Morphological Changes in Blood Cells in a Rat Model of Heatstroke: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Heatstroke Model
2.2. Blood Cell Count, Peripheral Blood Smear Stain, and Bone Marrow Smear Stain
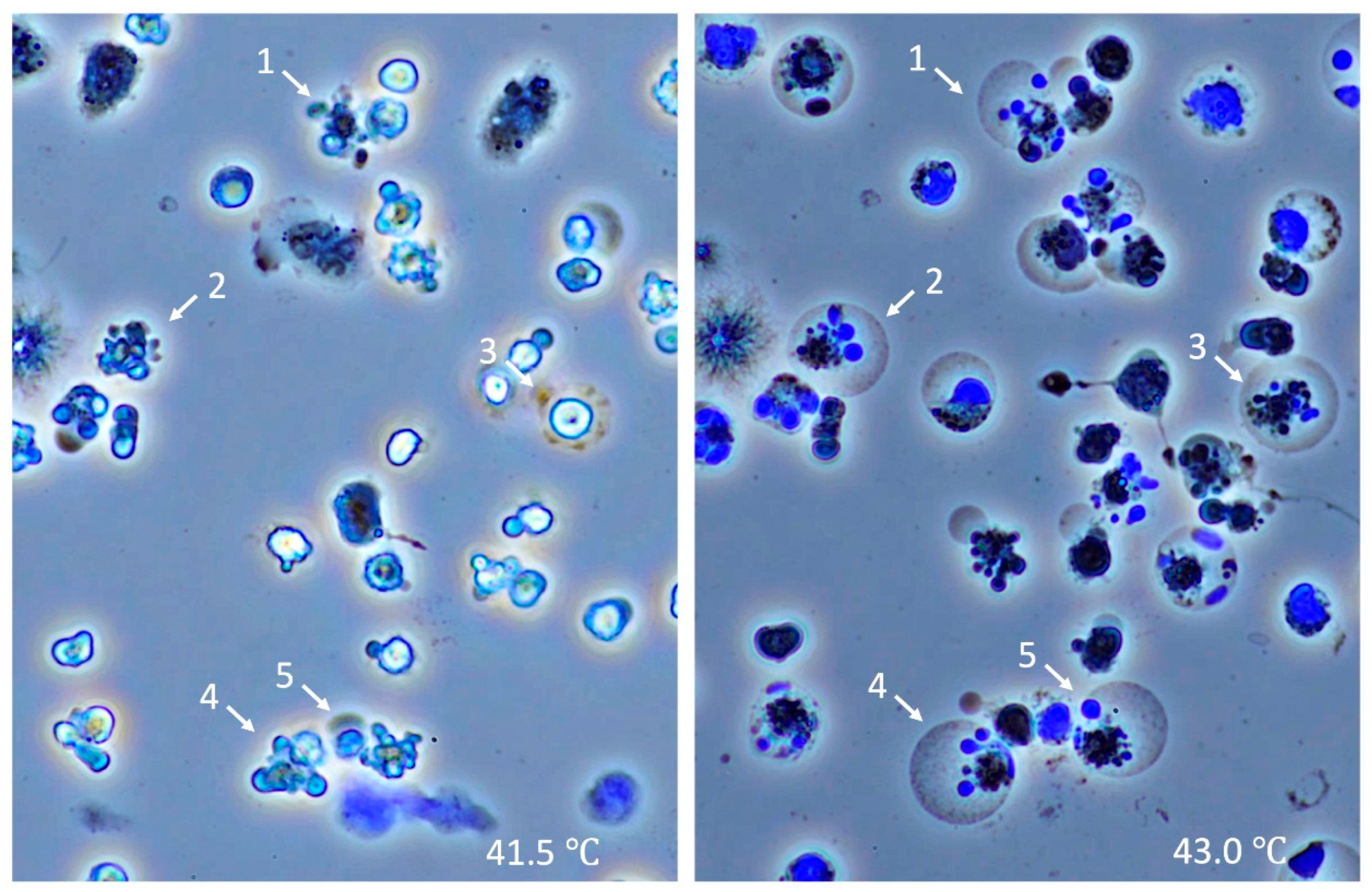
2.3. Time-Lapse Observation of Leukocytes Subjected to Heat In Vitro
2.4. Statistical Analysis
3. Results
3.1. Physiological Responses
3.2. Blood Cell Counts
3.3. Morphological Changes in the Peripheral Blood Cells
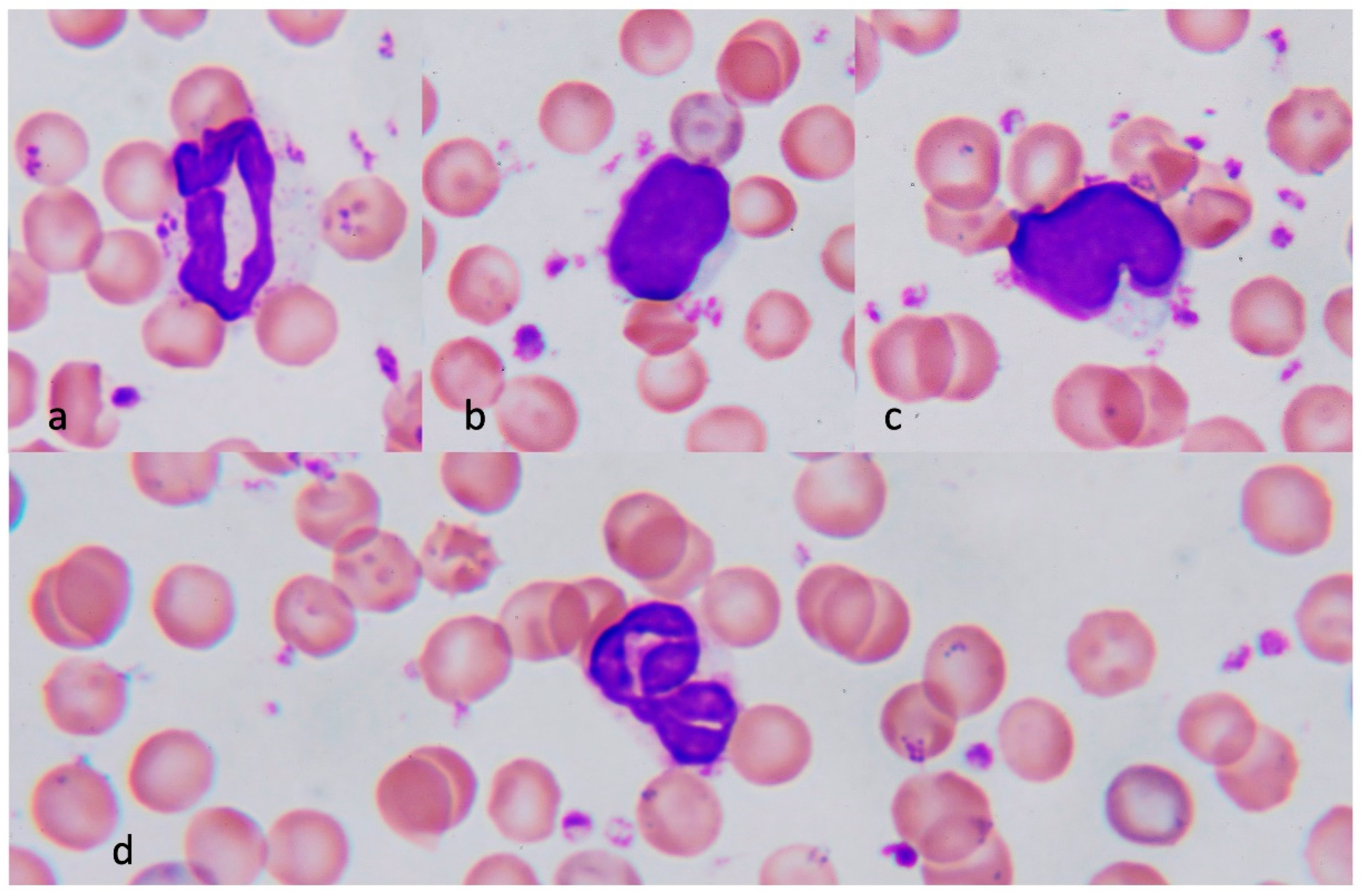
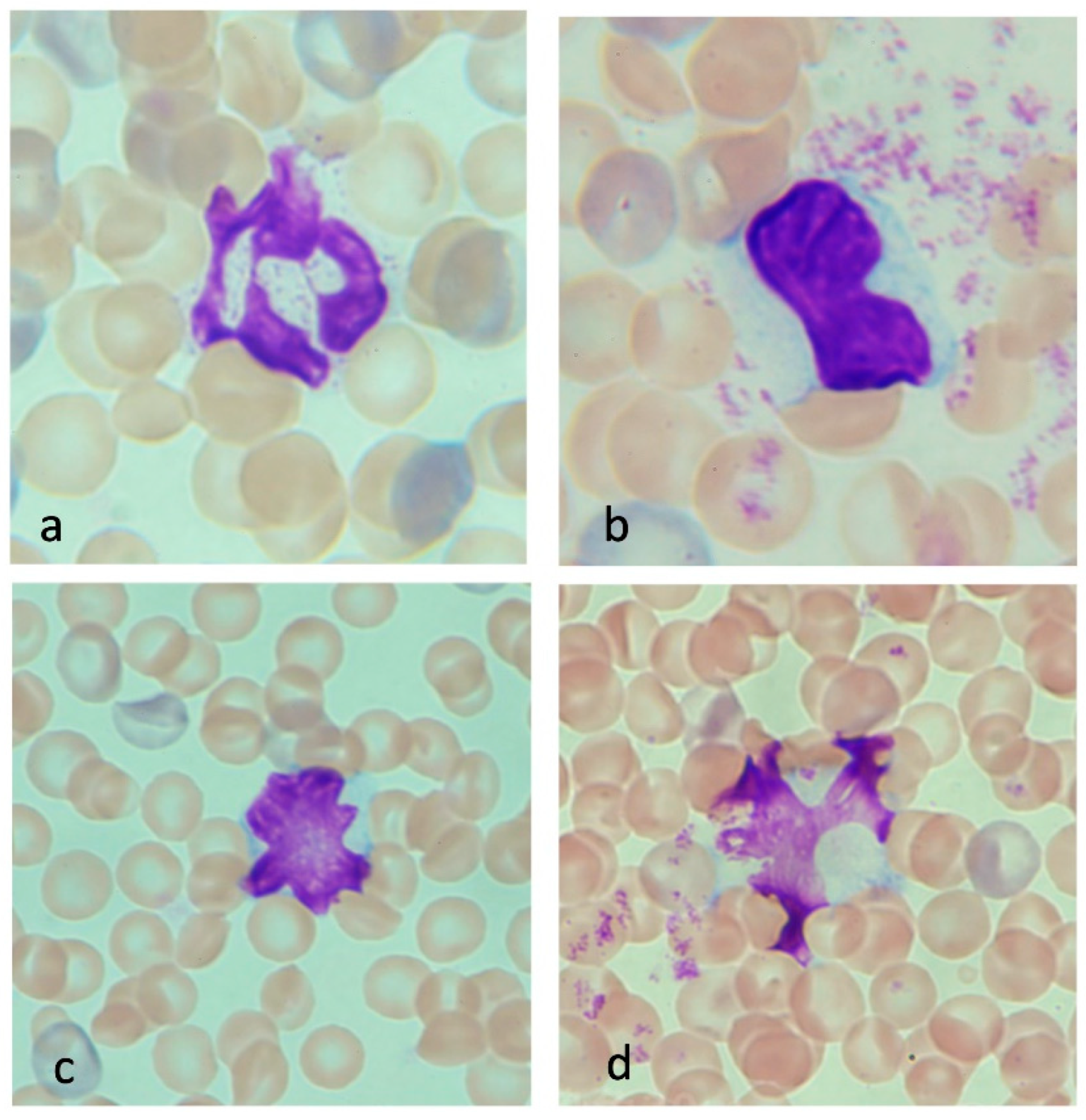
3.3.1. Neutrophils in Heatstroke
3.3.2. Monocytes in Heatstroke
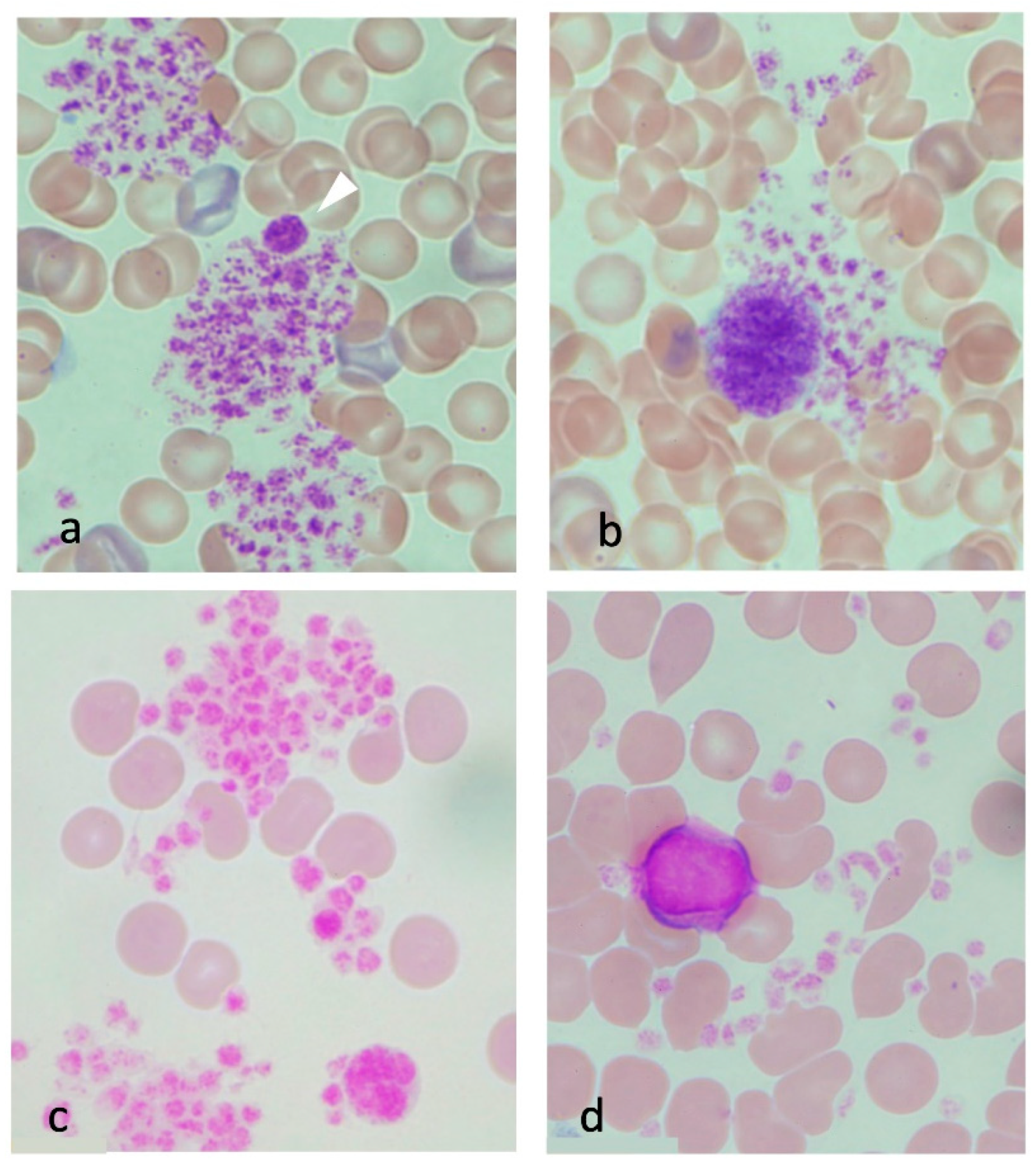
3.3.3. Erythrocytes in Heatstroke
3.3.4. Lymphocytes in Heatstroke
3.3.5. Platelets in Heatstroke
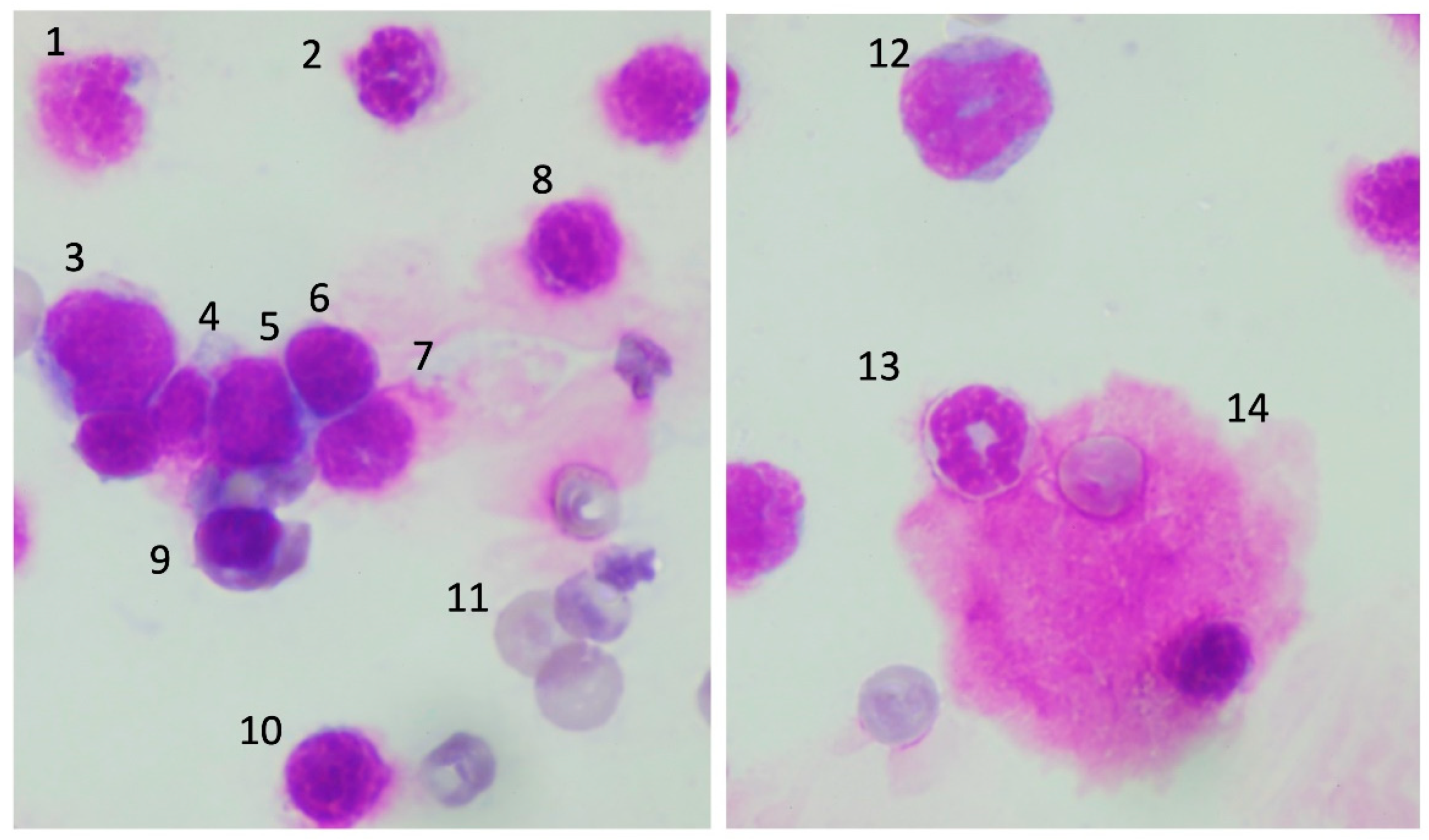
3.4. Morphological Changes of the Precursor Cells in Bone Marrow
3.5. Time-Lapse Observation of Leukocytes
4. Discussion
5. Strength and Limitations
6. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burkart, K.G.; Brauer, M.; Aravkin, A.Y.; Godwin, W.W.; Hay, S.I.; He, J.; Iannucci, V.C.; Larson, S.L.; Lim, S.S.; Liu, J.; et al. Health in a world of extreme heat. Lancet 2021, 398, 641. [Google Scholar]
