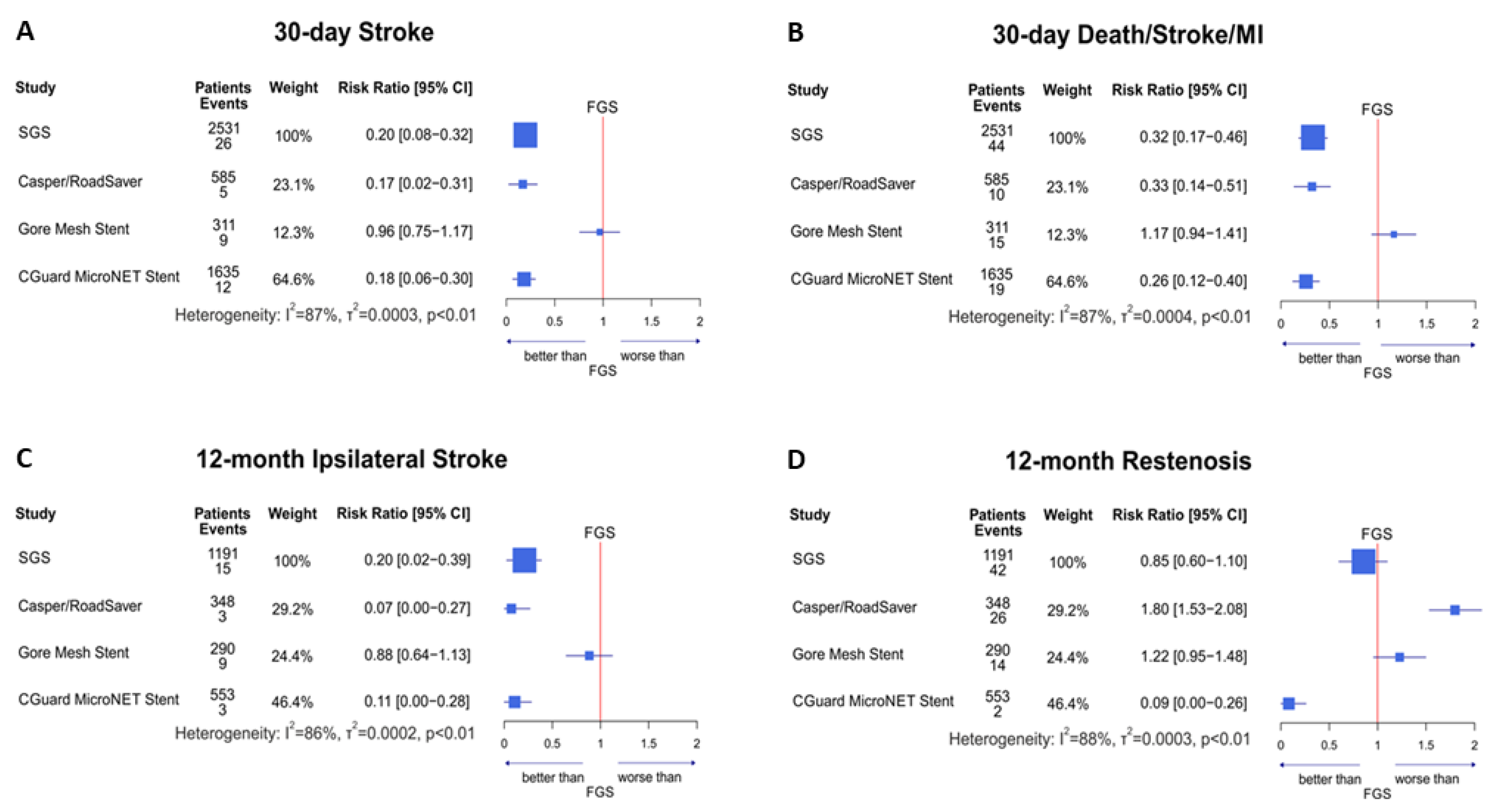
3.3. 30-Day Outcomes: SGS vs. FGS
3.4. 12-Month Outcomes: SGS vs. FGS
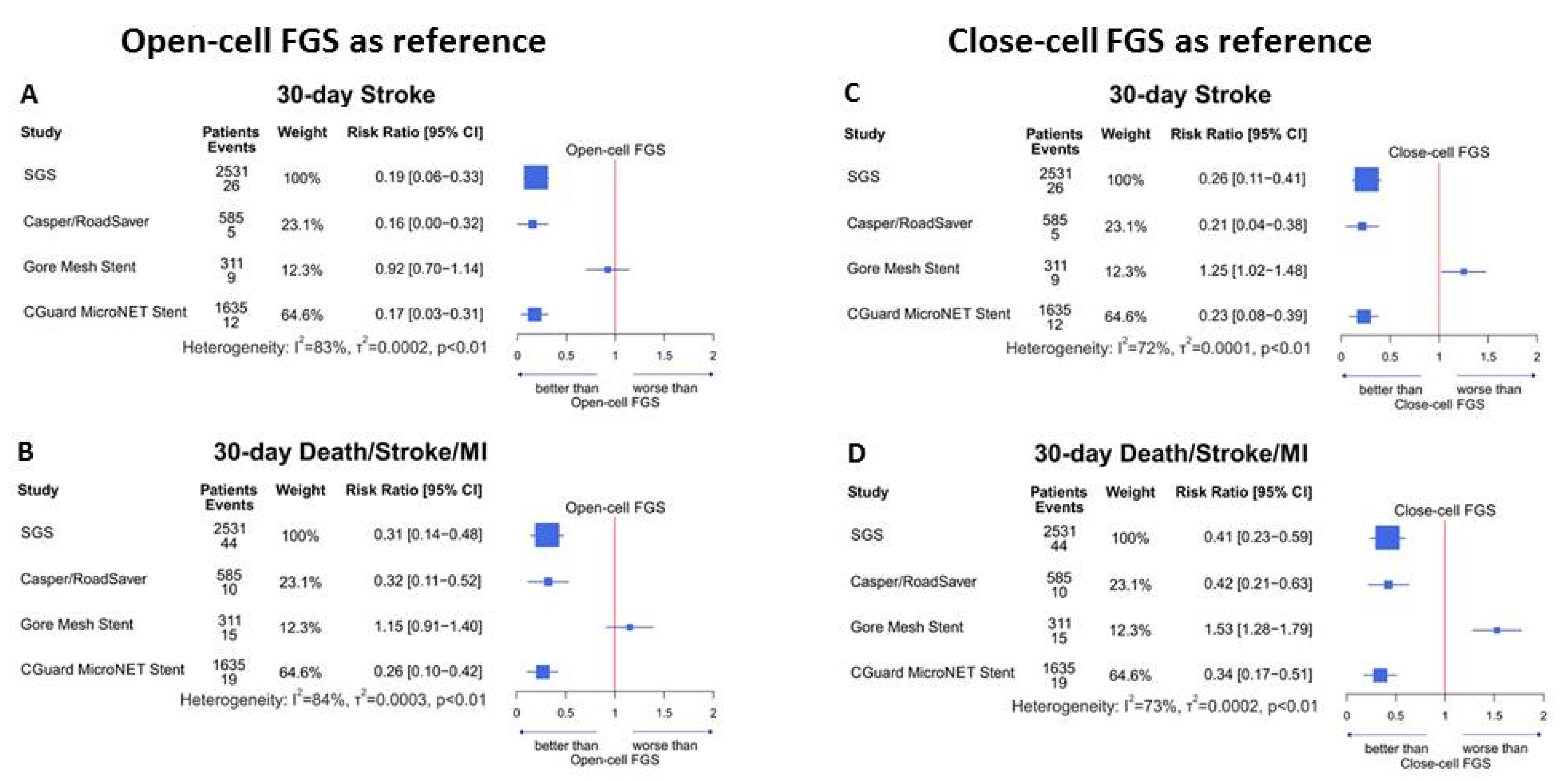
3.5. FGS Stent Type: Open- vs. Closed-Cell Design
3.6. SGS Stent Brand Comparisons
3.7. Heterogeneity
4. Discussion
5. Limitations
6. Conclusions
7. Perspectives
7.1. What Is Known?
7.2. What Is New?
7.3. What Is Next?
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CADIMA | Online evidence synthesis tool for the conduct and reporting of systematic reviews |
| CG | CGuard MicroNet-covered carotid stent (laser-cut nitinol frame covered with PET micro-mesh sleeve) |
| DSM | Death, stroke, myocardial infarction |
| FGS | First-generation (single-layer) carotid stent(s) |
| GS | Gore carotid stent (laser-cut nitinol frame covered by Teflon mesh layer) |
| IS | Ipsilateral stroke |
| ISR | In-stent restenosis |
| CR | Casper/RoadSaver dual metallic layer carotid stent (braided metallic mesh inside the metallic, braided frame) |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analysis |
| PROSPERO | International Prospective Register of Systematic Reviews |
| SGS | Second-generation (mesh-stent) carotid stent(s) |
References
- Sardar, P.; Chatterjee, S.; Aronow, H.D.; Kundu, A.; Ramchand, P.; Mukherjee, D.; Nairooz, R.; Gray, W.A.; White, C.J.; Jaff, M.R.; et al. Carotid Artery Stenting Versus Endarterectomy for Stroke Prevention: A Meta-Analysis of Clinical Trials. J. Am. Coll. Cardiol. 2017, 69, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.G.; Calvet, D.; Howard, G.; Gregson, J.; Algra, A.; Becquemin, J.-P.; de Borst, G.J.; Bulbulia, R.; Eckstein, H.-H.; Fraedrich, G.; et al. Long-term outcomes of stenting and endarterectomy for symptomatic carotid stenosis: A preplanned pooled analysis of individual patient data. Lancet Neurol. 2019, 18, 348–356. [Google Scholar] [CrossRef]
- Bonati, L.H.; Jongen, L.M.; Haller, S.; Flach, H.Z.; Dobson, J.; Nederkoorn, P.J.; Macdonald, S.; Gaines, P.A.; Waaijer, A.; Stierli, P.; et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: A substudy of the International Carotid Stenting Study (ICSS). Lancet Neurol. 2010, 9, 353–362. [Google Scholar] [CrossRef]
- Ikari, Y.; Misumi, K.; Yokoi, H.; Ogata, N.; Umemoto, T.; Uesugi, M.; Kinoshita, Y.; Nakano, M.; Higashitani, M.; Abe, H.; et al. Initial results of carotid artery stenting in Japan. Cardiovasc. Interv. Ther. 2013, 28, 37–44. [Google Scholar] [CrossRef]
- Fairman, R.; Gray, W.A.; Scicli, A.P.; Wilburn, O.; Verta, P.; Atkinson, R.; Yadav, J.S.; Wholey, M.; Hopkins, L.N.; Raabe, R.; et al. The CAPTURE registry-Analysis of strokes resulting from carotid artery stenting in the post approval setting: Timing, location, severity, and type. Ann. Surg. 2007, 246, 551–556. [Google Scholar] [CrossRef]
- Hill, M.; Brooks, W.H.; Mackey, A.; Clark, W.M.; Meschia, J.F.; Morrish, W.F.; Mohr, J.; Rhodes, J.D.; Popma, J.J.; Lal, B.K.; et al. Stroke after carotid stenting and endarterectomy in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Circulation 2012, 126, 3054–3061. [Google Scholar] [CrossRef]
- Kotsugi, M.; Takayama, K.; Myouchin, K.; Wada, T.; Nakagawa, I.; Nakagawa, H.; Taoka, T.; Kurokawa, S.; Nakase, H.; Kichikawa, K. Carotid Artery Stenting: Investigation of Plaque Protrusion Incidence and Prognosis. JACC Cardiovasc. Interv. 2017, 10, 824–831. [Google Scholar] [CrossRef]
- Shinozaki, N.; Ogata, N.; Ikari, Y. Plaque protrusion detected by intravascular ultrasound during carotid artery stenting. J. Stroke Cerebrovasc. Dis. 2014, 23, 2622–2625. [Google Scholar] [CrossRef]
- Paraskevas, K.I.; Mikhailidis, D.P.; Veith, F.J. Mechanisms to explain the poor results of carotid artery stenting (CAS) in symptomatic patients to date and options to improve CAS outcomes. J. Vasc. Surg. 2010, 52, 1367–1375. [Google Scholar] [CrossRef]
- Montorsi, P.; Caputi, L.; Galli, S.; Ciceri, E.; Ballerini, G.; Agrifoglio, M.; Ravagnani, P.; Trabattoni, D.; Pontone, G.; Fabbiocchi, F.; et al. Microembolization during carotid artery stenting in patients with high-risk, lipid-rich plaque. A randomized trial of proximal versus distal cerebral protection. J. Am. Coll. Cardiol. 2011, 58, 1656–1663. [Google Scholar] [CrossRef]
- Pieniazek, P.; Musialek, P.; Kablak-Ziembicka, A.; Tekieli, L.; Motyl, R.; Przewlocki, T.; Moczulski, Z.; Pasowicz, M.; Sokolowski, A.; Lesniak-Sobelga, A.; et al. Carotid artery stenting with patient- and lesion-tailored selection of the neuroprotection system and stent type: Early and 5-year results from a prospective academic registry of 535 consecutive procedures (TARGET-CAS). J. Endovasc. Ther. 2008, 15, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Schofer, J.; Arendt, M.; Tubler, T.; Sandstede, J.; Schluter, M. Late cerebral embolization after emboli-protected carotid artery stenting assessed by sequential diffusion-weighted magnetic resonance imaging. JACC Cardiovasc. Interv. 2008, 1, 571–577. [Google Scholar] [CrossRef]
- De Donato, G.; Setacci, F.; Sirignano, P.; Galzerano, G.; Cappelli, A.; Setacci, C. Optical coherence tomography after carotid stenting: Rate of stent malapposition, plaque prolapse and fibrous cap rupture according to stent design. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Sakamoto, S.; Shinagawa, K.; Ichinose, N.; Ishii, D.; Matsushige, T.; Kiura, Y.; Kurisu, K. Detection of in-stent protrusion (ISP) by intravascular ultrasound during carotid stenting: Usefulness of stent-in-stent placement for ISP. Eur. Radiol. 2019, 29, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I.; Kotsugi, M.; Park, H.S.; Furuta, T.; Sato, F.; Myochin, K.; Nishimura, F.; Yamada, S.; Motoyama, Y.; Nakase, H. Near-infrared spectroscopy carotid plaque characteristics and cerebral embolism in carotid artery stenting using first-generation stent. EuroIntervention 2021, 17, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Musialek, P.; Roubin, G.S. Double-Layer Carotid Stents: From the Clinical Need, through a Stent-in-Stent Strategy, to Effective Plaque Isolation… the Journey Toward Safe Carotid Revascularization Using the Endovascular Route. J. Endovasc. Ther. 2019, 26, 572–577. [Google Scholar] [CrossRef]
- Paraskevas, K.I.; Veith, F.J. Transcervical access, reversal of flow and mesh-covered stents: New options in the armamentarium of carotid artery stenting. World J. Cardiol. 2017, 9, 416–421. [Google Scholar] [CrossRef]
- Musiałek, P.; Hopkins, L.N.; Siddiqui, A. One swallow does not a summer make but many swallows do: Accumulating clinical evidence for nearly-eliminated peri-procedural and 30-day complications with meshcovered stents transforms the carotid revascularisation field. Adv. Interv. Cardiol. 2017, 13, 95–106. [Google Scholar] [CrossRef]
- Schönholz, C.; Yamada, R.; Montgomery, W.; Brothers, T.; Guimaraes, M. First-in-man implantation of a new hybrid carotid stent to prevent periprocedural neurological events during carotid artery stenting. J. Endovasc. Ther. 2014, 21, 601–604. [Google Scholar] [CrossRef]
- Wissgott, C.; Schmidt, W.; Brandt, C.; Behrens, P.; Andresen, R. Preliminary Clinical Results and Mechanical Behavior of a New Double-Layer Carotid Stent. J. Endovasc. Ther. 2015, 22, 634–639. [Google Scholar] [CrossRef]
- Wissgott, C.; Schmidt, W.; Brandt-Wunderlich, C.; Behrens, P.; Andresen, R. Clinical Results and Mechanical Properties of the Carotid CGUARD Double-Layered Embolic Prevention Stent. J. Endovasc. Ther. 2017, 24, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Musialek, P.; Mazurek, A.; Trystula, M.; Borratynska, A.; Lesniak-Sobelga, A.; Urbanczyk, M.; Banys, R.P.; Brzychczy, A.; Zajdel, W.; Partyka, L.; et al. Novel PARADIGM in carotid revascularisation: Prospective evaluation of All-comer peRcutaneous cArotiD revascularisation in symptomatic and Increased-risk asymptomatic carotid artery stenosis using CGuard™ MicroNet-covered embolic prevention stent system. EuroIntervention 2016, 12, e658–e670. [Google Scholar] [CrossRef] [PubMed]
- Schofer, J.; Musiałek, P.; Bijuklic, K.; Kolvenbach, R.; Trystula, M.; Siudak, Z.; Sievert, H. A Prospective, Multicenter Study of a Novel Mesh-Covered Carotid Stent the CGuard CARENET Trial (Carotid Embolic Protection Using MicroNet). JACC Cardiovasc. Interv. 2015, 8, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Ruffino, M.A.; Faletti, R.; Bergamasco, L.; Fonio, P.; Righi, D. Incidence of New Ischaemic Brain Lesions After Carotid Artery Stenting with the Micromesh Roadsaver Carotid Artery Stent: A Prospective Single-Centre Study. Cardiovasc. Intervent. Radiol. 2016, 39, 1541–1549. [Google Scholar] [CrossRef]
- Mazurek, A.; Borratynska, A.; Malinowski, K.P.; Brozda, M.; Gancarczyk, U.; Dluzniewska, N.; Czyz, L.; Duplicka, M.; Sobieraj, E.; Trystula, M.; et al. MicroNET-covered stents for embolic prevention in patients undergoing carotid revascularisation: Twelve-month outcomes from the PARADIGM study. EuroIntervention 2020, 16, E950–E952. [Google Scholar] [CrossRef]
- Montorsi, P.; Caputi, L.; Galli, S.; Ravagnani, P.M.; Teruzzi, G.; Annoni, A.; Calligaris, G.; Fabbiocchi, F.; Trabattoni, D.; de Martini, S.; et al. Carotid Wallstent Versus Roadsaver Stent and Distal Versus Proximal Protection on Cerebral Microembolization During Carotid Artery Stenting. JACC Cardiovasc. Interv. 2020, 13, 403–414. [Google Scholar] [CrossRef]
