Dual Targeting of the EGFR/HER2 Pathway in Combination with Systemic Chemotherapy in Refractory Pancreatic Cancer—The CONKO-008 Phase I Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Oversight
2.2. Patient Eligibility
2.3. Study Design
2.4. Patient Evaluation and Assessments
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
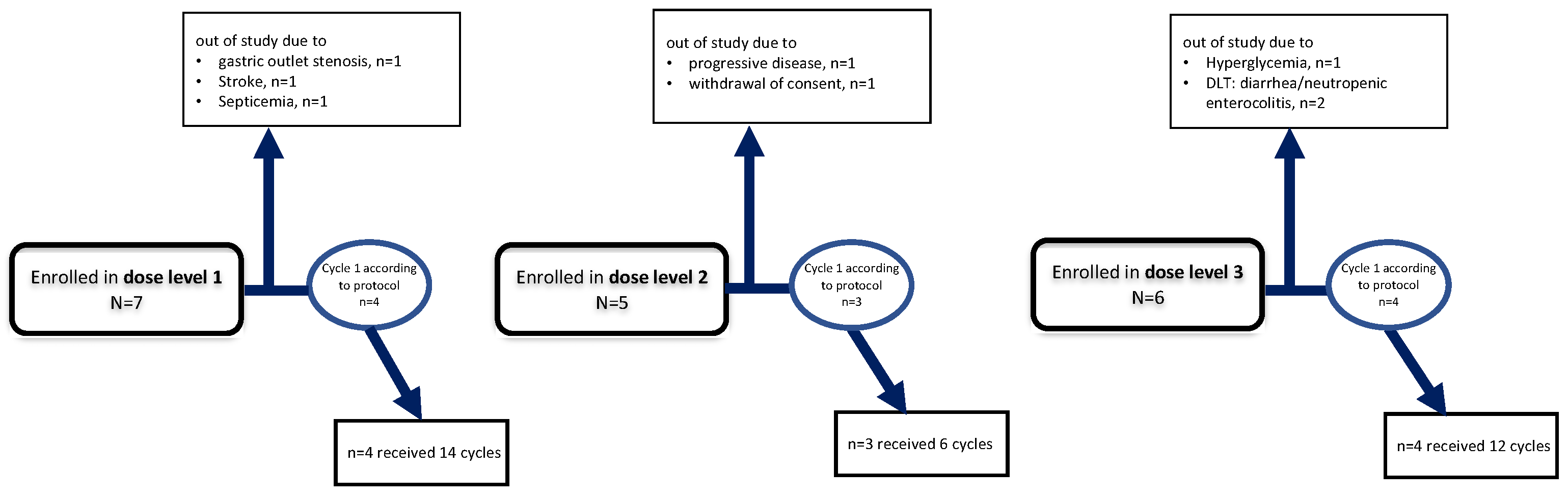
3.1.1. Dose Level 1: 1000 mg Lapatinib
3.1.2. Dose Level 2: 1250 mg Lapatinib
3.1.3. Dose Level 3: 1500 mg Lapatinib
3.2. Safety
3.2.1. Toxicity Summary
3.2.2. Toxicity Summary
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultheis, B.; Strumberg, D.; Kuhlmann, J.; Wolf, M.; Link, K.; Seufferlein, T.; Kaufmann, J.; Feist, M.; Gebhardt, F.; Khan, M.; et al. Safety, Efficacy and Pharcacokinetics of Targeted Therapy with The Liposomal RNA Interference Therapeutic Atu027 Combined with Gemcitabine in Patients with Pancreatic Adenocarcinoma. A Randomized Phase Ib/IIa Study. Cancers 2020, 12, 3130. [Google Scholar] [CrossRef] [PubMed]
- Renouf, D.; Knox, J.J.; Kavan, P.; Jonker, D.; Welch, S.; Couture, F.; Lemay, F.; Tehfe, M.; Harb, M.; Aucoin, N.; et al. LBA65 The Canadian Cancer Trials Group PA.7 trial: Results of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) vs GEM, nab-P, durvalumab (D) and tremelimumab (T) as first line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC). Ann. Oncol. 2020, 31, S1195. [Google Scholar] [CrossRef]
- Patel, K.; Siraj, S.; Smith, C.; Nair, M.; Vishwanatha, J.; Basha, R. Pancreatic Cancer: An Emphasis on Current Perspectives in Immunotherapy. Crit. Rev. Oncog. 2019, 24, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- Oettle, H.; Riess, H.; Stieler, J.M.; Heil, G.; Schwaner, I.; Seraphin, J.; Görner, M.; Mölle, M.; Greten, T.F.; Lakner, V.; et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: Outcomes from the CONKO-003 trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2423–2429. [Google Scholar] [CrossRef]
- Pelzer, U.; Schwaner, I.; Stieler, J.; Adler, M.; Seraphin, J.; Dörken, B.; Riess, H.; Oettle, H. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: A phase III-study from the German CONKO-study group. Eur. J. Cancer 2011, 47, 1676–1681. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Li, C.P.; Bodoky, G.; Dean, A.; Shan, Y.S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.A.; et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef]
- Yarden, Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur. J. Cancer Oxf. Engl. 2001, 37 (Suppl. S4), S3–S8. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Friess, H.; Kobrin, M.S.; Büchler, M.; Kunz, J.; Beger, H.G.; Korc, M. Overexpression of HER2/neu oncogene in human pancreatic carcinoma. Hum. Pathol. 1993, 24, 1127–1134. [Google Scholar] [CrossRef]
- Kimura, K.; Sawada, T.; Komatsu, M.; Inoue, M.; Muguruma, K.; Nishihara, T.; Yamashita, Y.; Yamada, N.; Ohira, M.; Hirakawa, K. Antitumor effect of trastuzumab for pancreatic cancer with high HER-2 expression and enhancement of effect by combined therapy with gemcitabine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 4925–4932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, S.; Appert, H.E.; Nakata, B.; Domenico, D.R.; Kim, K.; Howard, J.M. Overexpression of HER2/neu oncogene in pancreatic cancer correlates with shortened survival. Int. J. Pancreatol. Off. J. Int. Assoc. Pancreatol. 1995, 17, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tsiambas, E.; Karameris, A.; Dervenis, C.; Lazaris, A.C.; Giannakou, N.; Gerontopoulos, K.; Patsouris, E. HER2/neu expression and gene alterations in pancreatic ductal adenocarcinoma: A comparative immunohistochemistry and chromogenic in situ hybridization study based on tissue microarrays and computerized image analysis. JOP J. Pancreas 2006, 7, 283–294. [Google Scholar]