- Lewandowski, S.A.; Shaman, J.L. Heat stress morbidity among US military personnel: Daily exposure and lagged response (1998–2019). Int. J. Biometeorol. 2022, 66, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Bouchama, A.; Abuyassin, B.; Lehe, C.; Laitano, O.; Jay, O.; O’Connor, F.G.; Leon, L.R. Classic and exertional heatstroke. Nat. Rev. Dis. Primer. 2022, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.E.; Zhao, Y.Q.; Wang, H.; Fan, M. Pathophysiological factors underlying heatstroke. Med. Hypotheses 2006, 67, 609–617. [Google Scholar] [CrossRef]
- Epstein, Y.; Yanovich, R. Heatstroke. Reply. N. Engl. J. Med. 2019, 381, 1187. [Google Scholar]
- Iba, T.; Connors, J.M.; Levi, M.; Levy, J.H. Heatstroke-induced coagulopathy: Biomarkers, mechanistic insights and patient management. EClinicalMedicine 2022, 44, 101276. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Gu, X.L.; Zhao, X.; He, X.; Shi, H.W.; Zhang, K.; Zhang, Y.M.; Su, Y.N.; Zhu, J.B.; Li, Z.W.; et al. NLRP3 ablation enhances tolerance in heat stroke pathology by inhibiting IL-1β-mediated neuroinflammation. J. Neuroinflamm. 2021, 18, 128. [Google Scholar] [CrossRef]
