High PEEP Levels during CPR Improve Ventilation without Deleterious Haemodynamic Effects in Pigs
Abstract
:1. Introduction
2. Methods
Anaesthesia and Instrumentation
3. Trial Protocol and Data Collection
Scores and Statistics
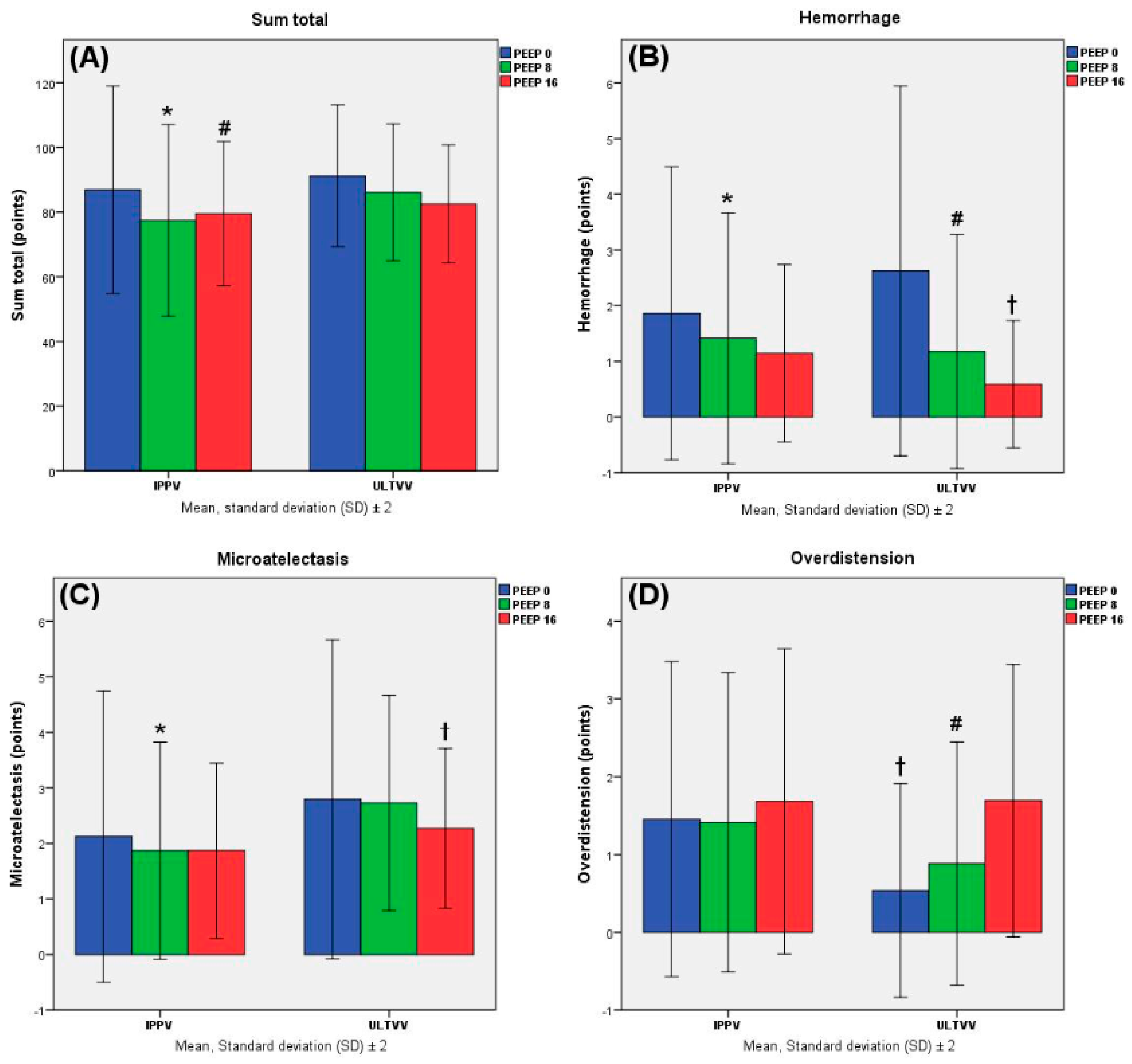
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newell, C.; Grier, S.; Soar, J. Airway and ventilation management during cardiopulmonary resuscitation and after successful resuscitation. Crit. Care 2018, 22, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soar, J.; Böttiger, B.W.; Carli, P.; Couper, K.; Deakin, C.D.; Djärv, T.; Lott, C.; Olasveengen, T.; Paal, P.; Pellis, T.; et al. European resuscitation council guidelines 2021: Adult advanced life support. Resuscitation 2021, 161, 115–151. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3 adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020, 142 (Suppl. S2), S366–S468. [Google Scholar] [CrossRef] [PubMed]
- McCaul, C.; Kornecki, A.; Engelberts, D.; McNamara, P.; Kavanagh, B.P. Positive end-expiratory pressure improves survival in a rodent model of cardiopulmonary resuscitation using high-dose epinephrine. Anesth. Analg. 2009, 109, 1202–1208. [Google Scholar] [CrossRef]
- Hodgkin, B.C.; Lambrew, C.T.; Lawrence, F.H., III; Angelakos, E.T. Effects of PEEP and of increased frequency of ventilation during CPR. Crit. Care Med. 1980, 8, 123–126. [Google Scholar] [CrossRef]
- Kill, C.; Hahn, O.; Dietz, F.; Neuhaus, C.; Schwarz, S.; Mahling, R.; Wallot, P.; Jerrentrup, A.; Steinfeldt, T.; Wulf, H.; et al. Mechanical ventilation during cardiopulmonary resuscitation with intermittent positive-pressure ventilation, bilevel ventilation, or chest compression synchronized ventilation in a pig model. Crit. Care Med. 2014, 42, e89–e95. [Google Scholar] [CrossRef]
- Ruemmler, R.; Ziebart, A.; Moellmann, C.; Garcia-Bardon, A.; Kamuf, J.; Kuropka, F.; Duenges, B.; Hartmann, E.K. Ultra-low tidal volume ventilation—A novel and effective ventilation strategy during experimental cardiopulmonary resuscitation. Resuscitation 2018, 132, 56–62. [Google Scholar] [CrossRef]
- Frerichs, I.; Schmitz, G.; Pulletz, S.; Schädler, D.; Zick, G.; Scholz, J.; Weiler, N. Reproducibility of regional lung ventilation distribution determined by electrical impedance tomography during mechanical ventilation. Physiol. Meas. 2007, 28, S261–S267. [Google Scholar] [CrossRef]
- Shono, A.; Katayama, N.; Fujihara, T.; Böhm, S.H.; Waldmann, A.D.; Ugata, K.; Nikai, T.; Saito, Y. Positive end-expiratory pressure and distribution of ventilation in pneumoperitoneum combined with steep trendelenburg position. Anesthesiology 2020, 132, 476–490. [Google Scholar] [CrossRef]