- Dancer, J.; Takei, H.; Ro, J.Y.; Lowery-Nordberg, M. Coexpression of EGFR and HER-2 in pancreatic ductal adenocarcinoma: A comparative study using immunohistochemistry correlated with gene amplification by fluorescencent in situ hybridization. Oncol. Rep. 2007, 18, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Pryczynicz, A.; Guzińska-Ustymowicz, K.; Kemona, A.; Czyzewska, J. Expression of EGF and EGFR strongly correlates with metastasis of pancreatic ductal carcinoma. Anticancer Res. 2008, 28, 1399–1404. [Google Scholar]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [Green Version]
- Cameron, D.; Casey, M.; Oliva, C.; Newstat, B.; Imwalle, B.; Geyer, C.E. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: Final survival analysis of a phase III randomized trial. Oncologist 2010, 15, 924–934. [Google Scholar] [CrossRef] [Green Version]
- Oettle, H.; Pelzer, U.; Stieler, J.; Hilbig, A.; Roll, L.; Schwaner, I.; Adler, M.; Detken, S.; Dörken, B.; Riess, H. Oxaliplatin/folinic acid/5-fluorouracil [24h] (OFF) plus best supportive care versus best supportive care alone (BSC) in second-line therapy of gemcitabine-refractory advanced pancreatic cancer (CONKO 003). J. Clin. Oncol. 2005, 23, 4031. [Google Scholar] [CrossRef]
- Siegel-Lakhai, W.S.; Beijnen, J.H.; Vervenne, W.L.; Boot, H.; Keessen, M.; Versola, M.; Koch, K.M.; Smith, D.A.; Pandite, L.; Richel, D.J.; et al. Phase I pharmacokinetic study of the safety and tolerability of lapatinib (GW572016) in combination with oxaliplatin/fluorouracil/leucovorin (FOLFOX4) in patients with solid tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13 Pt 1, 4495–4502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennie, T.W.; Fleming, R.A.; Bowen, C.J.; Dar, M.M.; Alberti, D.; Oliver, K.; Loconte, N.; Mulkerin, D.; Holen, K.D. A phase I study of capecitabine, oxaliplatin, and lapatinib in metastatic or advanced solid tumors. Clin. Colorectal Cancer 2011, 10, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Safran, H.; Miner, T.; Resnick, M.; Dipetrillo, T.; McNulty, B.; Evans, D.; Joseph, P.; Plette, A.; Millis, R.; Sears, D.; et al. Lapatinib/gemcitabine and lapatinib/gemcitabine/oxaliplatin: A phase I study for advanced pancreaticobiliary cancer. Am. J. Clin. Oncol. 2008, 31, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Fedele, C.; Riccio, G.; Coppola, C.; Barbieri, A.; Monti, M.G.; Arra, C.; Tocchetti, C.G.; D’Alessio, G.; Maurea, N.; De Lorenzo, C. Comparison of preclinical cardiotoxic effects of different ErbB2 inhibitors. Breast Cancer Res. Treat. 2012, 133, 511–521. [Google Scholar] [CrossRef]
- Azim, H.; Azim, H.A.; Escudier, B. Trastuzumab versus lapatinib: The cardiac side of the story. Cancer Treat. Rev. 2009, 35, 633–638. [Google Scholar] [CrossRef]