- Karpenko, A.; Bugurov, S.; Ignatenko, P.; Starodubtsev, V.; Popova, I.; Malinowski, K.; Musialek, P. Randomized Controlled Trial of Conventional Versus MicroNet-Covered Stent in Carotid Artery Revascularization. JACC Cardiovasc. Interv. 2021, 14, 2377–2387. [Google Scholar] [CrossRef]
- Stabile, E.; de Donato, G.; Musialek, P.; Deloose, K.; Nerla, R.; Sirignano, P.; Mazurek, A.; Mansour, W.; Fioretti, V.; Esposito, F.; et al. Use of Dual-Layered Stents in Endovascular Treatment of Extracranial Stenosis of the Internal Carotid Artery: Results of a Patient-Based Meta-Analysis of 4 Clinical Studies. JACC Cardiovasc. Interv. 2018, 11, 2405–2411. [Google Scholar] [CrossRef]
- Stabile, E.; de Donato, G.; Musialek, P.; Deloose, K.; Nerla, R.; Sirignano, P.; Mazurek, A.; Mansour, W.; Fioretti, V.; Esposito, F.; et al. Use of Dual-Layered Stents for Carotid Artery Angioplasty: 1-Year Results of a Patient-Based Meta-Analysis. JACC Cardiovasc. Interv. 2020, 13, 1709–1715. [Google Scholar] [CrossRef]
- Yadav, J.S.; Wholey, M.H.; Kuntz, R.E.; Fayad, P.; Katzen, B.T.; Mishkel, G.J.; Bajwa, T.K.; Whitlow, P.; Strickman, N.E.; Jaff, M.R.; et al. Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N. Engl. J. Med. 2004, 351, 1493–1501. [Google Scholar] [CrossRef]
- Arhuidese, I.J.; Rizwan, M.; Nejim, B.; Malas, M. Outcomes of Primary and Secondary Carotid Artery Stenting. Stroke 2017, 48, 3086–3092. [Google Scholar] [CrossRef] [PubMed]
- Aronow, H.D.; Gray, W.A.; Ramee, S.R.; Mishkel, G.J.; Schreiber, T.J.; Wang, H. Predictors of neurological events associated with carotid artery stenting in high-surgical-risk patients: Insights from the Cordis Carotid Stent Collaborative. Circ Cardiovasc. Interv. 2010, 3, 577–584. [Google Scholar] [CrossRef]
- Bibl, D.; Lampl, C.; Biberhofer, I.; Kerschner, K.; Kypta, A.; Bergmann, J.; Kaindlstorfer, A.; Roper, C.; Yazdi, K.; Engleder, C.; et al. Internal carotid artery stent placement without emboli protection: Results and long-term outcome. Neurology 2005, 65, 132–134. [Google Scholar] [CrossRef]
- Capoccia, L.; Sirignano, P.; Mansour, W.; d’Adamo, A.; Sbarigia, E.; Mariani, P.; di Biasi, C.; Speziale, F. Peri-procedural brain lesions prevention in CAS (3PCAS): Randomized trial comparing CGuard™ stent vs. Wallstent™. Int. J. Cardiol. 2019, 279, 148–153. [Google Scholar] [CrossRef]
- De Haro, J.; Michel, I.; Bleda, S.; Cañibano, C.; Acin, F. Carotid Stenting in Patients with High Risk Versus Standard Risk for Open Carotid Endarterectomy (REAL-1 Trial). Am. J. Cardiol. 2017, 120, 322–326. [Google Scholar] [CrossRef]
- Diamond, J.; Madhavan, M.V.; Sabik, J.F.; Serruys, P.W.; Kappetein, A.P.; Leon, M.B.; Taggart, D.P.; Berland, J.; Morice, M.; Gersh, B.J.; et al. Left Main Percutaneous Coronary Intervention Versus Coronary Artery Bypass Grafting in Patients with Prior Cerebrovascular Disease: Results from the EXCEL Trial. JACC Cardiovasc. Interv. 2018, 11, 2441–2450. [Google Scholar] [CrossRef]
- Ecker, R.D.; Lau, T.; Levy, E.I.; Hopkins, L.N. Thirty-day morbidity and mortality rates for carotid artery intervention by surgeons who perform both carotid endarterectomy and carotid artery angioplasty and stent placement. J. Neurosurg. 2007, 106, 217–221. [Google Scholar] [CrossRef]
- Eckstein, H.-H.; Ringleb, P.; Allenberg, J.-R.; Berger, J.; Fraedrich, G.; Hacke, W.; Hennerici, M.; Stingele, R.; Fiehler, J.; Zeumer, H.; et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: A multinational, prospective, randomised trial. Lancet Neurol. 2008, 7, 893–902. [Google Scholar] [CrossRef]
- Featherstone, R.L.; Dobson, J.; Ederle, J.; Doig, D.; Bonati, L.H.; Morris, S.; Patel, N.; Brown, M.M. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): A randomised controlled trial with cost-effectiveness analysis. Health Technol. Assess. 2016, 20, 1–94. [Google Scholar] [CrossRef]
- Gandini, R.; Del Giudice, C.; Da Ros, V.; Sallustio, F.; Altobelli, S.; D’Onofrio, A.; Abrignani, S.; Vasili, E.; Stanzione, P.; Simonetti, G. Long-term Results of Drug-Eluting Balloon Angioplasty for Treatment of Refractory Recurrent Carotid In-Stent Restenosis. J. Endovasc. Ther. 2014, 21, 671–677. [Google Scholar] [CrossRef]
- González, A.; Gil-Peralta, A.; Mayol, A.; Gonzalez-Marcos, J.; Moniche, F.; Aguilar, M.; Gutierrez, I. Internal Carotid Artery Stenting in Patients with Near Occlusion: 30-Day and Long-Term Outcome. Am. J. Neuroradiol. 2010, 32, 252–258. [Google Scholar] [CrossRef]
- Goode, S.D.; Cleveland, T.J.; Gaines, P.A. United Kingdom carotid artery stent registry: Short- and long-term outcomes. Cardiovasc. Intervent. Radiol. 2013, 36, 1221–1231. [Google Scholar] [CrossRef]
- Gory, B.; Piotin, M.; Haussen, D.C.; Steglich-Arnholm, H.; Holtmannspötter, M.; Labreuche, J.; Taschner, C.; Eiden, S.; Nogueira, R.G.; Papanagiotou, P.; et al. Thrombectomy in Acute Stroke with Tandem Occlusions From Dissection Versus Atherosclerotic Cause. Stroke 2017, 48, 3145–3148. [Google Scholar] [CrossRef]
- Heck, D. Thirty day results of 227 consecutive carotid stent procedures performed in carotid stenting clinical trials. J. NeuroInterv. Surg. 2009, 1, 154–158. [Google Scholar] [CrossRef]
- Higashida, R.T.; Popma, J.J.; Apruzzese, P.; Zimetbaum, P. MAVErIC I and II Investigators. Evaluation of the medtronic exponent self-expanding carotid stent system with the medtronic guardwire temporary occlusion and aspiration system in the treatment of carotid stenosis: Combined from the MAVErIC (Medtronic AVE Self-expanding CaRotid Stent System with distal protection in the treatment of Carotid stenosis) I and MAVErIC II trials. Stroke 2010, 41, 102–109. [Google Scholar]
- Hill, M.; Morrish, W.; Soulez, G.; Nevelsteen, A.; Maleux, G.; Rogers, C.; Hauptmann, K.; Bonafé, A.; Beyar, R.; Gruberg, L.; et al. Multicenter Evaluation of a Self-Expanding Carotid Stent System with Distal Protection in the Treatment of Carotid Stenosis. Am. J. Neuroradiol. 2006, 27, 759–765. [Google Scholar]
- Hopf-Jensen, S.; Marques, L.; Preiß, M.; Müller-Hülsbeck, S. Lesion-Related Carotid Angioplasty and Stenting with Closed-Cell Design without Embolic Protection Devices in High-Risk Elderly Patients-Can This Concept Work Out? A Single Center Experience Focusing on Stent Design. Int. J. Angiol. 2014, 23, 263–270. [Google Scholar]
- Howie, B.A.; Witek, A.M.; Hussain, M.S.; Bain, M.D.; Toth, G. Carotid Endarterectomy and Carotid Artery Stenting in a Predominantly Symptomatic Real-World Patient Population. World Neurosurg. 2019, 127, e722–e726. [Google Scholar] [CrossRef]
- Huang, H.; Chen, K.; Guo, T.; Zhang, Y.; Qu, W.; Zhou, Z.; Liu, G.; Chen, L. Treatment with carotid angioplasty stent placement for post-stroke depression compared to antidepressants. Neurosciences 2012, 17, 53–56. [Google Scholar]
- Itami, H.; Tokunaga, K.; Okuma, Y.; Hishikawa, T.; Sugiu, K.; Ida, K.; Date, I. Novel 3D-CT evaluation of carotid stent volume: Greater chronological expansion of stents in patients with vulnerable plaques. Neuroradiology 2013, 55, 1153–1160. [Google Scholar] [CrossRef]
- Ito, K.; Kai, Y.; Hyodo, A.; Ishiuchi, S. Long-Term Outcome of Angioplasty or Stent Placement for Stenosis of the Cavernous or Petrous Portion of the Internal Carotid Artery. Neurol. Med. -Chir. 2011, 51, 813–818. [Google Scholar] [CrossRef][Green Version]
- Jones, M.R.; Howard, G.; Roubin, G.S.; Blackshear, J.L.; Cohen, D.J.; Cutlip, D.E.; Leimgruber, P.P.; Rhodes, D.; Prineas, R.J.; Glasser, S.P.; et al. Periprocedural Stroke and Myocardial Infarction as Risks for Long-Term Mortality in CREST. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004663. [Google Scholar] [CrossRef]
- Jongen, L.M.; Hendrikse, J.; Waaijer, A.; van der Worp, H.B.; Leijdekkers, V.J.; Lo, R.T.; Mali, W.P.T.M.; Prokop, M. Frequency and consequences of early in-stent lesions after carotid artery stent placement. J. Vasc. Interv. Radiol. 2009, 20, 573–579. [Google Scholar] [CrossRef]
- Khan, A.A.; Chaudhry, S.A.; Sivagnanam, K.; Hassan, A.E.; Suri, M.F.K.; Qureshi, A.I. Cost-effectiveness of carotid artery stent placement versus endarterectomy in patients with carotid artery stenosis. J. Neurosurg. 2012, 117, 89–93. [Google Scholar] [CrossRef]
- Kougias, P.; Collins, R.; Pastorek, N.; Sharath, S.; Barshes, N.R.; McCulloch, K.; Pisimisis, G.; Berger, D.H. Comparison of domain-specific cognitive function after carotid endarterectomy and stenting. J. Vasc. Surg. 2015, 62, 355–362. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Han, J.; Yang, L.; Li, Y. Short-term Outcome of Straight vs Tapered Carotid Stenting for Symptomatic Carotid Artery Ste-nosis: A Prospective Study. J. Endovasc. Ther. 2018, 25, 765–770. [Google Scholar] [CrossRef]
- Malas, M.B.; Lorenzo, J.I.L.; Nejim, B.; Hanover, T.M.; Mehta, M.; Kashyap, V.; Kwolek, C.J.; Cambria, R. Analysis of the ROADSTER pivotal and extended-access cohorts shows excellent 1-year durability of transcarotid stenting with dynamic flow reversal. J. Vasc. Surg. 2019, 69, 1786–1796. [Google Scholar] [CrossRef]