- Bouchama, A.; Roberts, G.; Al Mohanna, F.; El-Sayed, R.; Lach, B.; Chollet-Martin, S.; Ollivier, V.; Al Baradei, R.; Loualich, A.; Nakeeb, S.; et al. Inflammatory, hemostatic, and clinical changes in a baboon experimental model for heatstroke. J. Appl. Physiol. 2005, 98, 697–705. [Google Scholar] [CrossRef]
- Huisse, M.G.; Pease, S.; Hurtado-Nedelec, M.; Arnaud, B.; Malaquin, C.; Wolff, M.; Gougerot-Pocidalo, M.A.; Kermarrec, N.; Bezeaud, A.; Guillin, M.C.; et al. Leukocyte activation: The link between inflammation and coagulation during heatstroke. A study of patients during the 2003 heat wave in Paris. Crit. Care Med. 2008, 36, 2288–2295. [Google Scholar] [CrossRef]
- Riond, B.; Weissenbacher, S.; Hofmann-Lehmann, R.; Lutz, H. Performance evaluation of the Sysmex pocH-100iV Diff hematology analyzer for analysis of canine, feline, equine, and bovine blood. Vet. Clin. Pathol. 2011, 40, 484–495. [Google Scholar] [CrossRef]
- Shimazaki, J.; Hifumi, T.; Shimizu, K.; Oda, Y.; Kanda, J.; Kondo, Y.; Shiraishi, S.; Takauji, S.; Hayashida, K.; Moriya, T.; et al. Clinical characteristics, prognostic factors, and outcomes of heat-related illness (Heatstroke Study 2017–2018). Acute Med. Surg. 2020, 7, e516. [Google Scholar] [CrossRef]
- Bruchim, Y.; Klement, E.; Saragusty, J.; Finkeilstein, E.; Kass, P.; Aroch, I. Heat stroke in dogs: A retrospective study of 54 cases (1999–2004) and analysis of risk factors for death. J. Vet. Intern. Med. 2006, 20, 38–46. [Google Scholar]
- Bouchama, A.; al Hussein, K.; Adra, C.; Rezeig, M.; Al Shail, E.; Al Sedairy, S. Distribution of peripheral blood leukocytes in acute heatstroke. J. Appl. Physiol. 1992, 73, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.T.; Ghebeh, H.; Chishti, M.A.; Al-Mohanna, F.; El-Sayed, R.; Al-Mohanna, F.; Bouchama, A. Microvascular injury, thrombosis, inflammation and apoptosis in the pathogenesis of heatstroke: A study in baboon model. Arter. Thromb. Vasc. Biol. 2008, 28, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Ranheim, E.A. Leukocyte abnormalities associated with hyperthermia. Int. J. Hematol. 