The Role of Left Atrial Longitudinal Strain in the Diagnosis of Acute Cellular Rejection in Heart Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Endomyocardial Biopsy
2.3. Echocardiographic Study
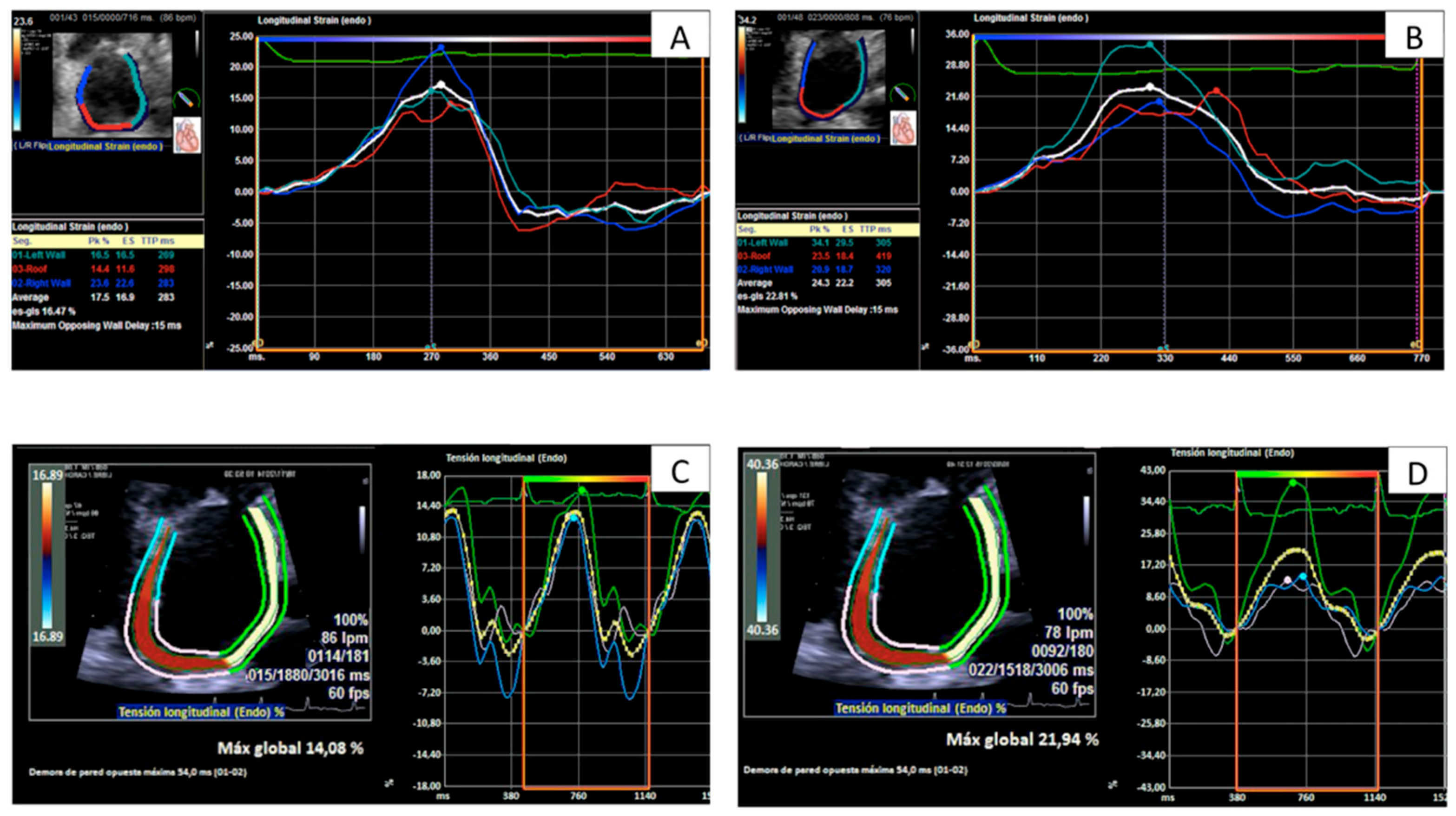
2.4. Strain Analysis
2.5. Groups of Comparison
2.6. Variability Analysis
2.7. Statistical Methods
3. Results
3.1. Patient Characteristics and Endomyocardial Biopsies Results
3.2. Association of LALS and Presence of ACR
3.3. Variability of LALS Parameters
3.4. Sensitivity and Specificity of Atrial Strain Parameters for Diagnosis of Rejection
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Todaro, M.C.; Choudhuri, I.; Belohlavek, M.; Jahangir, A.; Carerj, S.; Oreto, L.; Khandheria, B.K. New echocardiographic techniques for evaluation of left atrial mechanics. Eur. Heart J.-Cardiovasc. Imaging 2012, 13, 973–984. [Google Scholar] [CrossRef]
- Yang, L.-T.; Tsai, W.-C.; Luo, C.-Y.; Li, Y.-H.; Tsai, L.-M. Role of Left Atrial Reservoir Strain Rate in Left Atrial Remodeling in Severe Mitral Regurgitation. J. Med. Ultrasound 2017, 25, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Pastore, M.C.; Righini, F.M.; Mandoli, G.E.; D’Ascenzi, F.; Lisi, M.; Nistor, D.; Sparla, S.; Curci, V.; Di Tommaso, C.; et al. Prognostic value of left atrial strain in patients with moderate asymptomatic mitral regurgitation. Int. J. Cardiovasc. Imaging 2019, 35, 1597–1604. [Google Scholar] [CrossRef]
- García Martín, A.; Abellás Sequeiros, M.; González Gómez, A.G.; Rincón Díaz, L.M.; Monteagudo Ruiz, J.M.; Hinojar Baydés, R.; Moya Mur, J.L.; Zamorano, J.L.; Fernández-Golfín, C. Prognostic value of diastolic function parameters in significant aortic regurgitation: The role of the left atrial strain. J. Echocardiogr. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Pessoa-Amorim, G.; Mancio, J.; Vouga, L.; Ribeiro, J.; Gama, V.; Bettencourt, N.; Fontes-Carvalho, R. Impaired Left Atrial Strain as a Predictor of New-onset Atrial Fibrillation after Aortic Valve Replacement Independently of Left Atrial Size. Rev. Esp. Cardiol. 2018, 71, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Abou, R.; van Rosendael, P.; van der Bijl, P.; van Wijngaarden, S.E.; Regeer, M.V.; Podlesnikar, T.; Marsan, N.A.; Leung, D.Y.; Delgado, V.; et al. Relation of Echocardiographic Markers of Left Atrial Fibrosis to Atrial Fibrillation Burden. Am. J. Cardiol. 2018, 122, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ruiz, L.A.; Madrid-Miller, A.; Martínez-Flores, J.E.; González-Hermosillo, J.A.; Arenas-Fonseca, J.; Zamorano-Velázquez, N.; Mendoza-Pérez, B. Left atrial longitudinal strain by speckle tracking as independent predictor of recurrence after electrical cardioversion in persistent and long standing persistent non-valvular atrial fibrillation. Int. J. Cardiovasc. Imaging 2019, 35, 1587–1596. [Google Scholar] [CrossRef]
