State-of-the-Art Review: Technical and Imaging Considerations in Hybrid Transcatheter and Minimally Invasive Left Ventricular Reconstruction for Ischemic Heart Failure
Abstract
:1. Introduction
2. The History of LV Reconstruction Surgery
3. Transition from Conventional Surgical LV Reconstruction to an Alternative Hybrid Approach
4. LIVE Therapy Stitching Technique: What to Stitch to Achieve Optimal LV Reconstruction?
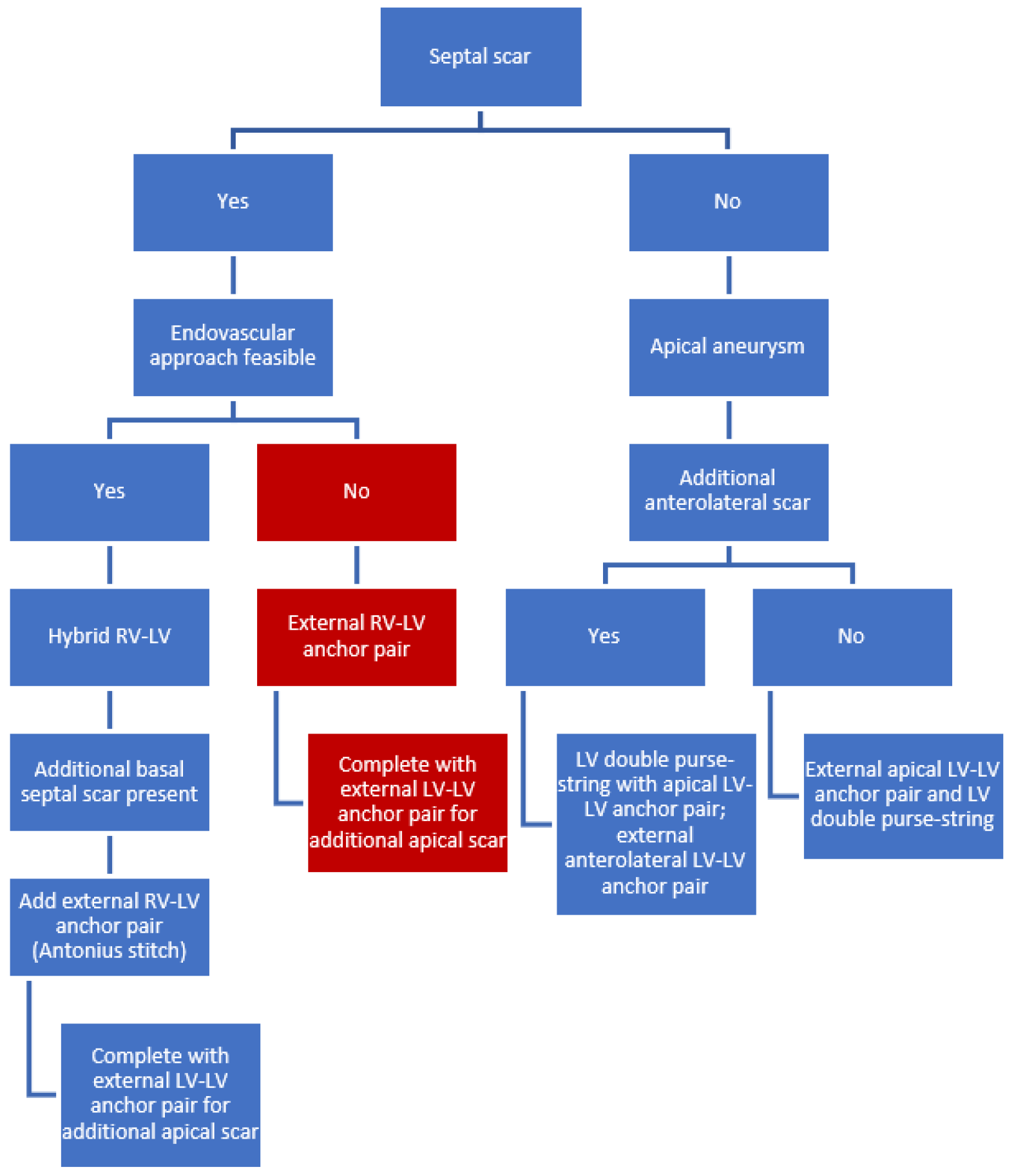
4.1. Septal Scar (Limited or Extended)
- Septal localization of myocardial scar requires a hybrid RV-LV approach. The internal anchor (from the RIJV) with its’ corresponding external anchor is the first pair to be implanted. This can be repeated several times.