- Mannheim, D.; Karmeli, R. A prospective randomized trial comparing endarterectomy to stenting in severe asymptomatic carotid stenosis. J. Cardiovasc. Surg. 2017, 58, 814–817. [Google Scholar] [CrossRef]
- Matsumura, J.S.; Gray, W.; Chaturvedi, S.; Gao, X.; Cheng, J.; Verta, P. CAPTURE 2 risk-adjusted stroke outcome benchmarks for carotid artery stenting with distal embolic protection. J. Vasc. Surg. 2010, 52, 576–583.e2. [Google Scholar] [CrossRef]
- Maud, A.; Vázquez, G.; Nyman, J.A.; Lakshminarayan, K.; Anderson, D.C.; Qureshi, A.I. Cost-effectiveness analysis of protected carotid artery stent placement versus endarterectomy in high-risk patients. J. Endovasc. Ther. 2010, 17, 224–229. [Google Scholar] [CrossRef]
- Moore, W.S.; Voeks, J.H.; Roubin, G.S.; Clark, W.M.; Howard, V.J.; Jones, M.R.; Brott, T.G. Duration of asymptomatic status and outcomes following carotid endarterectomy and carotid artery stenting in the Carotid Revascularization Endarterectomy vs Stenting Trial. J. Vasc. Surg. 2019, 69, 1797–1800. [Google Scholar] [CrossRef] [PubMed]
- Mozes, G.; Sullivan, T.M.; Torres-Russotto, D.; Bower, T.C.; Hoskin, T.L.; Sampaio, S.; Gloviczki, P.; Panneton, J.M.; Noel, A.A.; Cherry, K.J. Carotid endarterectomy in SAPPHIRE-eligible high-risk patients: Implications for selecting patients for carotid angioplasty and stenting. J. Vasc. Surg. 2004, 39, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Reiff, T.; Stingele, R.; Eckstein, H.H.; Fraedrich, G.; Jansen, O.; Mudra, H.; Mansmann, U.; Hacke, W.; Ringleb, P.; SPACE2-Study Group. Stent-protected angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy: SPACE2—A three-arm randomised-controlled clinical trial. Int. J. Stroke 2009, 4, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Reimers, B.; Tübler, T.; de Donato, G.; Della Barbera, M.; Cernetti, C.; Schlüter, M.; Mistrorigo, F.; Saccà, S.; Favero, L.; Setacci, F.; et al. Endovascular Treatment of In-Stent Restenosis After Carotid Artery Stenting: Immediate and Midterm Results. J. Endovasc. Ther. 2006, 13, 429–435. [Google Scholar] [CrossRef]
- Ringleb, P.A.; Allenberg, J.R.; Bruckmann, H.; Eckstein, H.H.; Fraedrich, G.; Hartmann, M.; Hennerici, M.G.; Jansen, O.; Klein, G.E.; Kunze, A.; et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: A randomised non-inferiority trial. Lancet 2006, 368, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, K.; Matsumura, J.S.; Chaturvedi, S.; Riles, T.; Ansel, G.M.; Metzger, D.C.; Wechsler, L.; Jaff, M.R.; Gray, W.; ACT I Investigators. Randomized Trial of Stent versus Surgery for Asym-ptomatic Carotid Stenosis. N. Engl. J. Med. 2016, 374, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.A.; Levy, E.; Bacharach, J.M.; Metzger, D.C.; Randall, B.; Garcia, A.; Siddiqui, A.; Schonholz, C.; Gray, W. A First-in-Human Evaluation of a Novel Mesh-Covered Stent for Treatment of Carotid Stenosis in Patients at High Risk for Endarterectomy: 30-Day Results of the SCAFFOLD Trial. JACC Cardiovasc. Interv. 2018, 11, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Setacci, C.; Speziale, F.; De Donato, G.; Sirignano, P.; Setacci, F.; Capoccia, L.; Galzerano, G.; Mansour, W. Physician-initiated prospective Italian Registry of carotid stenting with the C-Guard mesh-stent: The IRON-Guard registry. Rationale and design. J. Cardiovasc. Surg. 2015, 56, 787–791. [Google Scholar]
- Siddiq, F.; Adil, M.M.; Malik, A.A.; Qureshi, M.H.; Qureshi, A.I. Effect of Carotid Revascularization Endarterectomy Versus Stenting Trial Results on the Performance of Carotid Artery Stent Placement and Carotid Endarterectomy in the United States. Neurosurgery 2015, 77, 726–732. [Google Scholar] [CrossRef]
- Stabile, E.; Giugliano, G.; Cremonesi, A.; Bosiers, M.; Reimers, B.; Setacci, C.; Cao, P.; Schmidt, A.; Sievert, H.; Peeters, P.; et al. Impact on outcome of different types of carotid stent: Results from the European Registry of Carotid Artery Stenting. EuroIntervention 2016, 12, e265–e270. [Google Scholar] [CrossRef]
- Stingele, R.; Berger, J.; Alfke, K.; Eckstein, H.-H.; Fraedrich, G.; Allenberg, J.; Hartmann, M.; Ringleb, P.A.; Fiehler, J. Clinical and angiographic risk factors for stroke and death within 30 days after carotid endarterectomy and stent-protected angioplasty: A subanalysis of the SPACE study. Lancet Neurol. 2008, 7, 216–222. [Google Scholar] [CrossRef]
- Sugita, J.; Cremonesi, A.; Van Elst, F.; Stockx, L.; Mathias, K.; Schofer, J.; Suttorp, M.J.; Reul, J.; Lowens, S.; Sievert, H. European carotid PROCAR Trial: Prospective multicenter trial to evaluate the safety and performance of the ev3 Protégé stent in the treatment of carotid artery stenosis--1- and 6-month follow-up. J. Interv. Cardiol. 2006, 19, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Timaran, C.H.; Rosero, E.B.; Higuera, A.; Ilarraza, A.; Modrall, J.G.; Clagett, G.P. Randomized clinical trial of open-cell vs closed-cell stents for carotid stenting and effects of stent design on cerebral embolization. J. Vasc. Surg. 2011, 54, 1310–1316.e1. [Google Scholar] [CrossRef] [PubMed]
- Vitek, J.J.; Al-Mubarak, N.; Iyer, S.S.; Roubin, G.S. Carotid artery stent placement with distal balloon protection: Technical conside-rations. AJNR Am. J. Neuroradiol. 2005, 26, 854–861. [Google Scholar]
- Wang, Y.L.; Ma, J.; Li, Y.D.; Ding, P.X.; Han, X.W.; Wu, G. Application of the Willis covered stent for the management of posttraumatic carotid-cavernous fistulas: An initial clinical study. Neurol. India 2012, 60, 180–184. [Google Scholar]
- Watarai, H.; Kaku, Y.; Yamada, M.; Kokuzawa, J.; Tanaka, T.; Andoh, T.; Iwama, T. Follow-up study on in-stent thrombosis after carotid stenting using multidetector CT angiography. Neuroradiology 2009, 51, 243–251. [Google Scholar] [CrossRef]
- Witt, K.; Börsch, K.; Daniels, C.; Walluscheck, K.; Alfke, K.; Jansen, O.; Deuschl, G.; Stingele, R. Neuropsychological consequences of endarterectomy and endovascular angioplasty with stent placement for treatment of symptomatic carotid stenosis: A prospective randomised study. Aktuel- Neurol. 2006, 33, P435. [Google Scholar] [CrossRef]
- Zhao, W.; Meng, R.; Ma, C.; Hou, B.; Jiao, L.; Zhu, F.; Wu, W.; Shi, J.; Duan, Y.; Zhang, R.; et al. EfficSafety and Efficacy of Remote Ischemic Preconditioning in Patients with Severe Carotid Artery Stenosis Before Carotid Artery Stenting: A Proof-of-Concept, Randomized Controlled Trial.acy of Stent-Retriever Thrombectomy in Magnetic Resonance Imaging Versus Computed Tomographic Perfusion-Selected Patients in SWIFT PRIME Trial (Solitaire FR With the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke). Circulation 2017, 135, 1325–1335. [Google Scholar]
- Bianchi, C.; Ou, H.W.; Bishop, V.; Zhang, W.; Molkara, A.; Teruya, T.H.; Abou-Zamzam, A.M. Carotid Artery Stenting in High-Risk Patients: Midterm Mortality Analysis. Ann. Vasc. Surg. 2008, 22, 185–189. [Google Scholar] [CrossRef]
- Bonati, L.H.; Dobson, J.; Featherstone, R.L.; Ederle, J.; van der Worp, H.B.; de Borst, G.J.; Mali, W.P.T.M.; Beard, J.D.; Cleveland, T.; Engelter, S.T.; et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: The International Carotid Stenting Study (ICSS) randomised trial. Lancet 2014, 385, 529–538. [Google Scholar] [CrossRef]
- Bonati, L.H.; Ederle, J.; McCabe, D.J.; Dobson, J.; Featherstone, R.L.; Gaines, P.A.; Beard, J.D.; Venables, G.S.; Markus, H.S.; Clifton, A.; et al. Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): Long-term follow-up of a randomised trial. Lancet Neurol. 2009, 8, 908–917. [Google Scholar] [CrossRef]
- Bonati, L.H.; Gregson, J.; Dobson, J.; McCabe, D.J.H.; Nederkoorn, P.J.; van der Worp, H.B.; de Borst, G.J.; Richards, T.; Cleveland, T.; Müller, M.D.; et al. Restenosis and risk of stroke after stenting or endarterectomy for symptomatic carotid stenosis in the International Carotid Stenting Study (ICSS): Secondary analysis of a randomised trial. Lancet Neurol. 2018, 17, 587–596. [Google Scholar] [CrossRef]
- Cano, M.N.; Kambara, A.M.; De Cano, S.J.; Portela, L.A.P.; Paes, T.; Costa, J.R.; Abizaid, A.A.C.; Moreira, S.M.; Sousa, A.G.; Sousa, J.E.M.R. Randomized Comparison of Distal and Proximal Cerebral Protection During Carotid Artery Stenting. JACC Cardiovasc. Interv. 2013, 6, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- CaRESS Steering Committee. Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS) phase I clinical trial: 1-year results. J. Vasc. Surg. 2005, 42, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Chiam, P.; Roubin, G.S.; Panagopoulos, G.; Iyer, S.S.; Green, R.M.; Brennan, C.; Vitek, J.J. One-Year Clinical Outcomes, Midterm Survival, and Predictors of Mortality After Carotid Stenting in Elderly Patients. Circulation 2009, 119, 2343–2348. [Google Scholar] [CrossRef]
- De Borst, G.J.; Hellings, W.E.; Ackerstaff, R.G.; Moll, F.L.; Antonius Carotid Endarterectomy, Angioplasty and Stenting Study Group. Intrapatient comparison of restenosis between carotid artery angioplasty with stenting and carotid endarterectomy. J. Cardiovasc. Surg. 2006, 47, 49–54. [Google Scholar]
