Correlation between Lymphatic Surgery Outcome and Lymphatic Image-Staging or Clinical Severity in Patients with Lymphedema
Abstract
:1. Introduction
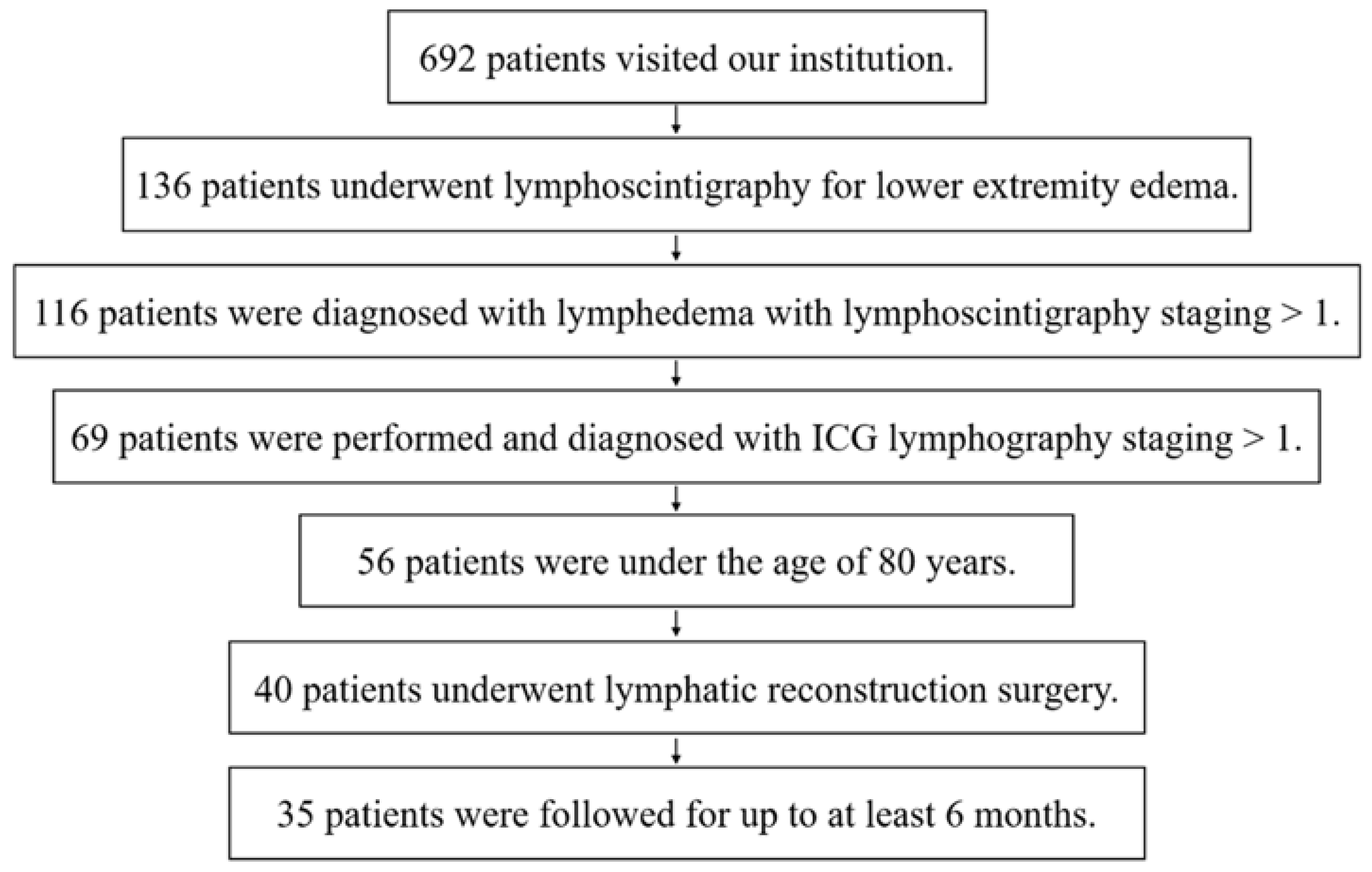
2. Materials and Methods
2.1. Patients
2.2. Technical Aspects of Lymphoscintigraphy
2.3. Technical Aspects of Indocyanine Green Study
2.4. Clinical Variables
2.5. Surgical Procedures
2.6. Statistical Analysis
3. Results
3.1. Clinical Parameters
3.2. Reproducibility and Correlation of the Clinical Severity Staging and Image Staging
3.3. Correlation between the Results of the First LVA and Lymphatic Images
3.4. Results and Comparison of Single LVA with the Multi-Surgery Group
3.5. Overall Results and Correlation with Clinical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rockson, S.G.; Rivera, K.K. Estimating the population burden of lymphedema. Ann. N. Y. Acad. Sci. 2008, 1131, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.A.; Shin, M.J.; Kim, J.H. Indocyanine green lymphography and lymphoscintigraphy severity stage showed strong correlation in lower limb lymphedema. Lymphat. Res. Biol. 2021, 19, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Matsuda, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: The modified dermal backflow stage and concept of subclinical lymphedema. Plast. Reconstr. Surg. 2011, 128, 314e–321e. [Google Scholar] [CrossRef]
- Maegawa, J.; Mikami, T.; Yamamoto, Y.; Satake, T.; Kobayashi, S. Types of lymphoscintigraphy and indications for lymphaticovenous anastomosis. Microsurgery 2010, 30, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Hara, H.; Hayashi, Y.; Narushima, M.; Yamamoto, T.; Todokoro, T.; Iida, T.; Sawamoto, N.; Araki, J.; Kikuchi, K.; et al. Pathological steps of cancer-related lymphedema: Histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS ONE 2012, 7, e41126. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Coroneos, C.J. Surgical treatment of lymphedema. Plast. Reconstr. Surg. 2019, 144, 738–758. [Google Scholar] [CrossRef]
- Imai, H.; Yoshida, S.; Mese, T.; Roh, S.; Fujita, A.; Uchiki, T.; Sasaki, A.; Nagamatsu, S.; Koshima, I. Technical tips for anastomosis of 0.2 mm diameter vessels during lymphatic venous anastomosis. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4304. [Google Scholar] [CrossRef]
- Koshima, I.; Narushima, M.; Mihara, M.; Yamamoto, T.; Hara, H.; Ohshima, A.; Kikuchi, K.; Todokoro, K.; Seki, Y.; Iida, T.; et al. Lymphadiposal flaps and lymphaticovenular anastomoses for severe leg edema: Functional reconstruction for lymph drainage system. J. Reconstr. Microsurg. 2016, 32, 50–55. [Google Scholar]
- Chang, D.W.; Dayan, J.; Greene, A.K.; MacDonald, J.K.; Masia, J.; Mehrara, B.; Neligan, P.C.; Nguyen, D. Surgical treatment of lymphedema: A systematic review and meta-analysis of controlled trials. Results of a consensus conference. Plast. Reconstr. Surg. 2021, 147, 975–993. [Google Scholar] [CrossRef]