- In case more septal scar is present basal to this pair/these pairs, an external RV-LV anchor pair can be placed. This external RV-LV stitch (“Antonius stitch”) is a modification of the hybrid approach, in which the anchor pair is deployed fully externally instead of a combination of internally (with transcatheter techniques through the RIJV) and externally.
- If scar tissue is present in the apex, additional external LV-LV anchor pairs should be placed and can be combined, if necessary, with a LV purse-string suture.
4.2. External Approach for Septal Scar in High-Risk Patients
4.3. Isolated Apical Aneurysm
5. The Role of Pre- and Post-Operative Imaging Modalities in the LIVE Procedure
5.1. Transthoracic Echocardiography
5.2. Cardiac Magnetic Resonance (CMR) Imaging with Late Gadolinium Enhancement (LGE)
5.3. Four-Dimensional Cardiac Computed Tomography
5.4. Three-Dimensional Reconstructive Imaging
6. A Guide through the LIVE Technique (Procedural Steps)
6.1. Patient Set-Up for the LIVE Procedure
6.2. Location of Incision
6.3. Insertion of the Endovascular Snare System in the RV
6.4. Insertion of the Needle through the RV Epicardium towards the Septum
6.5. Meeting in the Middle
6.6. Introduction of the Internal Anchor
6.7. Placement of the External Anchor
6.8. Imaging-Guided Release of the Anchor
6.9. Hybrid Placement of Additional Anchor Pairs
6.10. Additional External RV-LV Anchor Pair(s)
6.11. Additional External LV-LV Anchor Pair(s)
6.12. Closure
7. Insights from Previous Literature
7.1. Finding the Right Patient: Suggested Indications for Hybrid LVR
7.2. Finding the Right Patient: Suggested Contraindications for Hybrid LVR
7.3. Included Studies and Baseline Characteristics of Described Cohorts
7.4. Procedural Data and Clinical Outcome of Hybrid LVR
7.5. Echocardiographic Outcome of Hybrid LVR
7.6. Functional Outcome of Hybrid LVR
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Albakri, A. Ischemic heart failure: A review of clinical status and meta-analysis of diagnosis and clinical management methods. Clin. Med. Investig. 2018, 3, 1–15. [Google Scholar] [CrossRef]
- Kim, D.; Park, C.; Jin, E.; Hwang, H.; Sohn, I.; Cho, J.; Kim, C. Predictors of decreased left ventricular function subsequent to follow-up echocardiography after percutaneous coronary intervention following acute ST-elevation myocardial infarction. Exp. Ther. Med. 2018, 15, 4089–4096. [Google Scholar] [CrossRef]
- Zivelonghi, C.; Klein, P.; Swaans, M.J.; Agostoni, P. Hybrid transcatheter left ventricular reconstruction for the treatment of ischaemic cardiomyopathy. EuroIntervention 2018, 13, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.; Pillay, T.; Annest, L.; Bladel, K.; Kaiser, E.; Stahl, F.; Hanke, T.; Swaans, M.; Klein, P.; Ruf, T.; et al. Patient selection for LIVE therapy: From clinical indications to multimodality imaging individual case planning. Echocardiography 2021, 38, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Biffi, M.; Loforte, A.; Folesani, G.; Ziacchi, M.; Attinà, D.; Niro, F.; Pasquale, F.; Pacini, D. Hybrid transcatheter left ventricular reconstruction for the treatment of ischemic cardiomyopathy. Cardiovasc. Diagn. Ther. 2021, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Coltharp, W.H.; Hoff, S.J.; Stoney, W.S.; Alford, W.C.; Burrus, G.R.; Glassford, D.M.; Lea, J.W.; Petracek, M.R.; Starkey, T.D.; Shuman, T.A. Ventricular Aneurysmectomy A 25-Year Experience. Ann. Surg. 1994, 219, 707–714. [Google Scholar] [CrossRef]