- Santos, A.B.; Roca, G.Q.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Fang, J.C.; Zile, M.R.; Pitt, B.; Solomon, S.D.; et al. Prognostic Relevance of Left Atrial Dysfunction in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2016, 9, e002763. [Google Scholar] [CrossRef] [PubMed]
- Agoston, G.; Gargani, L.; Miglioranza, M.H.; Caputo, M.; Badano, L.P.; Moreo, A.; Muraru, D.; Mondillo, S.; Pignone, A.M.; Cerinic, M.M.; et al. Left atrial dysfunction detected by speckle tracking in patients with systemic sclerosis. Cardiovasc. Ultrasound 2014, 12, 30. [Google Scholar] [CrossRef]
- Esposito, R.; Russo, C.; Santoro, C.; Cocozza, S.; Riccio, E.; Sorrentino, R.; Pontillo, G.; Luciano, F.; Imbriaco, M.; Brunetti, A.; et al. Association between Left Atrial Deformation and Brain Involvement in Patients with Anderson-Fabry Disease at Diagnosis. J. Clin. Med. 2020, 9, 2741. [Google Scholar] [CrossRef]
- Morris, D.A.; Belyavskiy, E.; Aravind-Kumar, R.; Kropf, M.; Frydas, A.; Braunauer, K.; Marquez, E.; Krisper, M.; Lindhorst, R.; Osmanoglou, E.; et al. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1405–1415. [Google Scholar] [CrossRef]
- Nagueh, S.F. Non-invasive assessment of left ventricular filling pressure. Eur. J. Heart Fail. 2018, 20, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Medvedofsky, D.; Mediratta, A.; Balaney, B.; Kruse, E.; Ciszek, B.; Shah, A.P.; Blair, J.E.; Maffessanti, F.; Addetia, K.; et al. Peak left atrial strain as a single measure for the non-invasive assessment of left ventricular filling pressures. Int. J. Cardiovasc. Imaging 2019, 35, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, A.; Johnson, J.; Hage, C.; Bäck, M.; Merkely, B.; Venkateshvaran, A.; Lund, L.H.; Nagy, A.I.; Manouras, A. Left atrial strain improves estimation of filling pressures in heart failure: A simultaneous echocardiographic and invasive haemodynamic study. Clin. Res. Cardiol. 2019, 108, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Amende, I.; Simon, R.; Seegers, A.; Daniel, W.; Heublein, B.; Hetzer, R.; Haverich, A.; Hood, W.P.; Lichtlen, P.R.; Schützenmeister, R. Diastolic dysfunction during acute cardiac allograft rejection. Circulation 1990, 81, III66–III70. [Google Scholar] [PubMed]
- Chamberlain, R.; Edwards, N.F.A.; Scalia, G.M.; Chan, J. Novel left and right ventricular strain analysis to detect subclinical myocardial dysfunction in cardiac allograft rejection. Int. J. Cardiovasc. Imaging 2021, 38, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.R.; Dipchand, A.; Starling, R.; Anderson, A.; Chan, M.; Desai, S.; Fedson, S.; Fisher, P.; Gonzales-Stawinski, G.; Martinelli, L.; et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2010, 29, 914–956. [Google Scholar] [CrossRef]
- Stewart, S.; Winters, G.L.; Fishbein, M.C.; Tazelaar, H.D.; Kobashigawa, J.; Abrams, J.; Andersen, C.B.; Angelini, A.; Berry, G.J.; Burke, M.M.; et al. Revision of the 1990 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Heart Rejection. J. Heart Lung Transplant. 2005, 24, 1710–1720. [Google Scholar] [CrossRef]