2013, 97, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Im, D.D.; Ho, C.H.; Chan, R.Y. Hypersegmented Neutrophils in an Adolescent Male with Heatstroke. J. Pediatr. Hematol. Oncol. 2015, 37, 488. [Google Scholar] [CrossRef]
- Ward, P.C.; McKenna, R.W.; Kroft, S.H. White blood cell changes in hyperthermia. Br. J. Haematol. 2007, 138, 130. [Google Scholar] [CrossRef]
- Li, T.H.S.; Wong, M.S.; Wong, H.F.; Wong, W.S.; Chan, C.B. Hyperthermia associated morphological changes in white blood cells. Int. J. Lab. Hematol. 2021, 43, 888–889. [Google Scholar] [CrossRef]
- Mastrorilli, C.; Welles, E.G.; Hux, B.; Christopherson, P.W. Botryoid nuclei in the peripheral blood of a dog with heatstroke. Vet. Clin. Pathol. 2013, 42, 145–149. [Google Scholar] [CrossRef]
- Hu, J.; Kang, H.J.; Liu, C.; Hu, P.; Yang, M.M.; Zhou, F.H. Response of regulatory T cells to classic heat stroke in mice. Exp. Ther. Med. 2018, 16, 4609–4615. [Google Scholar] [CrossRef]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar] [PubMed]
- Aroch, I.; Segev, G.; Loeb, E.; Bruchim, Y. Peripheral nucleated red blood cells as a prognostic indicator in heatstroke in dogs. J. Vet. Intern. Med. 2009, 23, 544–551. [Google Scholar] [CrossRef]