- Demirel, S.; Attigah, N.; Bruijnen, H. Multicenter Experience on Eversion Versus Conventional Carotid Endarterectomy in Symptomatic Carotid Artery Stenosis: Observations from the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE-1) Trial. J. Vasc. Surg. 2012, 56, 1473–1474. [Google Scholar] [CrossRef][Green Version]
- Ederle, J.; Bonati, L.H.; Dobson, J.; Featherstone, R.L.; Gaines, P.A.; Beard, J.D.; Venables, G.S.; Markus, H.S.; Clifton, A.; Sandercock, P.; et al. Endovascular treatment with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): Long-term follow-up of a randomised trial. Lancet Neurol. 2009, 8, 898–907. [Google Scholar] [CrossRef]
- Gray, W.A.; Hopkins, L.N.; Yadav, S.; Davis, T.; Wholey, M.; Atkinson, R.; Cremonesi, A.; Fairman, R.; Walker, G.; Verta, P.; et al. Protected carotid stenting in high-surgical-risk patients: The ARCHeR results. J. Vasc. Surg. 2006, 44, 258–268. [Google Scholar] [CrossRef]
- Gurm, H.S.; Yadav, J.S.; Fayad, P.; Katzen, B.T.; Mishkel, G.J.; Bajwa, T.K.; Ansel, G.; Strickman, N.E.; Wang, H.; Cohen, S.A.; et al. Long-Term Results of Carotid Stenting versus Endarterectomy in High-Risk Patients. New Engl. J. Med. 2008, 358, 1572–1579. [Google Scholar] [CrossRef]
- Hopkins, L.N.; Myla, S.; Grube, E.; Wehman, J.C.; Levy, E.I.; Bersin, R.M.; Joye, J.D.; Allocco, D.J.; Kelley, L.; Baim, D.S. Carotid artery revascularization in high surgical risk patients with the NexStent and the Filterwire EX/EZ: 1-year results in the CABERNET trial. Catheter. Cardiovasc. Interv. 2008, 71, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; White, C.J.; Hopkins, L.N.; Katzen, B.T.; Safian, R.; Wholey, M.H.; Gray, W.A.; Ciocca, R.; Bachinsky, W.B.; Ansel, G.; et al. Carotid Artery Revascularization in High-Surgical-Risk Patients Using the Carotid WALLSTENT and FilterWire EX/EZ: 1-Year Outcomes in the BEACH Pivotal Group. J. Am. Coll. Cardiol. 2008, 51, 427–434. [Google Scholar] [CrossRef][Green Version]
- McCabe, D.J.; Pereira, A.C.; Clifton, A.; Bland, J.M.; Brown, M.M. Restenosis After Carotid Angioplasty, Stenting, or Endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS). Stroke 2005, 36, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.M.; Coon, A.L.; Kan, P.T.; Wakhloo, A.K.; Hanel, R.A. SCENT Trial. Stroke 2019, 50, 1473–1479. [Google Scholar] [CrossRef]
- Müller, M.D.; Gregson, J.; McCabe, D.J.; Nederkoorn, P.J.; Van Der Worp, H.B.; De Borst, G.J.; Cleveland, T.; Wolff, T.; Engelter, S.T.; Lyrer, P.A.; et al. Stent Design, Restenosis and Recurrent Stroke After Carotid Artery Stenting in the International Carotid Stenting Study. Stroke 2019, 50, 3013–3020. [Google Scholar] [CrossRef] [PubMed]
- Oteros, R.; Jimenez-Gomez, E.; Bravo-Rodriguez, F.; Ochoa, J.; Guerrero, R.; Delgado, F. Unprotected Carotid Artery Stenting in Symptomatic Patients with High-Grade Stenosis: Results and Long-Term Follow-Up in a Single-Center Experience. Am. J. Neuroradiol. 2012, 33, 1285–1291. [Google Scholar] [CrossRef]
- Ouriel, K.; Wholey, M.H.; Fayad, P.; Katzen, B.T.; Whitlow, P.; Frentzko, M.; Kuntz, R.E.; Wechsler, L.; Hopkins, N.; Satler, L.; et al. Feasibility Trial of Carotid Stenting with and Without an Embolus Protection Device. J. Endovasc. Ther. 2005, 12, 525–537. [Google Scholar] [CrossRef]
- Paukovits, T.M.; Haász, J.; Molnár, A.; Szeberin, Z.; Nemes, B.; Varga, D.; Hüttl, K.; Bérczi, V. Transfemoral endovascular treatment of proximal common carotid artery lesions: A single-center experience on 153 lesions. J. Vasc. Surg. 2008, 48, 80–87. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Kirmani, J.F.; Divani, A.A.; Hobson, R.W. Carotid Angioplasty with or without Stent Placement versus Carotid Endarterectomy for Treatment of Carotid Stenosis: A Meta-analysis. Neurosurgery 2005, 56, 1171–1181. [Google Scholar] [CrossRef]
- Raabe, R.D.; Burr, R.B.; Short, R. One-year Cognitive Outcomes Associated with Carotid Artery Stent Placement. J. Vasc. Interv. Radiol. 2010, 21, 983–988. [Google Scholar] [CrossRef]
- Randall, M.S.; McKevitt, F.M.; Kumar, S.; Cleveland, T.J.; Endean, K.; Venables, G.S.; Gaines, P.A. Long-Term Results of Carotid Artery Stents to Manage Symptomatic Carotid Artery Stenosis and Factors That Affect Outcome. Circ. Cardiovasc. Interv. 2010, 3, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Schermerhorn, M.L.; Liang, P.; Eldrup-Jorgensen, J.; Cronenwett, J.L.; Nolan, B.W.; Kashyap, V.; Wang, G.J.; Motaganahalli, R.L.; Malas, M.B. Association of Transcarotid Artery Revascularization vs Transfemoral Carotid Artery Stenting with Stroke or Death Among Patients with Carotid Artery Stenosis. JAMA 2019, 322, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, T.L.; Strickman, N.; Davis, T.; Kumar, V.; Mishkel, G.; Foster, M.; Donohoe, D.; Britto, S.; Ansel, G. Carotid Artery Stenting with Emboli Protection Surveillance Study: Outcomes at 1 Year. J. Am. Coll. Cardiol. 2010, 56, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Setacci, C.; Chisci, E.; de Donato, G.; Setacci, F.; Sirignano, P.; Galzerano, G. Carotid artery stenting in a single center: Are six years of experience enough to achieve the standard of care? Eur. J. Vasc. Endovasc. Surg. 2007, 34, 655–662. [Google Scholar] [CrossRef]
- SSYLVIA Study Investigators. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): Study results. Stroke 2004, 35, 1388–1392. [Google Scholar] [CrossRef]
- Weinberg, I.; Beckman, J.A.; Shu, Y.; Jaff, M.R. Response by Weinberg et al to Letter Regarding Article, “Carotid Stent Fractures Are Not Associated with Adverse Events: Results From the ACT-1 Multicenter Randomized Trial (Carotid Angioplasty and Stenting Versus Endarterectomy in Asymptomatic Subjects Who Are at Standard Risk for Carotid Endarterectomy With Significant Extracranial Carotid Stenotic Disease)”. Circulation 2018, 137, 2676–2677. [Google Scholar] [CrossRef]
- Yang, B.; Chen, W.; Yang, Y.; Lin, Y.; Duan, Y.; Li, J.; Wang, H.; Fu, F.; Zhuge, Q.; Chen, X. Short- and Long-Term Hemodynamic and Clinical Effects of Carotid Artery Stenting. Am. J. Neuroradiol. 2012, 33, 1170–1176. [Google Scholar] [CrossRef]
- Zhu, Q.; Fang, S.; Wang, G.; Zhou, Z.; Bian, S.; Cui, S.; Yu, S.; Wang, F.; Shan, L.; Kang, J. Clinical effects and safety review of self-expanding stent surgery for extracranial carotid artery stenosis treatment. Genet. Mol. Res. 2014, 13, 5128–5137. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Kohl, C.; McIntosh, E.J.; Unger, S.; Haddaway, N.R.; Kecke, S.; Schiemann, J.; Wilhelm, R. Online tools supporting the conduct and reporting of systematic reviews and systematic maps: A case study on CADIMA and review of existing tools. Environ. Evid. 2018, 7, 8. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Abu-Halimah, S.; Bensenhaver, J.; Nanjundappa, A.; Stone, P.A.; Dean, L.S.; Keiffer, T.; Emmett, M.; Tarakji, M.; AbuRahma, Z. Primary carotid artery stenting versus carotid artery stenting for postcarotid endarterectomy stenosis. J. Vasc. Surg. 2009, 50, 1031–1039. [Google Scholar] [CrossRef]
- Bayram, N.A.; Bozkurt, E.; Ayhan, H.; Gürkaş, E.; Orhan, G.; Ak, F.; Bilen, E.; Sari, C.; Akçay, M.; Durmaz, T.; et al. Early outcomes of carotid artery stenting. Perfusion 2012, 27, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Akkan, K.; Ilgit, E.; Onal, B.; Cindil, E.; Solak, E.P.; Oncu, F.; Geylan, D.E. Endovascular Treatment for Near Occlusion of the Internal Carotid Artery: 30-Day Outcome and Long-Term Follow-Up. Clin. Neuroradiol. 2018, 28, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Alkins, R.; Matouk, C.C.; Cruz, J.P.; Marotta, T.; Montanera, W.; Spears, J. Carotid Artery Angioplasty and Stenting for Patients Less than 70 Years-of-Age. Can. J. Neurol. Sci./J. Can. des Sci. Neurol. 2012, 39, 338–342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ansel, G.M.; Hopkins, L.N.; Jaff, M.R.; Rubino, P.; Bacharach, J.M.; Scheinert, D.; Myla, S.; Das, T.; Cremonesi, A.; Investigators for the ARMOUR Pivotal Trial. Safety and effectiveness of the INVATEC MO.MA proximal cerebral protection device during carotid artery stenting: Results from the ARMOUR pivotal trial. Catheter. Cardiovasc. Interv. 2010, 76, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.; Köklü, E.; Yüksel, I.Ö.; Çağırcı, G.; Bayar, N.; Yılmaz, A.; Biçer Gömceli, Y.; Erol, B. Two-year results of carotid artery stenting. Turk. Kardiyol. Dern. Ars. 2014, 42, 429–434. [Google Scholar] [CrossRef]
- Bergeron, P.; Roux, M.; Khanoyan, P.; Douillez, V.; Bras, J.; Gay, J. Long-term results of carotid stenting are competitive with surgery. J. Vasc. Surg. 2005, 41, 213–221. [Google Scholar] [CrossRef][Green Version]
- Biggs, N.G.; Rangarajan, S.; McClure, D.N. Has carotid artery stenting found its place? A 10-year regional centre perspective. ANZ J. Surg. 2014, 86, 179–183. [Google Scholar] [CrossRef]
- Bijuklic, K.; Wandler, A.; Hazizi, F.; Schofer, J. The PROFI Study (Prevention of Cerebral Embolization by Proximal Balloon Occlusion Compared to Filter Protection During Carotid Artery Stenting): A Prospective Randomized Trial. J. Am. Coll. Cardiol. 2012, 59, 1383–1389. [Google Scholar] [CrossRef]
- Bosiers, M.; De Donato, G.; Deloose, K.; Verbist, J.; Peeters, P.; Castriota, F.; Cremonesi, A.; Setacci, C. Does free cell area influence the outcome in carotid artery stenting? Eur. J. Vasc. Endovasc. Surg. 2007, 33, 135–141. [Google Scholar] [CrossRef]