- Peled, Y.; Lavee, J.; Ram, E.; Kassif, Y.; Peled, A.; Freimark, D.; Ofek, E.; Kogan, A. Recurrent acute cellular rejection graded ISHLT 1R early after heart transplantation negatively affects long-term outcomes: The prognostic significance of 1990 ISHLT grades 1B and 2. Transpl. Immunol. 2019, 55, 101204. [Google Scholar] [CrossRef]
- Estep, J.D.; Shah, D.J.; Nagueh, S.F.; Mahmarian, J.J.; Torre-Amione, G.; Zoghbi, W.A. The Role of Multimodality Cardiac Imaging in the Transplanted Heart. JACC Cardiovasc. Imaging 2009, 2, 1126–1140. [Google Scholar] [CrossRef] [Green Version]
- Clemmensen, T.S.; Løgstrup, B.B.; Eiskjaer, H.; Høyer, S.; Poulsen, S.H. The long-term influence of repetitive cellular cardiac rejections on left ventricular longitudinal myocardial deformation in heart transplant recipients. Transpl. Int. 2015, 28, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Ortiz, M.; Peña, M.L.; Mesa, D.; Delgado, M.; Romo, E.; Santisteban, M.; Puentes, M.; López Granados, A.; Castillo, J.C.; Arizón, J.M.; et al. Impact of Asymptomatic Acute Cellular Rejection on Left Ventricle Myocardial Function Evaluated by Means of Two-Dimensional Speckle Tracking Echocardiography in Heart Transplant Recipients. Echocardiography 2015, 32, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Goirigolzarri Artaza, J.; Mingo Santos, S.; Larrañaga, J.M.; Osa, A.; Sutil-Vega, M.; Ruiz Ortiz, M.; Corros, C.; Vidal, B.; Moñivas Palomero, V.; Maneiro, N.; et al. Validation of the usefulness of 2-dimensional strain parameters to exclude acute rejection after heart transplantation: A multicenter study. Rev. Esp. Cardiol. 2020, 74, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Elkaryoni, A.; Altibi, A.M.; Khan, M.S.; Okasha, O.; Ellakany, K.; Hassan, A.; Singh, A.; Qarajeh, R.; Mehta, S.; Nanda, N.C.; et al. Global longitudinal strain assessment of the left ventricle by speckle tracking echocardiography detects acute cellular rejection in orthotopic heart transplant recipients: A systematic review and meta-analysis. Echocardiography 2020, 37, 302–309. [Google Scholar] [CrossRef]
- Eleid, M.F.; Caracciolo, G.; Cho, E.J.; Scott, R.L.; Steidley, D.E.; Wilansky, S.; Arabia, F.A.; Khandheria, B.K.; Sengupta, P.P. Natural History of Left Ventricular Mechanics in Transplanted Hearts: Relationships with Clinical Variables and Genetic Expression Profiles of Allograft Rejection. JACC Cardiovasc. Imaging 2010, 3, 989–1000. [Google Scholar] [CrossRef]
- Ambardekar, A.V.; Alluri, N.; Patel, A.C.; Lindenfeld, J.; Dorosz, J.L. Myocardial Strain and Strain Rate from Speckle-Tracking Echocardiography are Unable to Differentiate Asymptomatic Biopsy-Proven Cellular Rejection in the First Year after Cardiac Transplantation. J. Am. Soc. Echocardiogr. 2015, 28, 478–485. [Google Scholar] [CrossRef]