- Beck, C.S. Operation for aneurysm of the heart. Ann. Surg. 1944, 120, 34–40. [Google Scholar] [CrossRef]
- Cooley, D.A. Ventricular aneurysm after myocardial infarction. J. Am. Med. Assoc. 1958, 167, 557. [Google Scholar] [CrossRef]
- Stoney, W.S.; Alford, W.C.; Burrus, G.R.; Thomas, C.S. Repair of Anteroseptal Ventricular Aneurysm. Ann. Thorac. Surg. 1973, 15, 394–404. [Google Scholar] [CrossRef]
- Dor, V.; Saab, M.; Coste, P.; Kornaszewska, M.; Montiglio, F. Left Ventricular Aneurysm: A New Surgical Approach. Thorac. Cardiovasc. Surg. 1989, 37, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Cooley, D.A. Ventricular Endoaneurysmorrhaphy: A Simplified Repair for Extensive Postinfarction Aneurysm. J. Card. Surg. 1989, 4, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Dor, V.; Di Donato, M.; Sabatier, M.; Montiglio, F.; Civaia, F. Left Ventricular Reconstruction by Endoventricular Circular Patch plasty Repair: A 17-Year Experience. Semin. Thorac. Cardiovasc. Surg. 2001, 13, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Menicanti, L.; Di Donato, M. The Dor procedure: What has changed after fifteen years of clinical practice? J. Thorac. Cardiovasc. Surg. 2002, 124, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Sabatier, M.; Dor, V.; Gensini, G.F.; Toso, A.; Maioli, M.; Stanley, A.W.H.; Athanasuleas, C.; Buckberg, G. Effects of the Dor procedure on left ventricular dimension and shape and geometric correlates of mitral regurgitation one year after surgery. J. Thorac. Cardiovasc. Surg. 2001, 121, 91–96. [Google Scholar] [CrossRef]
- Lee, K.-K. Hand-made sizing balloon in surgical ventricular restoration. Interact. Cardiovasc. Thorac. Surg. 2006, 5, 301–302. [Google Scholar] [CrossRef]
- Klein, P.; Anker, S.D.; Wechsler, A.; Skalsky, I.; Neuzil, P.; Annest, L.S.; Bifi, M.; McDonagh, T.; Frerker, C.; Schmidt, T.; et al. Less invasive ventricular reconstruction for ischaemic heart failure. Eur. J. Heart Fail. 2019, 21, 1638–1650. [Google Scholar] [CrossRef]
- Wechsler, A.S.; Sadowski, J.; Kapelak, B.; Bartus, K.; Kalinauskas, G.; Rucinskas, K.; Samalavicius, R.; Annest, L. Durability of epicardial ventricular restoration without ventriculotomy. Eur. J. Cardio Thorac. Surg. 2013, 44, e189–e192. [Google Scholar] [CrossRef]
- Loforte, A.; Alfonsi, J.; Gliozzi, G.; Folesani, G.; Fiorentino, M.; Biffi, M.; Marinelli, G.; Di Bartolomeo, R.; Pacini, D. Less invasive ventricular enhancement (LIVE) as potential therapy for ischaemic cardiomyopathy end-stage heart failure. J. Thorac. Dis. 2019, 11, S921–S928. [Google Scholar] [CrossRef]
- Dweck, M.R.; Williams, M.C.; Moss, A.J.; Newby, D.E.; Fayad, Z.A. Computed Tomography and Cardiac Magnetic Resonance in Ischemic Heart Disease. J. Am. Coll. Cardiol. 2016, 68, 2201–2216. [Google Scholar] [CrossRef]
- Pillay, T.; Neves, P.; Benetti, F.; Van Bladel, K.; Wechsler, A.; Annest, L. Minimal access left ventricular reconstruction. J. Card. Surg. 2021, 36, 300–306. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, R.B.A.; Bouma, B.J.; Cate, F.J.T.; Cheriex, E.C.; van Dantzig, J.M.; Elzenga, N.J.; Hamer, J.P.M.; Hoendernis, E.S.; Kamp, O.; Koster, J.; et al. Praktische Echocardiografie, 2nd ed.; Hamer, J.P.M., Pieper, P.G., Eds.; Bohn Stafleu van Loghum: Houten, The Netherlands, 2004; Volume 2. [Google Scholar]