- Bosiers, M.; Deloose, K.; Torsello, G.; Scheinert, D.; Maene, L.; Peeters, P.; Müller-Hülsbeck, S.; Sievert, H.; Langhoff, R.; Bosiers, M.; et al. The CLEAR-ROAD study: Evaluation of a new dual layer micromesh stent system for the carotid artery. EuroIntervention 2016, 12, e671–e676. [Google Scholar] [CrossRef] [PubMed]
- Bosiers, M.; Scheinert, D.; Mathias, K.; Langhoff, R.; Mudra, H.; Diaz-Cartelle, J. Carotid Stenting with Distal Protection in High-Surgical-Risk Patients: One-Year Results of the ASTI Trial. Cardiovasc. Interv. Radiol. 2014, 38, 295–303. [Google Scholar] [CrossRef]
- Brewster, L.P.; Beaulieu, R.; Corriere, M.A.; Veeraswamy, R.; Niazi, K.A.; Robertson, G.; Dodson, T.F.; Kasirajan, K. Carotid revascularization outcomes comparing distal filters, flow reversal, and endarterectomy. J. Vasc. Surg. 2011, 54, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.G.; Hobson, R.W.; Howard, G.; Roubin, G.S.; Clark, W.M.; Brooks, W.; Mackey, A.; Hill, M.D.; Leimgruber, P.P.; Sheffet, A.J.; et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N. Engl. J. Med. 2010, 363, 11–23. [Google Scholar] [CrossRef]
- Buszman, P.P.; Szymański, R.; Dębiński, M.; Milewski, K.; Krol, M.; Nowakowski, P.; Kiesz, R.S.; Radvany, M.G.; Wiernek, S.; Wiernek, B.; et al. Long-term results of cephalad arteries percutanoeus transluminal angioplasty with stent implantation (The CAPTAS registry). Catheter. Cardiovasc. Interv. 2012, 79, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Casana, R.; Tolva, V.; Odero, A.; Malloggi, C.; Paolucci, A.; Triulzi, F.; Silani, V. Safety and Efficacy of the New Micromesh-Covered Stent CGuard in Patients Undergoing Carotid Artery Stenting: Early Experience from a Single Centre. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 681–687. [Google Scholar] [CrossRef] [PubMed]
- De Castro-Afonso, L.H.; Nakiri, G.S.; Monsignore, L.M.; dos Santos, D.; Camilo, M.R.; Dias, F.A.; Cougo-Pinto, P.T.; Barreira, C.M.A.; Alessio-Alves, F.F.; Fábio, S.R.C.; et al. Outcomes of carotid artery stenting at a high-volume Brazilian interventional neuroradiology center. Clinics 2015, 70, 180–184. [Google Scholar] [CrossRef]
- Chung, C.; Cayne, N.S.; Adelman, M.A.; Riles, T.S.; Lamparello, P.; Han, D.; Marin, M.L.; Faries, P.L.; Lamparello, P. Improved Hemodynamic Outcomes with Glycopyrrolate Over Atropine in Carotid Angioplasty and Stenting. Perspect. Vasc. Surg. Endovasc. Ther. 2010, 22, 164–170. [Google Scholar] [CrossRef]
- Chung, G.; Jeong, J.; Kwak, H.; Hwang, S. Associations between Cerebral Embolism and Carotid Intraplaque Hemorrhage during Protected Carotid Artery Stenting. Am. J. Neuroradiol. 2015, 37, 686–691. [Google Scholar] [CrossRef]
- Cieri, E.; De Rango, P.; Maccaroni, M.R.; Spaccatini, A.; Caso, V.; Cao, P. Is haemodynamic depression during carotid stenting a predictor of peri-procedural complications? Eur. J. Vasc. Endovasc. Surg. 2008, 35, 399–404. [Google Scholar] [CrossRef]
- Clair, D.G.; Hopkins, L.N.; Mehta, M.; Kasirajan, K.; Schermerhorn, M.; Schönholz, C.; Kwolek, C.J.; Eskandari, M.K.; Powell, R.J.; Ansel, G.M.; et al. Neuroprotection during carotid artery stenting using the GORE flow reversal system: 30-day outcomes in the EMPiRE Clinical Study. Catheter. Cardiovasc. Interv. 2010, 77, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.G.; Saad, W.E. Impact of Elevated Perioperative Fasting Blood Glucose on Carotid Artery Stenting Outcomes. Ann. Vasc. Surg. 2014, 28, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- De Donato, G.; Setacci, C.; Deloose, K.; Peeters, P.; Cremonesi, A.; Bosiers, M. Long-term results of carotid artery stenting. J. Vasc. Surg. 2008, 48, 1431–1440. [Google Scholar] [CrossRef]
- Eskandari, M.K.; Usman, A.A.; Garcia-Toca, M.; Matsumura, J.S.; Kibbe, M.R.; Morasch, M.D.; Rodriguez, H.E.; Pearce, W.H. Eight-year institutional review of carotid artery stenting. J. Vasc. Surg. 2010, 51, 1145–1151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Faggioli, G.; Pini, R.; Rapezzi, C.; Mauro, R.; Freyrie, A.; Gargiulo, M.; Reggiani, L.B.; Stella, A. Carotid Revascularization in Patients with Ongoing Oral Anticoagulant Therapy: The Advantages of Stent Placement. J. Vasc. Interv. Radiol. 2013, 24, 370–377. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Khan, H.U.; Sambol, E.B.; Kent, K.C.; Faries, P.L.; Vouyouka, A.G. Carotid artery stenting is safe and associated with comparable outcomes in men and women. J. Vasc. Surg. 2009, 49, 315–324. [Google Scholar] [CrossRef]
- Gray, W.A. 1-Year Results of a New Mesh-Covered Carotid Stent: SCAFFOLD Trial. 2018. Available online: https://linc2018.cncptdlx.com/media/0944_William_Gray_30_01_2018_Room_3_-_Technical_Forum.pdf (accessed on 1 June 2022).
- Gray, W.A.; Levy, E.; Bacharach, J.M.; Metzger, D.C.; Randall, B.; Siddiqui, A.; Schonholz, C.; Alani, F.; Schneider, P.A. Evaluation of a novel mesh-covered stent for treatment of carotid stenosis in patients at high risk for endarterectomy: 1-year results of the SCAFFOLD trial. Catheter. Cardiovasc. Interv. 2019, 96, 121–127. [Google Scholar] [CrossRef]
- Gray, W.A.; Mehta, M.; Alani, F.; Kasirajan, K.; Begg, R.J.; Bacharach, J.M.; Soukas, P.A.; EMBOLDEN Clinical Study Investigators. Use of a novel embolic filter in carotid artery stenting: 30-Day results from the EMBOLDEN Clinical Study. Catheter. Cardiovasc. Interv. 2018, 92, 1128–1135. [Google Scholar] [CrossRef]
- Gray, W.A.; Rosenfield, K.A.; Jaff, M.R.; Chaturvedi, S.; Peng, L.; Verta, P. Influence of Site and Operator Characteristics on Carotid Artery Stent Outcomes: Analysis of the CAPTURE 2 (Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events) Clinical Study. JACC Cardiovasc. Interv. 2011, 4, 235–246. [Google Scholar] [CrossRef]
- Gray, W.A.; Yadav, J.S.; Verta, P.; Scicli, A.; Fairman, R.; Wholey, M.; Hopkins, L.N.; Atkinson, R.; Raabe, R.; Barnwell, S.; et al. The CAPTURE registry: Predictors of outcomes in carotid artery stenting with embolic protection for high surgical risk patients in the early post-approval setting. Catheter. Cardiovasc. Interv. 2007, 70, 1025–1033. [Google Scholar] [CrossRef]
- Gruberg, L.; Jeremias, A.; Rundback, J.H.; Anderson, H.V.; Spertus, J.A.; Kennedy, K.F.; Rosenfield, K.A. Impact of Glomerular filtration rate on clinical outcomes after carotid artery revascularization in 11,832 patients from the CARE registry®. Catheter. Cardiovasc. Interv. 2014, 84, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Hammer, F.D.; Lacroix, V.; Duprez, T.; Grandin, C.; Verhelst, R.; Peeters, A.; Cosnard, G. Cerebral microembolization after protected carotid artery stenting in surgical high-risk patients: Results of a 2-year prospective study. J. Vasc. Surg. 2005, 42, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Gopalakrishnan, L.; Rajagopal, S.; Rath, P.; Henry, I.; Hugel, M. Bilateral carotid angioplasty and stenting. Catheter. Cardiovasc. Interv. 2005, 64, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Henry, I.; Polydorou, A.; Hugel, M. Carotid angioplasty and stenting in octogenarians: Is it safe? Catheter. Cardiovasc. Interv. 2008, 72, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Polydorou, A.; Henry, I.; Liasis, N.; Polydorou, A.; Polydorou, V.; Demesticha, T.; Skandalakis, P.; Kotsiomitis, E.; Hugel, M.; et al. New distal embolic protection device the FiberNet® 3 dimensional filter: First carotid human study. Catheter. Cardiovasc. Interv. 2007, 69, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, F.; Parrilla, G.; Garcia-Villalba, B.; de Rueda, M.E.; Zamarro, J.; Garrote, M.; Moreno, A. Comparison between proximal versus distal protection devices in 287 cases of carotid revascularization using angioplasty and stenting: Periprocedure complications, morbidity, and mortality. Cardiovasc. Intervent. Radiol. 2014, 37, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, L.N.; White, C.J.; Foster, M.T.; Powell, R.J.; Zemel, G.; Diaz-Cartelle, J. Carotid artery stenting and patient outcomes: The CABANA surveillance study. Catheter. Cardiovasc. Interv. 2014, 84, 997–1004. [Google Scholar] [CrossRef]
- Hussain, M.A.; Mamdani, M.; Tu, J.V.; Saposnik, G.; Aljabri, B.; Bhatt, D.L.; Verma, S.; Al-Omran, M. Long-term Outcomes of Carotid Endarterectomy Versus Stenting in a Multicenter Population-based Canadian Study. Ann. Surg. 2018, 268, 364–373. [Google Scholar] [CrossRef]
- Ielasi, A.; Latib, A.; Godino, C.; Sharp, A.S.P.; Al Lamee, R.; Montorfano, M.; Airoldi, F.; Carlino, M.; Chieffo, A.; Sangiorgi, G.; et al. Clinical Outcomes Following Protected Carotid Artery Stenting in Symptomatic and Asymptomatic Patients. J. Endovasc. Ther. 2010, 17, 298–307. [Google Scholar] [CrossRef]
- Jiang, X.J.; Dong, H.; Peng, M.; Zou, Y.B.; Song, L.; Xu, B.; Zhang, H.-M.; Wu, H.-Y.; Zhou, X.-L.; Yang, Y.-J.; et al. Simultaneous Bilateral vs Unilateral Carotid Artery Stenting: 30-Day and 1-Year Results. J. Endovasc. Ther. 2016, 23, 258–266. [Google Scholar] [CrossRef]