- Hemmelgarn, C.; Gannon, K. Heatstroke: Clinical signs, diagnosis, treatment and prognosis. Compend. Contin. Educ. Vet. 2013, 35, E3. [Google Scholar] [PubMed]
- Iba, T.; Helms, J.; Levi, M.; Levy, J.H. The role of platelets in heat-related illness and heat-induced coagulopathy. Thromb. Res. 2022; in press. [Google Scholar]
- Martinod, K.; Deppermann, C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets 2021, 32, 314–324. [Google Scholar] [CrossRef]
- Umemura, Y.; Ogura, H.; Matsuura, H.; Ebihara, T.; Shimizu, K.; Shimazu, T. Bone marrow-derived mononuclear cell therapy can attenuate systemic inflammation in rat heatstroke. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 97. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Huang, K.F.; Lin, M.T.; Chang, F.M. Human umbilical cord blood cells or estrogen may be beneficial in treating heatstroke. Taiwan J. Obstet. Gynecol. 2007, 46, 15–25. [Google Scholar] [CrossRef]
- Liu, W.S.; Chen, C.T.; Foo, N.H.; Huang, H.R.; Wang, J.J.; Chen, S.H.; Chen, T.J. Human umbilical cord blood cells protect against hypothalamic apoptosis and systemic inflammation response during heatstroke in rats. Pediatr. Neonatol. 2009, 50, 208–216. [Google Scholar] [CrossRef]