- Richard, J.C.M.; Lefebvre, J.C.; Tassaux, D.; Brochard, L. Update in mechanical ventilation 2010. Am. J. Respir. Crit. Care Med. 2011, 184, 32–36. [Google Scholar] [CrossRef]
- Pinsky, M.R.; Summer, W.R.; Wise, R.A.; Permutt, S.; Bromberger-Barnea, B. Augmentation of cardiac function by elevation of intrathoracic pressure. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 54, 950–955. [Google Scholar] [CrossRef]
- Fichtner, F.; Moerer, O.; Weber-Carstens, S.; Nothacker, M.; Kaisers, U.; Laudi, S.; Guideline Group. Clinical guideline for treating acute respiratory insufficiency with invasive ventilation and extracorporeal membrane oxygenation: Evidence-based recommendations for choosing modes and setting parameters of mechanical ventilation. Respiration 2019, 98, 357–372. [Google Scholar] [CrossRef]
- Ziebart, A.; Hartmann, E.K.; Thomas, R.; Liu, T.; Duenges, B.; Schad, A.; Bodenstein, M.; Thal, S.C.; David, M. Low tidal volume pressure support versus controlled ventilation in early experimental sepsis in pigs. Respir. Res. 2014, 15, 101. [Google Scholar] [CrossRef] [Green Version]
- Amato, M.B.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.; Mercat, A.; et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Cheifetz, I.M.; Craig, D.M.; Quick, G.; McGovern, J.J.; Cannon, M.L.; Ungerleider, R.M.; Smith, P.K.; Meliones, J.N. Increasing tidal volumes and pulmonary overdistention adversely affect pulmonary vascular mechanics and cardiac output in a pediatric swine model. Crit. Care Med. 1998, 26, 710–716. [Google Scholar] [CrossRef]
- Acosta, P.; Santisbon, E.; Varon, J. The use of positive end-expiratory pressure in mechanical ventilation. Crit. Care Clin. 2007, 23, 251–261. [Google Scholar] [CrossRef]
- Levenbrown, Y.; Hossain, M.J.; Keith, J.P.; Burr, K.; Hesek, A.; Shaffer, T.H. Effect of positive end-expiratory pressure on additional passive ventilation generated by CPR compressions in a porcine model. Intensive Care Med. Exp. 2021, 9, 37. [Google Scholar] [CrossRef]
- Hartmann, E.K.; Duenges, B.; Boehme, S.; Szczyrba, M.; Liu, T.; Klein, K.U.; Baumgardner, J.E.; Markstaller, K.; David, M. Ventilation/perfusion ratios measured by multiple inert gas elimination during experimental cardiopulmonary resuscitation. Acta Anaesthesiol. Scand. 2014, 58, 1032–1039. [Google Scholar] [CrossRef]
- Charbonney, E.; Grieco, D.L.; Cordioli, R.L.; Badat, B.; Savary, D.; Richard, J.C.M. Ventilation during cardiopulmonary resuscitation: What have we learned from models? Respir. Care 2019, 64, 1132–1138. [Google Scholar] [CrossRef]