- Jiao, L.-Q.; Song, G.; Li, S.-M.; Miao, Z.-R.; Zhu, F.-S.; Ji, X.-M.; Yin, G.-Y.; Chen, Y.-F.; Wang, Y.-B.; Ma, Y.; et al. Thirty-day outcome of carotid artery stenting in Chinese patients: A single-center experience. Chin. Med. J. 2013, 126, 3915–3920. [Google Scholar] [PubMed]
- Jim, J.; Rubin, B.G.; Landis, G.S.; Kenwood, C.T.; Siami, F.S.; Sicard, G.A. Society for Vascular Surgery Vascular Registry evaluation of stent cell design on carotid artery stenting outcomes. J. Vasc. Surg. 2011, 54, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Katzen, B.T.; Criado, F.J.; Ramee, S.R.; Massop, D.W.; Hopkins, L.N.; Donohoe, D.; Cohen, S.A.; Mauri, L.; on behalf of the CASES-PMS Investigators. Carotid artery stenting with emboli protection surveillance study: Thirty-day results of the CASES-PMS study. Catheter. Cardiovasc. Interv. 2007, 70, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Kimiagar, I.; Gur, A.Y.; Auriel, E.; Peer, A.; Sacagiu, T.; Bass, A. Long-Term Follow-Up of Patients After Carotid Stenting with or Without Distal Protective Device in a Single Tertiary Medical Center. Vasc. Endovasc. Surg. 2012, 46, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Lago, A.; Parkhutik, V.; Tembl, J.I.; Aparici, F.; Mainar, E.; Alcalá, C.; Vázquez-Añón, V. Long-term outcome in patients with carotid artery stenting and contralateral carotid occlusion: A single neurovascular center prospective analysis. Neuroradiology 2011, 54, 965–972. [Google Scholar] [CrossRef]
- Ledwoch, J.; Staubach, S.; Segerer, M.; Strohm, H.; Mudra, H. Carotid artery stenting in clinical practice depending on patient age. Catheter. Cardiovasc. Interv. 2017, 90, 451–460. [Google Scholar] [CrossRef]
- Lian, X.; Lin, M.; Liu, M.; Huang, J.; He, X. Complications and predictors associated with persistent hemodynamic depression after carotid artery stenting. Clin. Neurol. Neurosurg. 2014, 124, 81–84. [Google Scholar] [CrossRef]
- Lindström, D.; Jonsson, M.; Formgren, J.; Delle, M.; Rosfors, S.; Gillgren, P. Outcome After 7 Years of Carotid Artery Stenting and Endarterectomy in Sweden—Single Centre and National Results. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 499–503. [Google Scholar] [CrossRef]
- Liu, H.; Chu, J.; Zhang, L.; Liu, C.; Yan, Z.; Zhou, S. Early Carotid Artery Stenting for Cerebral Watershed Infarction Is Safe and Effective: A Retrospective Study. Eur. Neurol. 2016, 76, 256–260. [Google Scholar] [CrossRef]
- Liu, Y.M.; Qin, H.; Zhang, B.; Wang, Y.J.; Feng, J.; Wu, X. Efficacy of different types of self-expandable stents in carotid artery stenting for carotid bifurcation stenosis. J. Huazhong. Univ. Sci. Technolog. Med. Sci. 2016, 36, 95–98. [Google Scholar] [CrossRef]
- Loghmanpour, N.A.; Siewiorek, G.M.; Wanamaker, K.M.; Muluk, S.C.; Chaer, R.; Wholey, M.H.; Finol, E.A. Assessing the impact of distal protection filter design characteristics on 30-day outcomes of carotid artery stenting procedures. J. Vasc. Surg. 2013, 57, 309–317.e2. [Google Scholar] [CrossRef] [PubMed]
- Macharzina, R.R.; Claus, C.; Messé, S.R.; Boehme, T.; Vach, W.; Winker, T.; Rastan, A.; Beschorner, U.; Noory, E.; Neumann, F.-J.; et al. History of transient ischaemic attack, myocardial infarction and hyperlipidaemia affects outcome following carotid artery stenting. EuroIntervention 2015, 11, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Machnik, R.; Paluszek, P.; Tekieli, Ł.; Dzierwa, K.; Maciejewski, D.; Trystuła, M.; Brzychczy, A.; Banaszkiewicz, K.; Musiał, R.; Pieniążek, P. Mesh-covered (Roadsaver) stent as a new treatment modality for symptomatic or high-risk carotid stenosis. Adv. Interv. Cardiol. 2017, 2, 130–134. [Google Scholar] [CrossRef]
- Mammo, D.F.; Cheng, C.-I.; Ragina, N.P.; Alani, F. Factors affecting cardiovascular and cerebrovascular complications of carotid artery stenting in Northern Michigan: A retrospective study. Cardiovasc. Revascularization Med. 2017, 18, S18–S21. [Google Scholar] [CrossRef] [PubMed]
- Mannheim, D.; Falah, B.; Karmeli, R. Endarterectomy or Stenting in Severe Asymptomatic Carotid Stenosis. Isr. Med Assoc. J. IMAJ 2017, 19, 289–292. [Google Scholar]
- Marine, L.A.; Rubin, B.G.; Reddy, R.; Sanchez, L.A.; Parodi, J.C.; Sicard, G.A. Treatment of asymptomatic carotid artery disease: Similar early outcomes after carotid stenting for high-risk patients and endarterectomy for standard-risk patients. J. Vasc. Surg. 2006, 43, 953–958. [Google Scholar] [CrossRef][Green Version]
- Mas, J.-L.; Arquizan, C.; Calvet, D.; Viguier, A.; Albucher, J.-F.; Piquet, P.; Garnier, P.; Viader, F.; Giroud, M.; Hosseini, H.; et al. Long-Term Follow-Up Study of Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis Trial. Stroke 2014, 45, 2750–2756. [Google Scholar] [CrossRef]
- Massop, D.; Dave, R.; Metzger, C.; Bachinsky, W.; Solis, M.; Shah, R.; Schultz, G.; Schreiber, T.; Ashchi, M.; Hibbard, R.; et al. Stenting and Angioplasty with Protection in Patients at High-Risk for Endarterectomy: SAPPHIRE Worldwide Registry First 2,001 Patients. Catheter. Cardiovasc. Interv. 2009, 73, 129–136. [Google Scholar] [CrossRef]
- Matsumura, J.S.; Gray, W.; Chaturvedi, S.; Yamanouchi, D.; Peng, L.; Verta, P. Results of carotid artery stenting with distal embolic protection with improved systems: Protected Carotid Artery Stenting in Patients at High Risk for Carotid Endarterectomy (PROTECT) trial. J. Vasc. Surg. 2012, 55, 968–976.e5. [Google Scholar] [CrossRef]
- Mayoral Campos, V.; Guirola Órtiz, J.A.; Tejero Juste, C.; Gimeno Peribáñez, M.J.; Serrano, C.; Pérez Lázaro, C.; Giral, I.D.; Ariza, M.Á.d. Carotid artery stenting in a single center, single operator, single type of device and 15 years of follow-up. CVIR Endovasc. 2018, 1, 3. [Google Scholar] [CrossRef]
- Meller, S.M.; Al-Damluji, M.S.; Gutierrez, A.; Stilp, E.; Mena-Hurtado, C. Carotid stenting versus endarterectomy for the treatment of carotid artery stenosis: Contemporary results from a large single center study. Catheter. Cardiovasc. Interv. 2016, 88, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.A.; Gandhi, C.D.; Johnson, D.M.; Winn, H.R.; Patel, A.B. Outcomes of carotid artery stenting in high-risk patients with carotid artery stenosis: A single neurovascular center retrospective review of 101 consecutive patients. Neurosurgery 2010, 66, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, S.; The Japanese CAS Survey Investigators; Taki, W.; Sakai, N.; Nakahara, I. Historical perspective of carotid artery stenting in Japan: Analysis of 8,092 cases in The Japanese CAS survey. Acta Neurochir. 2012, 154, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Montorsi, P.; Galli, S.; Ravagnani, P.; Ruchin, P.; Lualdi, A.; Fabbiocchi, F.; Trabattoni, D.; Veglia, F.; Ali, S.G.; Bartorelli, A.L. Randomized trial of predilation versus direct stenting for treatment of carotid artery stenosis. Int. J. Cardiol. 2010, 138, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Krasniqi, N.; Turgut, M.; Husmann, M.; Roffi, M.; Schwarz, U.; Greutmann, M.; Lüscher, T.F.; Amann-Vest, B.; Corti, R. Carotid artery stenting: A single center “real world” experience. PLoS ONE 2012, 7, 35300. [Google Scholar] [CrossRef]
- Mudra, H.; Staubach, S.; Hein-Rothweiler, R.; Segerer, M.; Strohm, H.; Weber, H.; Ledwoch, J. Long-Term Outcomes of Carotid Artery Stenting in Clinical Practice. Circ. Cardiovasc. Interv. 2016, 9, e003940. [Google Scholar] [CrossRef]
- Mazurek, A.; Borratynska, A.; Tomaszewski, T.; Leśniak-Sobelga, A.; Wilkołek, P.; Gancarczyk, U.; Brózda, M.; Sobieraj, E.; Sikorska, M.; Czyż, L.; et al. Long-Term Outcomes of the MicroNetcovered Stent System Routine Use for Carotid Revascularization in Stroke Prevention: PARADIGM-Extend 5 Year Evidence—ESC Best Poster. Available online: https://esc365.escardio.org/vgn-ext-templating/Congress/216009-long-term-outcomes-of-the-micronet-coveredstent-system-routine-use-for-carotid-revsacularization-in-stroke-prevention-paradigm-extend-5-year-evidence#abstract (accessed on 1 June 2022).
- Myla, S.; Bacharach, J.M.; Ansel, G.M.; Dippel, E.J.; McCormick, D.J.; Popma, J.J. Carotid artery stenting in high surgical risk patients using the FiberNet embolic protection system: The EPIC trial results. Catheter. Cardiovasc. Interv. 2010, 75, 817–822. [Google Scholar] [CrossRef]
- Nerla, R.; Castriota, F.; Micari, A.; Sbarzaglia, P.; Secco, G.G.; Ruffino, M.A.; de Donato, G.; Setacci, C.; Cremonesi, A. Carotid artery stenting with a new-generation double-mesh stent in three high-volume Italian centres: Clinical results of a multidisciplinary approach. EuroIntervention 2016, 12, e677–e683. [Google Scholar] [CrossRef]
- Nikas, D.; Reith, W.; Schmidt, A.; Duda, S.; Mathias, K.; Cremonesi, A.; Dill, H.; Formgren, J.; Pieniążek, P.; Musialek, P.; et al. Prospective, multicenter European study of the GORE flow reversal system for providing neuroprotection during carotid artery stenting. Catheter. Cardiovasc. Interv. 2012, 80, 1060–1068. [Google Scholar] [CrossRef]
- Odrowaz-Pieniazek, P. What We Learned after First 122 CAS Procedures Using Dual-Layer Micromesh Roadsaver Stents. EuroPCR Congress: Paris, France, 2018; Available online: https://abstractbook.pcronline.com/index/slide/abstract/100046/search/pieniazek (accessed on 9 May 2022).