- Abdullah, S.E.; Haigentz, M.; Piperdi, B. Dermatologic Toxicities from Monoclonal Antibodies and Tyrosine Kinase Inhibitors against EGFR: Pathophysiology and Management. Chemother. Res. Pract. 2012, 2012, 351210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardone, B.; Nicholson, K.; Newman, M.; Guitart, J.; Gerami, P.; Talarico, N.; Yang, X.J.; Rademaker, A.; West, D.P.; Lacouture, M.E. Histopathologic and immunohistochemical characterization of rash to human epidermal growth factor receptor 1 (HER1) and HER1/2 inhibitors in cancer patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 4452–4460. [Google Scholar] [CrossRef] [Green Version]
| (A) | |
| Characteristic | |
| No. of patients, n = | 18 |
| Age, median (range), years | 62 (50–75) |
| Karnofsky Performance Status, median (range), % | 80 (70–90) |
| Female, n | 6 |
| Male, n | 12 |
| BMI median (range) | 18.3 (16.1–27.9) |
| Previous 1st line therapies | |
| Gemcitabine | 7 |
| Gemcitabine + Erlotinib | 6 |
| Gemcitabine + Aflibercept | 13 |
| Gemcitabine + Sorafenib | 3 |
| Gemcitabine + Capecitabine | 1 |
| (B) | |
| Tumor Characteristic | |
| Stage | |
| localized n | 0 |
| metastasized n | 18 |
| Initially curative intended resection | |
| yes, n | 9 |
| no, n | 9 |
| Histology | |
| Adenocarcinoma ductal | 17 |
| Adenocarcinoma papillary | 1 |
| Tumor Grading | |
| G1 | 0 |
| G2 | 12 |
| G3 | 6 |
| Pattern of Progression | |
| pulmonary | 5 |
| liver | 7 |
| both | 9 |
| Dose Level 1 (Lapatinib 1000 mg) | Dose Level 2 (Lapatinib 1250 mg) | Dose Level 3 (Lapatinib 1500 mg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTC AE 4.0 Grade | I | II | III | IV | I | II | III | IV | I | II | III | IV |
| Anemia | 6 | 1 | 0 | 0 | 3 | 1 | 1 | 0 | 4 | 1 | 0 | 0 |
| Leukopenia | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Thrombocytopenia | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Potassium | 2 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 |
| Sodium | 2 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| Albumin | 3 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 3 | 0 | 0 |
| ALT | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| AST | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| GGT | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 0 | 0 | 1 | 2 | 1 |
| Alkaline phosphatase | 0 | 2 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Creatinine | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bilirubin | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| QTc-time | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypertension | 0 | 3 | 0 | 0 | 1 | 2 | 0 | 0 | 3 | 2 | 1 | 0 |
| Nausea | 3 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 3 | 1 | 0 | 0 |
| Vomiting | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Diarrhea | 4 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 2 | 1 | 1 |
| Infection | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 3 | 2 | 0 | 0 | 3 | 2 | 0 | 0 | 1 | 2 | 0 | 0 |
| Hand-Foot Syndrome | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Synkope | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastric outlet stenosis | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stroke | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GI bleeding | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pain | 1 | 2 | 1 | 0 | 3 | 0 | 2 | 0 | 1 | 0 | 0 | 0 |
| Toxicity | Dose Level 1: Lapatinib 1000 mg | Dose Level 2: Lapatinib 1250 mg | Dose Level 3: Lapatinib 1500 mg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTC AE 4.0 Grade | I | II | III | IV | I | II | III | IV | I | II | III | IV |
| Anemia | 8 | 7 | 0 | 0 | 4 | 2 | 0 | 0 | 10 | 0 | 0 | 0 |
| Leukopenia | 6 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 3 | 0 | 0 | 0 |