- Cordioli, R.L.; Grieco, D.L.; Charbonney, E.; Richard, J.C.; Savary, D. New physiological insights in ventilation during cardiopulmonary resuscitation. Curr. Opin. Crit. Care 2019, 25, 37–44. [Google Scholar] [CrossRef]
- Levenbrown, Y.; Hossain, M.J.; Keith, J.P.; Burr, K.; Hesek, A.; Shaffer, T. The effect of positive end-expiratory pressure on cardiac output and oxygen delivery during cardiopulmonary resuscitation. Intensive Care Med. Exp. 2020, 8, 36. [Google Scholar] [CrossRef]
- Kamuf, J.; Garcia-Bardon, A.; Duenges, B.; Liu, T.; Jahn-Eimermacher, A.; Heid, F.; David, M.; Hartmann, E.K. Endexpiratory lung volume measurement correlates with the ventilation/perfusion mismatch in lung injured pigs. Respir. Res. 2017, 18, 101. [Google Scholar] [CrossRef] [Green Version]
- Bodenstein, M.; David, M.; Markstaller, K. Principles of electrical impedance tomography and its clinical application. Crit. Care Med. 2009, 37, 713–724. [Google Scholar] [CrossRef]
- Muders, T.; Luepschen, H.; Putensen, C. Impedance tomography as a new monitoring technique. Curr. Opin. Crit. Care 2010, 16, 269–275. [Google Scholar] [CrossRef]
- Luepschen, H.; Meier, T.; Grossherr, M.; Leibecke, T.; Karsten, J.; Leonhardt, S. Protective ventilation using electrical impedance tomography. Physiol. Meas. 2007, 28, S247–S260. [Google Scholar] [CrossRef]
- Walsh, B.K.; Smallwood, C.D. Electrical impedance tomography during mechanical ventilation. Respir. Care 2016, 61, 1417–1424. [Google Scholar] [CrossRef]
- Ruemmler, R.; Ziebart, A.; Kuropka, F.; Duenges, B.; Kamuf, J.; Garcia-Bardon, A.; Hartmann, E.K. Bi-Level ventilation decreases pulmonary shunt and modulates neuroinflammation in a cardiopulmonary resuscitation model. PeerJ 2020, 8, e9072. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.C.; Salcido, D.D.; Menegazzi, J.J. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehosp. Emerg. Care 2010, 14, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hüpfl, M.; Selig, H.F.; Nagele, P. Chest-compression-only versus standard cardiopulmonary resuscitation: A meta-analysis. Lancet 2010, 376, 1552–1557. [Google Scholar] [CrossRef] [Green Version]
- Kiguchi, T.; Okubo, M.; Nishiyama, C.; Maconochie, I.; Ong, M.E.H.; Kern, K.B.; Wyckoff, M.H.; McNally, B.; Christensen, E.F.; Tjelmeland, I.; et al. Out-of-hospital cardiac arrest across the World: First report from the international liaison committee on resuscitation (ILCOR). Resuscitation 2020, 152, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Ji, C.; Deakin, C.D.; Quinn, T.; Nolan, J.P.; Scomparin, C.; Regan, S.; Long, J.; Slowther, A.; Pocock, H.; et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N. Engl. J. Med. 2018, 379, 711–721. [Google Scholar] [CrossRef]