- Parlani, G.; De Rango, P.; Cieri, E.; Verzini, F.; Giordano, G.; Simonte, G.; Isernia, G.; Cao, P. Diabetes is not a predictor of outcome for carotid revascularization with stenting as it may be for carotid endarterectomy. J. Vasc. Surg. 2012, 55, 79–89. [Google Scholar] [CrossRef]
- Pieniążek, P.; Tekieli, L.; Musiałek, P.; Kablak-Ziembicka, A.; Przewłocki, T.; Motyl, R.; Dzierwa, K.; Paluszek, P.; Hlawaty, M.; Żmudka, K.; et al. Carotid artery stenting according to the tailored-CAS algorithm is associated with a low complication rate at 30 days: Data from the TARGET-CAS study. Polish Heart J. 2012, 70, 378–386. [Google Scholar]
- Powell, R.J.; Alessi, C.; Nolan, B.; Rzucidlo, E.; Fillinger, M.; Walsh, D.; Wyers, M.; Zwolak, R.; Cronenwett, J.L. Comparison of embolization protection device-specific technical difficulties during carotid artery stenting. J. Vasc. Surg. 2006, 44, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Protack, C.D.; Bakken, A.M.; Xu, J.; Saad, W.A.; Lumsden, A.B.; Davies, M.G. Metabolic syndrome: A predictor of adverse outcomes after carotid revascularization. J. Vasc. Surg. 2009, 49, 1172–1180.e1. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.; Sugita, J.; Gödel, H.; Sievert, H. Flow-Reversal Device for Cerebral Protection During Carotid Artery Stenting-Acute and Long-Term Results. J. Interv. Cardiol. 2006, 19, 55–62. [Google Scholar] [CrossRef]
- Rhee-Moore, S.J.; Derubertis, B.G.; Lam, R.C.; Hynecek, R.L.; Lee, L.; McKinsey, J.F.; Morrissey, N.J.; Karwowski, J.; Mureebe, L.; Kent, K.C.; et al. Periprocedural Complication Rates Are Equivalent between Symptomatic and Asymptomatic Patients Undergoing Carotid Angioplasty and Stenting. Ann. Vasc. Surg. 2008, 22, 233–237. [Google Scholar] [CrossRef]
- Roffi, M.; Greutmann, M.; Eberli, F.R.; Rainoni, L.; Lüscher, T.F.; Amann-Vesti, B.; Schwarz, U. Starting a carotid artery stenting program is safe. Catheter. Cardiovasc. Interv. 2008, 71, 469–473. [Google Scholar] [CrossRef]
- Safian, R.D.; Bresnahan, J.F.; Jaff, M.R.; Foster, M.; Bacharach, J.M.; Maini, B.; Turco, M.; Myla, S.; Eles, G.; Ansel, G.M. Protected Carotid Stenting in High-Risk Patients with Severe Carotid Artery Stenosis. J. Am. Coll. Cardiol. 2006, 47, 2384–2389. [Google Scholar] [CrossRef]
- Safian, R.D.; Jaff, M.R.; Bresnahan, J.F.; Foster, M.; Bacharach, J.M.; Yadav, J.; Joye, J.; Myla, S.; Kassab, E.; Mann, J.T.; et al. Protected Carotid Stenting in High-Risk Patients: Results of the SpideRX Arm of the Carotid Revascularization with ev3 Arterial Technology Evolution Trial. J. Interv. Cardiol. 2010, 23, 491–498. [Google Scholar] [CrossRef]
- Sahin, M.; Acar, G.; Özkan, B.; AlıcIı, G.; Yazıcıoglu, M.V.; Bulut, M.; Kalkan, M.E.; Demir, S.; Acar, R.D.; Boztosun, B. Comparison of short-term outcomes after carotid artery stenting according to different stent designs. Adv. Interv. Cardiol. 2013, 2, 121–125. [Google Scholar] [CrossRef]
- Şahin, M.; Yazicioglu, M.V.; Acar, G.; Demir, S.; Kalkan, M.E.; Özkan, B.; Alici, G.; Akgun, T.; Akcakoyun, M.; Boztosun, B. Safety of balloon pre-dilatation in the treatment of severe carotid artery stenosis. Eur. Rev. Med Pharmacol. Sci. 2013, 17, 788–793. [Google Scholar]
- Sakamoto, S.; Kiura, Y.; Okazaki, T.; Shinagawa, K.; Ichinose, N.; Shibukawa, M.; Orita, Y.; Shimonaga, K.; Kajihara, Y.; Kurisu, K. Usefulness of dual protection combined with blood aspiration for distal embolic protection during carotid artery stenting. Acta Neurochir. 2014, 157, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Bajzer, C.; Casserly, I.P.; Exaire, E.; Haery, C.; Sachar, R.; Lee, D.; Abou-Chebl, A.; Yadav, J.S. Evaluating the Optimal Activated Clotting Time During Carotid Artery Stenting. Am. J. Cardiol. 2006, 97, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Scheinert, D.; Reimers, B.; Cremonesi, A.; Schmidt, A.; Sievert, H.; Rohde, S.; Schofer, J.; Mudra, H.G.; Bosiers, M.; Zeller, T.; et al. Independent Modular Filter for Embolic Protection in Carotid Stenting. Circ. Cardiovasc. Interv. 2017, 10, 004244. [Google Scholar] [CrossRef] [PubMed]
- Setacci, C.; de Donato, G.; Chisci, E.; Stella, A.; Faggioli, G.; Reimers, B.; Cernetti, C.; Quijada, M.L.; Cappi, B.; Sangiorgi, G. Deferred Urgency Carotid Artery Stenting in Symptomatic Patients: Clinical Lessons and Biomarker Patterns from a Prospective Registry. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 644–651. [Google Scholar] [CrossRef]
- Shen, S.; Jiang, X.; Dong, H.; Peng, M.; Wang, Z.; Che, W.; Zou, Y.; Yang, Y. Effect of aortic arch type on technical indicators in patients undergoing carotid artery stenting. J. Int. Med Res. 2018, 47, 682–688. [Google Scholar] [CrossRef]
- Shin, S.H.; Stout, C.L.; Richardson, A.I.; DeMasi, R.J.; Shah, R.M.; Panneton, J.M. Carotid angioplasty and stenting in anatomically high-risk patients: Safe and durable except for radiation-induced stenosis. J. Vasc. Surg. 2009, 50, 762–767. [Google Scholar] [CrossRef][Green Version]
- Simonetti, G.; Gandini, R.; Versaci, F.; Pampana, E.; Fabiano, S.; Stefanini, M.; Spinelli, A.; Reale, C.A.; Di Primio, M.; Gaspari, E. Carotid artery stenting: A single-centre experience with up to 8 years’ follow-up. Eur. Radiol. 2008, 19, 982–989. [Google Scholar] [CrossRef]
- Sirignano, P.; Stabile, E.; Mansour, W.; Speziale, F. One-month results from a prospective experience on CAS using C-GUARD stent system: The IRONGUARD-2 study. Eur. Heart J. 2020, 41, 2170–2177. [Google Scholar] [CrossRef]
- Spacek, M.; Zimolova, P.; Veselka, J. Carotid Artery Stenting Without Post-Dilation. J. Interv. Cardiol. 2011, 25, 190–196. [Google Scholar] [CrossRef]
- Speziale, F.; Capoccia, L.; Sirignano, P.; Mansour, W.; Pranteda, C.; Casana, R.; Setacci, C.; Accrocca, F.; Alberti, D.; de Donato, G.; et al. Thirty-day results from prospective multi-specialty evaluation of carotid artery stenting using the CGuard MicroNet-covered Embolic Prevention System in real-world multicentre clinical practice: The IRON-Guard study. EuroIntervention 2018, 13, 1714–1720. [Google Scholar] [CrossRef]
- Stabile, E.; Salemme, L.; Sorropago, G.; Tesorio, T.; Nammas, W.; Miranda, M.; Popusoi, G.; Cioppa, A.; Ambrosini, V.; Cota, L.; et al. Proximal Endovascular Occlusion for Carotid Artery Stenting: Results from a Prospective Registry of 1,300 Patients. J. Am. Coll. Cardiol. 2010, 55, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Stanziale, S.F.; Marone, L.K.; Boules, T.N.; Brimmeier, J.A.; Hill, K.; Makaroun, M.S.; Wholey, M.H. Carotid artery stenting in octogenarians is associated with increased adverse outcomes. J. Vasc. Surg. 2006, 43, 297–304. [Google Scholar] [CrossRef]
- Tadros, R.O.; Spyris, C.T.; Vouyouka, A.G.; Chung, C.; Krishnan, P.; Arnold, M.W.; Marin, M.L.; Faries, P.L. Comparing the embolic potential of open and closed cell stents during carotid angioplasty and stenting. J. Vasc. Surg. 2012, 56, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, T.; Matsumaru, Y.; Hayakawa, M.; Nemoto, S.; Matsumura, A. Cilostazol reduces restenosis after carotid artery stenting. J. Vasc. Surg. 2010, 51, 51–56. [Google Scholar] [CrossRef]
- Tang, G.L.; Matsumura, J.S.; Morasch, M.D.; Pearce, W.H.; Nguyen, A.; Amaranto, D.; Eskandari, M.K. Carotid angioplasty and stenting vs carotid endarterectomy for treatment of asymptomatic disease: Single-center experience. Arch. Surg. 2008, 143, 653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tas, M.H.; Simsek, Z.; Colak, A.; Koza, Y.; Demir, P.; Demir, R.; Kaya, U.; Tanboga, I.H.; Gundogdu, F.; Sevimli, S. Comparison of Carotid Artery Stenting and Carotid Endarterectomy in Patients with Symptomatic Carotid Artery Stenosis: A Single Center Study. Adv. Ther. 2013, 30, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Tatli, E.; Tokatli, A.; Vatan, M.B.; Agac, M.T.; Gunduz, H.; Akdemir, R.; Kilic, H. Comparison of closed-cell and hybrid-cell stent designs in carotid artery stenting: Clinical and procedural outcomes. Adv. Interv. Cardiol. 2017, 2, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ullery, B.W.; Orlova, K.; Shang, E.K.; Jackson, B.M.; Wang, G.J.; Fairman, R.M.; Woo, E.Y. Results of carotid angioplasty and stenting are equivalent for critical versus high-grade lesions in patients deemed high risk for carotid endarterectomy. J. Surg. Res. 2013, 185, 21–26. [Google Scholar] [CrossRef]
- Van der Heyden, J.; Wolters, F.J.; Garin, N.; Blant, S.A.; Inglin, M.; Bal, E.T.; Suttorp, J.M. The role of embolic protection devices during carotid stenting prior to cardiac surgery in asymptomatic patients: Empty filters? Catheter. Cardiovasc. Interv. 2012, 80, 112–119. [Google Scholar] [CrossRef]
- Veselka, J.; Zimolová, P.; Martinkovičová, L.; Tomašov, P.; Hájek, P.; Malý, M.; Zemanek, D.; Tesar, D.; Tomasov, P. Comparison of mid-term outcomes of carotid artery stenting for moderate versus critical stenosis. Arch. Med. Sci. 2012, 8, 75–80. [Google Scholar] [CrossRef]
- Veselka, J.; Zimolová, P.; Špaček, M.; Hájek, P.; Malý, M.; Tomašov, P.; Martinkovičová, L.; Zemánek, D. Comparison of carotid artery stenting in patients with single versus bilateral carotid artery disease and factors affecting midterm outcome. Ann. Vasc. Surg. 2011, 25, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Scheinert, D.; Borghesi, R.; Cremonesi, A.; Rosenschein, U.; Scheinert, S.; Bräunlich, S.; Bausback, Y.; Ülrich, M.; Schmidt, A. First clinical experience with the GARDEX EPD: A novel embolic protection device for carotid artery stenting. EuroIntervention 2013, 8, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Werner, N.; Zeymer, U.; Mark, B.; Hochadel, M.; Hauptmann, K.E.; Jung, J.; Hoffmann, E.; Elsässer, A.; Fürste, T.; Leschke, M.; et al. Carotid Artery Stenting in Clinical Practice: Does Sex Matter? Results From the Carotid Artery Stenting Registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (ALKK). Clin. Cardiol. 2012, 35, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wieker, C.M.; Demirel, S.; Attigah, N.; Hakimi, M.; Hinz, U.; Böckler, D. Outcome of carotid artery stenting in the hands of vascular surgeons. Langenbecks Arch. Surg. 2017, 402, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Wissgott, C.; Brandt-Wunderlich, C.; Kopetsch, C.; Schmidt, W.; Andresen, R. Initial Clinical Results and In Vitro Testing of the New CGuard MicroNet-Covered “One-Size-Fits-All” Carotid Stent. J. Endovasc. Ther. 2019, 26, 578–582. [Google Scholar] [CrossRef]