- Hara, H.; Mihara, M. Multilymphosome injection indocyanine green lymphography can detect more lymphatic vessels than lymphoscintigraphy in lymphedematous limbs. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1025–1030. [Google Scholar] [CrossRef]
- Yamamoto, T.; Matsuda, N.; Todokoro, T.; Yoshimatsu, H.; Narushima, M.; Mihara, M.; Uchida, G.; Koshima, I. Lower extremity lymphedema index: A simple method for severity evaluation of lower extremity lymphedema. Ann. Plast. Surg. 2011, 67, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hamada, Y.; Koshima, I.; Imai, H.; Uchiki, T.; Sasaki, A.; Fujioka, Y.; Nagamatsu, S.; Yokota, K.; Harima, M.; et al. Role of lymphatico venular anastomosis for treatment of lymphorrhea in lower limbs. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1357–1404. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Koshima, I.; Imai, H.; Uchiki, T.; Sasaki, A.; Nagamatsu, S.; Yokota, K. Investigation of flow velocity in recipient perforator artery for a reliable indicator for the flap transfer with perforator to perforator anastomosis. Microsurgery 2021, 41, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Koshima, I.; Imai, H.; Roh, S.; Mese, T.; Uchiki, T.; Sasaki, A.; Nagamatsu, S. Effect of postoperative compression therapy on the success of liposuction in patients with advanced lower limb lymphedema. J. Clin. Med. 2021, 10, 4852. [Google Scholar] [CrossRef]
- Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef]
- Akita, S.; Mitsukawa, N.; Kazama, T.; Kuriyama, M.; Kubota, Y.; Omori, N.; Koizumi, T.; Kosaka, K.; Uno, T.; Satoh, K. Comparison of lymphoscintigraphy and indocyanine green lymphography for the diagnosis of extremity lymphoedema. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 792–798. [Google Scholar] [CrossRef]
- Pappalardo, M.; Lin, C.; Ho, O.A.; Kuo, C.F.; Lin, C.Y.; Cheng, M.H. Staging and clinical correlations of lymphoscintigraphy for unilateral gynecological cancer-related lymphedema. J. Surg. Oncol. 2020, 121, 422–434. [Google Scholar] [CrossRef]
- Maclellan, R.A.; Zurakowski, D.; Voss, S.; Greene, A.K. Correlation between lymphedema disease severity and lymphoscintigraphic findings: A clinical-radiologic study. J. Am. Coll. Surg. 2017, 225, 366–370. [Google Scholar] [CrossRef]
- Garza, R.M.; Ooi, A.S.H.; Falk, J.; Chang, D.W. The relationship between clinical and lndocyanine green staging in lymphedema. Lymphat. Res. Biol. 2019, 17, 329–333. [Google Scholar] [CrossRef]
- Koshima, I.; Inagawa, K.; Urushibara, K.; Moriguchi, T. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J. Reconstr. Microsurg. 2000, 16, 437–442. [Google Scholar] [CrossRef]
- Imai, H.; Yoshida, S.; Uchiki, T.; Sasaki, A.; Nagamatsu, S.; Koshima, I. Successful treatment of rheumatoid lymphedema with lymphatic venous anastomosis. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3763. [Google Scholar] [CrossRef] [PubMed]