- Panchal, A.R.; Berg, K.M.; Hirsch, K.G.; Kudenchuk, P.J.; Del Rios, M.; Cabañas, J.G.; Link, M.S.; Kurz, M.C.; Chan, P.S.; Morley, P.T.; et al. 2019 American heart association focused update on advanced cardiovascular life support: Use of advanced airways, vasopressors, and extracorporeal cardiopulmonary resuscitation during cardiac arrest: An update to the american heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2019, 140, e881–e894. [Google Scholar] [CrossRef]



| Group Parameters | Group 1–3 | Group 4–6 |
|---|---|---|
| Ventilation mode | IPPV | ULTVV |
| PEEP level | 0, 8, 16 mbar (I0, I8, I16) | 0, 8, 16 mbar (U0, U8, U16) |
| Tidal volume (Vt) | 9–10 mL/kgBW | 2–3 mL/kgBW |
| Respiratory rate (RR) | 10 breaths/min | 50 breaths/min |
| FiO2 | 1.0 | 1.0 |
| Parameter | Intervention | CPR 5 min | CPR 15 min | CPR 25 min | p Values |
|---|---|---|---|---|---|
| Groups | |||||
| MEAN (SD) | |||||
| Pdrive | IPPV | 18.56 (5.82) | 19.27 (6.08) | 18.40 (6.35) | |
| [mbar] | ULTVV * | 14.82 (4.96) | 13.73 (6.21) | 14.31 (6.10) | * vs. IPPV, 0.002 |
| PEEP 0 mbar | 20.93 (5.57) | 20.51 (7.82) | 21.77 (4.14) | ||
| PEEP 8 mbar * | 16.12 (4.28) | 16.58 (4.37) | 16.05 (4.57) | * vs. PEEP 0, 0.014 | |
| PEEP 16 mbar #,† | 13.02 (4.20) | 12.40 (5.04) | 11.25 (5.93) | # vs. PEEP 0, 0.000; † vs. PEEP 8, 0.045 | |
| Ppeak | IPPV | 26.92 (6.38) | 27.46 (6.03) | 26.58 (6.03) | |
| [mbar] | ULTVV * | 22.79 (4.57) | 21.74 (6.33) | 22.29 (3.59) | * vs. IPPV, 0.001 |
| PEEP 0 mbar | 21.39 (5.60) | 20.48 (7.70) | 21.80 (4.21) | ||
| PEEP 8 mbar | 23.86 (4.45) | 24.61 (4.35) | 24.07 (4.61) | ||
| PEEP 16 mbar #,† | 29.31 (4.70) | 28.71 (5.41) | 27.44 (5.87) | # vs. PEEP 0, 0.000; † vs. PEEP 8, 0.031 | |
| shunt | IPPV | 19.60 (21.67) | 17.42 (14.99) | 19.00 (19.85) | |
| [%] | ULTVV | 15.80 (13.56) | 17.99 (12.00) | 23.06 (11.48) | |
| PEEP 0 mbar | 29.06 (26.04) | 21.59 (12.47) | 19.08 (10.87) | ||
| PEEP 8 mbar | 8.78 (4.11) | 19.47 (15.43) | 14.54 (7.92) | ||
| PEEP 16 mbar | 15.94 (10.95) | 12.05 (10.94) | 29.47 (22.70) | ||
| paCO2 | IPPV | 44.60 (19.45) | 45.32 (24.26) | 80.27 (26.36) | |
| [mmHg] | ULTVV * | 64.70 (15.80) | 71.43 (20.84) | 88.80 (24.27) | * vs. IPPV, 0.001 |
| PEEP 0 mbar | 59.05 (15.75) | 66.18 (26.13) | 74.02 (28.09) | ||
| PEEP 8 mbar | 56.60 (19.96) | 53.35 (24.48) | 82.00 (23.79) | ||