- Yan, D.; Tang, X.; Shi, Z.; Wang, L.; Lin, C.; Guo, D.; Fu, W. Perioperative and Follow-up Results of Carotid Artery Stenting and Carotid Endarterectomy in Patients with Carotid Near-Occlusion. Ann. Vasc. Surg. 2019, 59, 21–27. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Qi, G.; Li, Y.; Liu, Y. The middle-term outcome of carotid endarterectomy and stenting for treatment of ischemic stroke in Chinese patients. Sci. Rep. 2018, 8, 4697. [Google Scholar] [CrossRef]
- Yen, M.H.; Lee, D.S.; Kapadia, S.; Sachar, R.; Bhatt, D.L.; Bajzer, C.T.; Yadav, J.S. Symptomatic patients have similar outcomes compared with asymptomatic patients after carotid artery stenting with emboli protection. Am. J. Cardiol. 2005, 95, 297–300. [Google Scholar] [CrossRef]
- Yoon, W.; Kim, S.K.; Park, M.; Chae, H.; Kang, H. Safety of Protected Carotid Artery Stenting in Patients with Severe Carotid Artery Stenosis and Carotid Intraplaque Hemorrhage. Am. J. Neuroradiol. 2012, 33, 1027–1031. [Google Scholar] [CrossRef]
- Yoshida, S.; Bensley, R.P.; Glaser, J.D.; Nabzdyk, C.S.; Hamdan, A.D.; Wyers, M.C.; Chaikof, E.L.; Schermerhorn, M.L. The current national criteria for carotid artery stenting overestimate its efficacy in patients who are symptomatic and at high risk. J. Vasc. Surg. 2013, 58, 120–127. [Google Scholar] [CrossRef][Green Version]
- Yoshimura, S.; Yamada, K.; Kawasaki, M.; Asano, T.; Kanematsu, M.; Miyai, M.; Enomoto, Y.; Egashira, Y.; Iwama, T. Selection of Carotid Artery Stenting or Endarterectomy Based on Magnetic Resonance Plaque Imaging Reduced Periprocedural Adverse Events. J. Stroke Cerebrovasc. Dis. 2012, 22, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.P.; Bosiers, M.; Deloose, K.; Uflacker, R.; Schönholz, C.J. Impact of stent design on the outcome of intervention for carotid bifurcation stenosis. J. Cardiovasc. Surg. 2010, 51, 799–806. [Google Scholar]
- Halliday, A.; Bulbulia, R.; Bonati, L.H.; Chester, J.; Cradduck-Bamford, A.; Peto, R.; Pan, H. ACST-2 Collaborative Group. Second asymptomatic carotid surgery trial (ACST-2): A randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet 2021, 398, 1065–1073. [Google Scholar] [CrossRef]
- Machnik, R.A.; Pieniążek, P.; Misztal, M.; Plens, K.; Kazibudzki, M.; Tomaszewski, T.; Brzychczy, A.; Musiał, R.; Trystuła, M.; Tekieli, Ł.M. Carotid artery stenting with Roadsaver stent. Early and four-year results from a single-center registry. Adv. Interv. Cardiol. 2020, 16, 444–451. [Google Scholar] [CrossRef]
- Imamura, H.; Sakai, N.; Matsumoto, Y.; Yamagami, H.; Terada, T.; Fujinaka, T.; Yoshimura, S.; Sugiu, K.; Ishii, A.; Matsumaru, Y.; et al. Clinical trial of carotid artery stenting using dual-layer CASPER stent for carotid endarterectomy in patients at high and normal risk in the Japanese population. J. Neurointerv. Surg. 2021, 13, 524–529. [Google Scholar] [CrossRef]
- Sýkora, J.; Zeleňák, K.; Vorčák, M.; Števík, M.; Sýkorová, M.; Sivák, J.; Rovňák, M.; Zapletalová, J.; Mužík, J.; Šinák, I.; et al. Comparison of Restenosis Risk in Single-Layer versus Dual-Layer Carotid Stents: A Duplex Ultrasound Evaluation. Cardiovasc. Intervent. Radiol. 2022. ahead of print. [Google Scholar] [CrossRef]
- Tigkiropoulos, K.; Papoutsis, I.; Abatzis-Papadopoulos, M.; Kousidis, P.; Mpismpos, D.; Melas, N.; Stavridis, K.; Karamanos, D.; Lazaridis, I.; Saratzis, N. Thirty-Day Results of the Novel CGuard-Covered Stent in Patients Undergoing Carotid Artery Stenting. J. Endovasc. Ther. 2021, 28, 542–548. [Google Scholar] [CrossRef]
- Sirignano, P.; Stabile, E.; Mansour, W.; Speziale, F. 1-Month Results from a Prospective Experience on CAS Using CGuard Stent System: The IRONGUARD 2 Study. JACC Cardiovasc. Interv. 2020, 13, 2170–2177. [Google Scholar] [CrossRef]
- Sirignano, P.; Stabile, E.; Mansour, W.; Capoccia, L.; Faccenna, F.; Intrieri, F.; Ferri, M.; Saccà, S.; Sponza, M.; Mortola, P.; et al. 1-Year Results from a Prospective Experience on CAS Using the CGuard Stent System: The IRONGUARD 2 Study. JACC Cardiovasc. Interv. 2021, 14, 1917–1923. [Google Scholar] [CrossRef]
- Dakour-Aridi, H.; Mathlouthi, A.; Locham, S.; Goodney, P.; Schermerhorn, M.L.; Malas, M.B. Predictors of midterm high-grade restenosis after carotid revascularization in a multicenter national database. J. Vasc. Surg. 2020, 71, 1972–1981. [Google Scholar] [CrossRef]
- Galyfos, G.C.; Tsoutsas, I.; Konstantopoulos, T.; Galanopoulos, G.; Sigala, F.; Filis, K.; Papavassiliou, V. Early and Late Outcomes after Transcarotid Revascularisation for Internal Carotid Artery Stenosis: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 725–738. [Google Scholar] [CrossRef]
- Trystula, M.; Musialek, P. Transient flow reversal combined with sustained embolic prevention in transcervical revascularization of symptomatic and highly-emboligenic carotid stenoses for optimized endovascular lumen reconstruction and improved peri- and post-procedural outcomes. Adv. Interv. Cardiol. 2020, 16, 495–506. [Google Scholar]
- Musialek, P.; Mazurek, A.; Kolvenbach, R.; Malinowski, K.; Brinkmann, C.; Sievert, H.; Schofer, J. 5-year Clinical and Ultrasound Outcomes in CARENET Prospective Multicenter Trial of CGuard MicroNET-Covered Carotid Stent (CARotid Embolic Prevention using MicroNET-Covered Stent System in Patients with Symptomatic and Asymptomatic Carotid Artery Stenosis). JACC Cardiovasc. Interv. 2022, in press. [Google Scholar]
- Texakalidis, P.; Giannopoulos, S.; Kokkinidis, D.G.; Lanzino, G. Effect of Open- vs Closed-Cell Stent Design on Periprocedural Outcomes and Restenosis After Carotid Artery Stenting: A Systematic Review and Comprehensive Meta-analysis. J. Endovasc. Ther. 2018, 25, 523–533. [Google Scholar] [CrossRef] [PubMed]
- White, C.J.; Brott, T.G.; Gray, W.A.; Heck, D.; Jovin, T.; Lyden, S.P.; Metzger, D.C.; Rosenfield, K.; Roubin, G.; Sachar, R.; et al. Carotid Artery Stenting: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 155–170. [Google Scholar] [CrossRef] [PubMed]

| FGS | SGS | p FGS vs. SGS | Open-Cell FGS | Closed-Cell FGS | p Open-Cell vs. Closed-Cell FGS | p Open-Cell FGS vs. SGS | p Closed-Cell FGS vs. SGS | |
|---|---|---|---|---|---|---|---|---|
| No. of studies | 98 | 14 | - | 29 | 12 | - | - | - |
| No. of patients | 65,891 | 2531 | - | 21,351 | 7598 | - | - | - |
| Age (SD) | 70.1 (2.8) | 71.9 (2.5) | 0.02 | 70.4 (3.2) | 69.3 (3.4) | 0.60 | 0.32 | 0.13 |
| Male | 68% | 73% | 0.046 | 68% | 66% | 0.92 | 0.12 | 0.15 |
| Symptomatic | 45% | 41% | 0.40 | 43% | 50% | 0.61 | 0.94 | 0.45 |
| Diabetic | 34% | 32% | 0.43 | 35% | 36% | 0.71 | 0.88 | 0.61 |
| CAD | 51% | 47% | 0.55 | 48% | 55% | 0.59 | 0.98 | 0.98 |
| AF | 6% | 3% | 0.37 | 3% | ND | - | 0.99 | - |
| Contralateral occlusion | 10% | 16% | 0.22 | 10% | 12% | 0.87 | 0.63 | 0.99 |
| Embolic protection in CAS | 95.8% | 97.1% | 0.656 | 97.3% | 99.4% | 0.09 | 0.85 | 0.2 |
| FGS | SGS | Casper/ Roadsaver | Gore | CGuard | |
|---|---|---|---|---|---|
| 30-day Stroke (%) (95% CI) | 3.01 (2.63–3.38) | 0.60 (0.28–0.92) | 0.50 (0–1.15) | 2.89 (1.03–4.76) | 0.54 (0.17–0.92) |
| 30-day Death/Stroke/MI (%) (95% CI) | 4.11 (3.65–4.56) | 1.30 (0.64–1.96) | 1.33 (0–2.66) | 4.82 (2.44–7.2) | 1.08 (0.55–1.60) |
| 12-mo Ipsilateral Stroke (%) (95% CI) | 3.51 (2.52–4.50) | 0.7 (0–1.47) | 0.26 (0–1.27) | 3.1 (1.11–5.1) | 0.38 (0–0.9) |
| 12-mo Restenosis (%) (95% CI) | 3.97 (0.28–5.14) | 3.38 (1.39–5.37) | 7.16 (5.45–9.86) | 4.83 (2.36–7.29) | 0.34 (0–0.82) |
| 12-mo Ipsilateral Stroke/Restenosis (%) (95% CI) | 8.15 (6.63–9.96) | 5.12 (3.14–6.10) | 7.86 (5.04–10.68) | 7.93 (4.82–11.04) | 0.73 (0–1.44) |
| p FGS vs. SGS | p FGS vs. Roadsaver | p FGS vs. Gore | p FGS vs. CGuard | |
|---|---|---|---|---|
| 30-day Stroke | <0.001 | 0.011 | 0.954 | 0.002 |
| 30-day Death/Stroke/MI | <0.001 | 0.022 | 0.750 | <0.001 |
| 12-mo Ipsilateral Stroke | 0.001 | 0.007 | 0.846 | 0.013 |
| 12-mo Restenosis | 0.569 | 0.041 | 0.658 | 0.009 |
| 12-mo Ipsilateral Stroke/Restenosis | 0.027 | 0.998 | 0.961 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, A.; Malinowski, K.; Rosenfield, K.; Capoccia, L.; Speziale, F.; de Donato, G.; Setacci, C.; Wissgott, C.; Sirignano, P.; Tekieli, L.; et al. Clinical Outcomes of Second- versus First-Generation Carotid Stents: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 4819. https://doi.org/10.3390/jcm11164819
Mazurek A, Malinowski K, Rosenfield K, Capoccia L, Speziale F, de Donato G, Setacci C, Wissgott C, Sirignano P, Tekieli L, et al. Clinical Outcomes of Second- versus First-Generation Carotid Stents: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(16):4819. https://doi.org/10.3390/jcm11164819
Chicago/Turabian StyleMazurek, Adam, Krzysztof Malinowski, Kenneth Rosenfield, Laura Capoccia, Francesco Speziale, Gianmarco de Donato, Carlo Setacci, Christian Wissgott, Pasqualino Sirignano, Lukasz Tekieli, and et al. 2022. "Clinical Outcomes of Second- versus First-Generation Carotid Stents: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 16: 4819. https://doi.org/10.3390/jcm11164819
APA StyleMazurek, A., Malinowski, K., Rosenfield, K., Capoccia, L., Speziale, F., de Donato, G., Setacci, C., Wissgott, C., Sirignano, P., Tekieli, L., Karpenko, A., Kuczmik, W., Stabile, E., Metzger, D. C., Amor, M., Siddiqui, A. H., Micari, A., Pieniążek, P., Cremonesi, A., ... Musialek, P., on behalf of CARMEN (CArotid Revascularization Systematic Reviews and MEta-aNalyses) Investigators. (2022). Clinical Outcomes of Second- versus First-Generation Carotid Stents: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(16), 4819. https://doi.org/10.3390/jcm11164819