- Tan, C.; Rubenson, D.; Srivastava, A.; Mohan, R.; Smith, M.R.; Billick, K.; Bardarian, S.; Thomas Heywood, J. Left ventricular outflow tract velocity time integral outperforms ejection fraction and Doppler-derived cardiac output for predicting outcomes in a select advanced heart failure cohort. Cardiovasc. Ultrasound 2017, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Dabic, P.; Castelvecchio, S.; Santambrogio, C.; Brankovic, J.; Collarini, L.; Joussef, T.; Frigiola, A.; Buckberg, G.; Menicanti, L. Left ventricular geometry in normal and post-anterior myocardial infarction patients: Sphericity index and ‘new’ conicity index comparisons. Eur. J. Cardio Thorac. Surg. 2006, 29, S225–S230. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zheng, Z.; Feng, W.; Zhang, Y.; Jin, L.; Li, P.; Hu, S. Apical conicity ratio: A new index on left ventricular apical geometry after myocardial infarction. J. Thorac. Cardiovasc. Surg. 2010, 140, 1402–1407.e3. [Google Scholar] [CrossRef]
- Matusik, P.S.; Bryll, A.; Matusik, P.T.; Popiela, T.J. Ischemic and non-ischemic patterns of late gadolinium enhancement in heart failure with reduced ejection fraction. Cardiol. J. 2021, 28, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef]
- Chuang, M.L.; Gona, P.; Hautvast, G.L.T.F.; Salton, C.J.; Breeuwer, M.; O’Donnell, C.J.; Manning, W.J. CMR reference values for left ventricular volumes, mass, and ejection fraction using computer-aided analysis: The Framingham Heart Study. J. Magn. Reson. Imaging 2014, 39, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Grothues, F.; Smith, G.C.; Moon, J.C.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D.J. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef]
- Nakamori, S.; Ismail, H.; Ngo, L.H.; Manning, W.J.; Nezafat, R. Left ventricular geometry predicts ventricular tachyarrhythmia in patients with left ventricular systolic dysfunction: A comprehensive cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 2017, 19, 79. [Google Scholar] [CrossRef]
- Naar, J.; Skalský, I.; Krűger, A.; Málek, F.; Van Bladel, K.; Annest, L.S.; Moučka, P.; Mráz, T.; Reddy, V.Y.; Neužil, P. Long-Term Results of Hybrid Left Ventricular Reconstruction in the Treatment of Ischemic Cardiomyopathy. J. Cardiovasc. Transl. Res. 2021, 14, 1043–1050. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, G.; Zhang, G.; Wang, B.; Lin, Z.; Saiwha, H.D.; You, H.; Lai, K.; Su, M.; Wen, H.; et al. A transcatheter procedure for direct modification of the aneurysmatic left ventricle. EuroIntervention 2021, 16, e1541–e1543. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.; Agostoni, P.; Van Boven, W.J.; De Winter, R.J.; Swaans, M.J. Transcatheter and minimally invasive surgical left ventricular reconstruction for the treatment of ischaemic cardiomyopathy: Preliminary results. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 441–446. [Google Scholar] [CrossRef] [PubMed]



| Naar 2021 (n = 23) | Klein 2019 (n = 89) | Loforte 2019 (n = 7) | Klein 2019 (n = 9) | Wang 2021 (n = 26) | |
|---|---|---|---|---|---|
| Inclusion criteria | |||||
| 15–45% | 15–45% | <35% | <40% | <40% |
| II–IV | II–IV | III–IV | II–IV | II–IV |
| + | + | + | NR | + |
| + | + | + | + | + |
| + | + | + | + | + |
| − | + | + | − | + |
| Exclusion criteria | |||||
| + | + | + | + | + |
| + | + | + | + | + |
| + | + | + | + | − |
| + | − | + | + | − |
| − | + | + | + | − |
| Study ID | Naar 2021 | Klein 2019 | Loforte 2019 | Klein 2019 | Wang 2021 |
|---|---|---|---|---|---|
| Country of Origin | Czech Republic | 11 countries | Italy | The Netherlands | China |