| Characteristics | Value |
|---|---|
| Age, years | 62.4 (21–78) |
| BMI, kg/m2 | 25.7 (16.4–36.4) |
| Sex, Women/Men | 22/13 |
| The cause of lymphedema | |
| Complication of malignant tumor treatment | 24 (68.6%) |
| Primary | 6 (17.1%) |
| Cellulitis | 5 (14.3%) |
| Duration of edema, m | 52.5 (1–360) |
| ≥1 past cellulitis episode(s) | 11 (31.4%) |
| LEL index | 272.4 (180.7–384.8) |
| Lymphoscintigraphy staging | |
| I | 7 (20%) |
| II | 5 (14.3%) |
| III | 3 (8.6%) |
| IV | 12 (34.3%) |
| V | 8 (22.9%) |
| ICG lymphography staging | |
| I | 8 (22.9%) |
| II | 3 (8.6%) |
| III | 14 (40%) |
| IV | 4 (11.4%) |
| V | 6 (17.1%) |
| NECST classification | |
| 0 | 3 (8.6%) |
| I | 8 (22.9%) |
| II | 8 (22.9%) |
| III | 13 (37.1%) |
| IV | 3 (8.6%) |
| The type of surgery | |
| LVA | 25 (71.4%) |
| LVA and VLT | 5 (14.3%) |
| LVA and LS | 2 (5.7%) |
| LVA, VLT and LS | 3 (8.6%) |
| Regression Coefficients | p-Value | 95% Confidence Interval | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Preoperative LEL index | 0.13 | 0.01 | 0.03 | 0.23 |
| Episodes of cellulitis | −0.60 | 0.80 | −5.42 | 4.24 |
| Episodes of radiation | 2.53 | 0.28 | −2.15 | 7.22 |
| Periods of edema | 0.01 | 0.66 | −0.03 | 0.05 |
| Lymphoscintigraphy | −0.92 | 0.59 | −4.40 | 2.56 |
| ICG lymphography | −0.89 | 0.68 | −5.33 | 3.56 |
| NECST classification | −0.30 | 0.90 | −5.20 | 4.60 |
| Number of LVA | −0.97 | 0.47 | −3.72 | 1.78 |
| Regression Coefficients | p Value | 95% Confidence Interval | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Preoperative LEL index | 0.16 | 0.001 | 0.07 | 0.25 |
| Episodes of cellulitis | 0.41 | 0.86 | −4.21 | 5.02 |
| Episodes of radiation | 1.90 | 0.39 | −2.57 | 6.39 |
| Periods of edema | 0.00 | 0.90 | −0.04 | 0.04 |
| Lymphoscintigraphy | −1.72 | 0.30 | −5.05 | 1.61 |
| ICG lymphography | −0.17 | 0.93 | −4.42 | 4.08 |
| NECST classification | −0.73 | 0.75 | −5.42 | 3.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, H.; Yoshida, S.; Mese, T.; Roh, S.; Fujita, A.; Sasaki, A.; Nagamatsu, S.; Koshima, I. Correlation between Lymphatic Surgery Outcome and Lymphatic Image-Staging or Clinical Severity in Patients with Lymphedema. J. Clin. Med. 2022, 11, 4979. https://doi.org/10.3390/jcm11174979
Imai H, Yoshida S, Mese T, Roh S, Fujita A, Sasaki A, Nagamatsu S, Koshima I. Correlation between Lymphatic Surgery Outcome and Lymphatic Image-Staging or Clinical Severity in Patients with Lymphedema. Journal of Clinical Medicine. 2022; 11(17):4979. https://doi.org/10.3390/jcm11174979
Chicago/Turabian StyleImai, Hirofumi, Shuhei Yoshida, Toshiro Mese, Solji Roh, Asuka Fujita, Ayano Sasaki, Shogo Nagamatsu, and Isao Koshima. 2022. "Correlation between Lymphatic Surgery Outcome and Lymphatic Image-Staging or Clinical Severity in Patients with Lymphedema" Journal of Clinical Medicine 11, no. 17: 4979. https://doi.org/10.3390/jcm11174979