| PEEP 16 mbar | 48.30 (24.03) | 55.60 (27.22) | 97.57 (19.22) | ||
| paO2 | IPPV | 318.38 (197.12) | 281.12 (192.31) | 72.85 (129.10) | |
| [mmHg] | ULTVV | 242.60 (169.22) | 191.30 (163.44) | 82.73 (118.34) | |
| PEEP 0 mbar | 164.26 (123.97) | 155.42 (136.91) | 87.27 (124.16) | ||
| PEEP 8 mbar # | 330.57 (170.61) | 301.68 (173.26) | 122.38 (161.54) | # vs. PEEP 0, 0.025 | |
| PEEP 16 mbar | 346.63 (205.03) | 251.53 (209.41) | 23.71 (19.15) | ||
| MAP | IPPV | 27.38 (7.42) | 21.22 (7.17) | 14.19 (5.45) | |
| [mmHg] | ULTVV | 27.46 (6.82) | 18.38 (6.70) | 11.43 (5.69) | |
| PEEP 0 mbar | 27.48 (4.84) | 18.99 (7.77) | 10.73 (5.21) | ||
| PEEP 8 mbar | 27.62 (9.02) | 20.79 (8.22) | 14.20 (6.51) | ||
| PEEP 16 mbar | 27.17 (7.18) | 19.62 (4.98) | 13.49 (5.01) | ||
| Post mortem | |||||
| DAD. | IPPV | 1.95 (1.04) | |||
| atelectasis | ULTVV * | 2.59 (1.10) | * vs. IPPV, 0.000 | ||
| [points] | PEEP 0 mbar | 2.45 (1.40) | |||
| PEEP 8 mbar # | 2.29 (1.06) | # vs. PEEP 0, 0.047 | |||
| PEEP 16 mbar | 2.06 (0.77) | ||||
| DAD. | IPPV | 1.51 (0.98) | |||
| overdistens. | ULTVV * | 1.03 (0.91) | # vs. IPPV, 0.001 | ||
| [points] | PEEP 0 mbar | 0.99 (0.97) | |||
| PEEP 8 mbar | 1.14 (0.91) | ||||
| PEEP 16 mbar #,† | 1.68 (0.92) | # vs. PEEP 0, 0.000; † vs. PEEP 8, 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renz, M.; Müllejans, L.; Riedel, J.; Mohnke, K.; Rissel, R.; Ziebart, A.; Duenges, B.; Hartmann, E.K.; Ruemmler, R. High PEEP Levels during CPR Improve Ventilation without Deleterious Haemodynamic Effects in Pigs. J. Clin. Med. 2022, 11, 4921. https://doi.org/10.3390/jcm11164921
Renz M, Müllejans L, Riedel J, Mohnke K, Rissel R, Ziebart A, Duenges B, Hartmann EK, Ruemmler R. High PEEP Levels during CPR Improve Ventilation without Deleterious Haemodynamic Effects in Pigs. Journal of Clinical Medicine. 2022; 11(16):4921. https://doi.org/10.3390/jcm11164921
Chicago/Turabian StyleRenz, Miriam, Leah Müllejans, Julian Riedel, Katja Mohnke, René Rissel, Alexander Ziebart, Bastian Duenges, Erik Kristoffer Hartmann, and Robert Ruemmler. 2022. "High PEEP Levels during CPR Improve Ventilation without Deleterious Haemodynamic Effects in Pigs" Journal of Clinical Medicine 11, no. 16: 4921. https://doi.org/10.3390/jcm11164921
APA StyleRenz, M., Müllejans, L., Riedel, J., Mohnke, K., Rissel, R., Ziebart, A., Duenges, B., Hartmann, E. K., & Ruemmler, R. (2022). High PEEP Levels during CPR Improve Ventilation without Deleterious Haemodynamic Effects in Pigs. Journal of Clinical Medicine, 11(16), 4921. https://doi.org/10.3390/jcm11164921