- Drobatz, K.J.; Macintire, D.K. Heat-induced illness in dogs: 42 cases (1976–1993). J. Am. Vet. Med. Assoc. 1996, 209, 1894–1899. [Google Scholar]
- Bruchim, Y.; Loeb, E.; Saragusty, J.; Aroch, I. Pathological findings in dogs with fatal heatstroke. J. Comp. Pathol. 2009, 140, 97–104. [Google Scholar] [CrossRef]


| Control Group (n = 6) | Moderate Heatstroke Group, 90 min (n = 6) | Moderate Heatstroke Group, 180 min (n = 6) | Severe Heatstroke Group (n = 9) | |
|---|---|---|---|---|
| WBC count (×102/μL) | 68 ± 2 | 66 ± 1 | 61 ± 4 | 58 ± 6 ** |
| Lymphocyte (×102/μL) | 53 ± 5 | 52 ± 5 | 50 ± 4 | 42 ± 5 ** |
| Granulocyte (×102/μL) | 15 ± 3 | 14 ± 3 | 11 ± 3 | 16 ± 1 |
| RBC count (×104/μL) | 714 ± 16 | 723 ± 18 | 744 ± 31 | 785 ± 14 ** |
| Hemoglobin (g/dL) | 14.1 ± 2.0 | 13.5 ± 3.0 | 14.4 ± 2.9 | 15.1 ± 2.8 |
| Hematocrit (%) | 41.4 ± 6.6 | 44.2 ± 4.6 | 45.0 ± 5.1 | 45.5 ± 7.1 |
| MCV (fL) | 62.4 ± 2.4 | 64.2 ± 2.6 | 64.2 ± 2.5 | 62.5 ± 2.3 |
| MCH (pg) | 21.7 ± 0.7 | 21.7 ± 0.6 | 21.3 ± 0.5 | 21.1 ± 0.6 |