| Study period | September 2013–March 2019 | August 2010–March 2016 | January 2015–November 2018 | October 2016–July 2017 | January 2017–January 2019 |
| Study design | Prospective mono-center single-arm study | Prospective multi-center single-arm study | Mono-center single-arm study | Prospective multi-center single-arm study | Prospective mono-center single-arm study |
| Surgical technique | Hybrid * | Non-hybrid * Hybrid * | Hybrid * | Hybrid * | Hybrid * |
| No of patients | 23 | 89 | 7 | 9 | 26 |
| No of hybrid patients | 23 | 35 | 7 | 9 | 26 |
| Reference | Naar 2021 | Klein 2019 | Loforte 2019 | Klein 2019 | Wang 2021 | Total |
|---|---|---|---|---|---|---|
| Patients (n) | 23 | 35 | 7 | 9 | 26 | 100 |
| Age (years) | 59 ± 11 | 63 ± 10 | 72 ± 9 | 60 ± 8 | 58 ± 13 | 61 ± 11 |
| Male (%) | 65% | 91% | 71% | 89% | - | 81% |
| NYHA class (1–4) | 2.3 ± 0.5 | 2.6 ± 0.5 | 3.4 ± 0.6 | 2.7 ± 0.4 | 2.7 ± 0.6 | 2.6 ± 0.5 |
| 6MWT (m) | 381 ± 103 | 365 ± 90 | - | - | 369 ± 40 | 371 ± 82 |
| LVEF (%) | 32 ± 7 | 30 ± 8 | 23 ± 8 | 28 ± 8 | 36 ± 9 | 31 ± 8 |
| LVESVI (mL/m2) | 73 ± 27 | 75 ± 32 | 93 ± 11 | 53 ± 8 | 85 ± 26 | 76 ± 27 |
| LVEDVI (mL/m2) | 107 ± 27 | 110 ± 39 | 137 ± 20 | 75 ± 23 | 108 ± 33 | 108 ± 33 |
| TR (0–4) | 0.6 ± 0.6 | - | - | 0.5 ± 0.6 | - | 0.6 ± 0.6 |
| MR (0–4) | 1.2 | 1.1 ± 0.9 | - | 0.4 ± 1.2 | - | 1.0 |
| Reference | Naar 2021 | Klein 2019 | Loforte 2019 | Klein 2019 | Wang 2021 | Total |
|---|---|---|---|---|---|---|
| Patients (n) | 23 | 35 | 7 | 9 | 26 | 100 |
| Anchor pairs | 2.9 | - | 3.0 ± 0.9 | 2.6 ± 0.7 | 2.7 ± 0.7 | 2.8 |
| Operating time (min) | 204 ± 50 | - | 195 ± 115 | 21 ± 100 | 304 ± 69 | 218 ± 74 |
| Conversion to full sternotomy (n) | 1 (4%) | 0 (0%) | 1 (14%) | 1 (11%) | 0 (0%) | 3 (3%) |
| Re-operation (n) | 2 (9%) | 0 (0%) | 0 (0%) | 1 (11%) | 0 (0%) | 3 (3%) |
| Procedure-related mortality | 2 (9%) | 3 (9%) | 0 (0%) | 0 (0%) | 1 (4%) | 6 (6%) |
| ICU stay (days), median (IQR) | - | 2 (2–5) * | 4 (1.5–12.5) * | 2 (1–46) | - | - |
| Hospital stay (days), median (IQR) | - | 9 (7–15) * | 14 (11.5–32) * | 9 (3–57) | - | - |
| Ventilation time (hours), median (IQR) | - | 9.5 (7–15.5) * | 4 (4–17.5) * | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegeman, R.R.M.J.J.; Swaans, M.J.; van Kuijk, J.-P.; Klein, P. State-of-the-Art Review: Technical and Imaging Considerations in Hybrid Transcatheter and Minimally Invasive Left Ventricular Reconstruction for Ischemic Heart Failure. J. Clin. Med. 2022, 11, 4831. https://doi.org/10.3390/jcm11164831
Hegeman RRMJJ, Swaans MJ, van Kuijk J-P, Klein P. State-of-the-Art Review: Technical and Imaging Considerations in Hybrid Transcatheter and Minimally Invasive Left Ventricular Reconstruction for Ischemic Heart Failure. Journal of Clinical Medicine. 2022; 11(16):4831. https://doi.org/10.3390/jcm11164831
Chicago/Turabian StyleHegeman, Romy Roosmarijn Maria Jacqueline Josepha, Martin John Swaans, Jan-Peter van Kuijk, and Patrick Klein. 2022. "State-of-the-Art Review: Technical and Imaging Considerations in Hybrid Transcatheter and Minimally Invasive Left Ventricular Reconstruction for Ischemic Heart Failure" Journal of Clinical Medicine 11, no. 16: 4831. https://doi.org/10.3390/jcm11164831
APA StyleHegeman, R. R. M. J. J., Swaans, M. J., van Kuijk, J.-P., & Klein, P. (2022). State-of-the-Art Review: Technical and Imaging Considerations in Hybrid Transcatheter and Minimally Invasive Left Ventricular Reconstruction for Ischemic Heart Failure. Journal of Clinical Medicine, 11(16), 4831. https://doi.org/10.3390/jcm11164831