| Neutropenia | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 |
| Febrile Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 5 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Potassium | 4 | 1 | 2 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 |
| Sodium | 3 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 4 | 0 | 0 | 0 |
| Calcium | 5 | 7 | 0 | 0 | 2 | 3 | 1 | 0 | 5 | 1 | 0 | 0 |
| Magnesium | 9 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| Albumin | 5 | 4 | 1 | 0 | 2 | 4 | 0 | 0 | 5 | 0 | 0 | 0 |
| ALAT | 3 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 |
| ASAT | 7 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| GGT | 9 | 2 | 2 | 0 | 3 | 2 | 1 | 0 | 1 | 1 | 2 | 0 |
| Alkaline phosphatase | 8 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 3 | 1 | 1 | 0 |
| Creatinine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bilirubin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ejection fraction | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QTc-time | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypertension | 3 | 4 | 0 | 0 | 3 | 2 | 1 | 0 | 5 | 1 | 0 | 0 |
| Nausea | 4 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 5 | 2 | 0 | 0 |
| Vomiting | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Diarrhea | 6 | 3 | 2 | 0 | 3 | 1 | 0 | 0 | 3 | 2 | 1 | 0 |
| Infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 4 | 7 | 0 | 0 | 3 | 3 | 0 | 0 | 6 | 5 | 0 | 0 |
| Hand-Foot-Syndrome | 7 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 7 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Pain | 2 | 1 | 1 | 0 | 2 | 1 | 2 | 0 | 6 | 0 | 0 | 0 |
| Fracture | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypoglycemia | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Thromboembolism | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Striefler, J.K.; Stieler, J.M.; Neumann, C.C.M.; Geisel, D.; Ghadjar, P.; Sinn, M.; Malinka, T.; Pratschke, J.; Stintzing, S.; Oettle, H.; et al. Dual Targeting of the EGFR/HER2 Pathway in Combination with Systemic Chemotherapy in Refractory Pancreatic Cancer—The CONKO-008 Phase I Investigation. J. Clin. Med. 2022, 11, 4905. https://doi.org/10.3390/jcm11164905
Striefler JK, Stieler JM, Neumann CCM, Geisel D, Ghadjar P, Sinn M, Malinka T, Pratschke J, Stintzing S, Oettle H, et al. Dual Targeting of the EGFR/HER2 Pathway in Combination with Systemic Chemotherapy in Refractory Pancreatic Cancer—The CONKO-008 Phase I Investigation. Journal of Clinical Medicine. 2022; 11(16):4905. https://doi.org/10.3390/jcm11164905
Chicago/Turabian StyleStriefler, Jana K., Jens M. Stieler, Christopher C. M. Neumann, Dominik Geisel, Pirus Ghadjar, Marianne Sinn, Thomas Malinka, Johann Pratschke, Sebastian Stintzing, Helmut Oettle, and et al. 2022. "Dual Targeting of the EGFR/HER2 Pathway in Combination with Systemic Chemotherapy in Refractory Pancreatic Cancer—The CONKO-008 Phase I Investigation" Journal of Clinical Medicine 11, no. 16: 4905. https://doi.org/10.3390/jcm11164905
APA StyleStriefler, J. K., Stieler, J. M., Neumann, C. C. M., Geisel, D., Ghadjar, P., Sinn, M., Malinka, T., Pratschke, J., Stintzing, S., Oettle, H., Riess, H., & Pelzer, U. (2022). Dual Targeting of the EGFR/HER2 Pathway in Combination with Systemic Chemotherapy in Refractory Pancreatic Cancer—The CONKO-008 Phase I Investigation. Journal of Clinical Medicine, 11(16), 4905. https://doi.org/10.3390/jcm11164905