| MCHC (g/dL) | 37.4 ± 1.4 | 37.2 ± 1.6 | 37.2 ± 1.0 | 37.2 ± 1.3 |
| Platelet count (×104/μL) | 127.9 ± 18.1 | 107.5 ± 14.9 | 116.5 ± 12.1 | 82.9 ± 21.2 ** |
| PDW (fL) | 6.6 ± 0.9 | 8.4 ± 1.2 * | 8.3 ± 0.9 * | 8.7± 1.1 ** |
| MPV (%) | 6.5 ± 0.8 | 7.4 ± 1.1 | 7.4 ± 1.1 | 7.5 ± 2.1 * |
| P-LCR (%) | 5.5 ± 0.8 | 8.6 ± 0.9 ** | 8.3 ± 0.7 ** | 9.3 ± 1.2 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iba, T.; Sawada, T.; Kondo, Y.; Kondo, K.; Levy, J.H. Morphological Changes in Blood Cells in a Rat Model of Heatstroke: A Pilot Study. J. Clin. Med. 2022, 11, 4821. https://doi.org/10.3390/jcm11164821
Iba T, Sawada T, Kondo Y, Kondo K, Levy JH. Morphological Changes in Blood Cells in a Rat Model of Heatstroke: A Pilot Study. Journal of Clinical Medicine. 2022; 11(16):4821. https://doi.org/10.3390/jcm11164821
Chicago/Turabian StyleIba, Toshiaki, Tomohiro Sawada, Yutaka Kondo, Kenta Kondo, and Jerrold H. Levy. 2022. "Morphological Changes in Blood Cells in a Rat Model of Heatstroke: A Pilot Study" Journal of Clinical Medicine 11, no. 16: 4821. https://doi.org/10.3390/jcm11164821