- Yeh, J.; Aiyagari, R.; Gajarski, R.J.; Zamberlan, M.C.; Lu, J.C. Left Atrial Deformation Predicts Pulmonary Capillary Wedge Pressure in Pediatric Heart Transplant Recipients. Echocardiography 2015, 32, 535–540. [Google Scholar] [CrossRef]
- Loar, R.W.; Pignatelli, R.H.; Morris, S.A.; Colquitt, J.L.; Feagin, D.K.; Denfield, S.W.; Tunuguntla, H.P. Left Atrial Strain Correlates with Elevated Filling Pressures in Pediatric Heart Transplantation Recipients. J. Am. Soc. Echocardiogr. 2020, 33, 504–511.e11. [Google Scholar] [CrossRef]
- Ruiz-Ortiz, M.; Rodriguez-Diego, S.; Delgado, M.; Kim, J.; Weinsaft, J.W.; Ortega, R.; Carnero, L.; Sánchez, J.J.; Carrasco, F.; López-Aguilera, J.; et al. Myocardial deformation and acute cellular rejection after heart transplantation: Impact of inter-vendor variability in diagnostic effectiveness. Echocardiography 2019, 36, 2185–2194. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Bech-Hanssen, O.; Pergola, V.; Al-Admawi, M.; Fadel, B.M.; Di Salvo, G. Atrial function in heart transplant recipients operated with the bicaval technique. Scand. Cardiovasc. J. 2015, 50, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; García-Izquierdo, E.; Chetrit, M.; Moñivas-Palomero, V.; Mingo-Santos, S.; Andersen, S.; Gude, E.; et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur. Heart J.-Cardiovasc. Imaging 2021, 23, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Flachskampf, F.A.; Baron, T. Left atrial strain: Evaluating left ventricular filling pressure from an upstream vantage point. Eur. Heart J.-Cardiovasc. Imaging 2021, 23, 71–73. [Google Scholar] [CrossRef]
- Lu, J.C.; Magdo, H.S.; Yu, S.; Lowery, R.; Aiyagari, R.; Zamberlan, M.; Gajarski, R.J. Usefulness of Diastolic Strain Measurements in Predicting Elevated Left Ventricular Filling Pressure and Risk of Rejection or Coronary Artery Vasculopathy in Pediatric Heart Transplant Recipients. Am. J. Cardiol. 2016, 117, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Ortiz, M.; Rodríguez Diego, S.; Delgado Ortega, M.; Sánchez Fernández, J.J.; Ortega Salas, R.; Carnero Montoro, L.; Carrasco Ávalos, F.; López Aguilera, J.; López Granados, A.; Arizón Del Prado, J.M.; et al. Tissue Doppler velocities for ruling out rejection in heart transplant recipients in the context of myocardial strain imaging: A multivariate, prospective, single-center study. Int. J. Cardiovasc. Imaging 2020, 36, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Robinet, S.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Kacharava, G.; Athanassopoulos, G.D.; et al. Echocardiographic reference ranges for normal left atrial function parameters: Results from the EACVI NORRE study. Eur. Heart J.-Cardiovasc. Imaging 2018, 19, 630–638. [Google Scholar] [CrossRef] [Green Version]




| Grade of Rejection (ISHLT 2005) | p Value | ||||||
|---|---|---|---|---|---|---|---|
| Software-Variable | 0R | 1R | 2R–3R | Overall * | 0R vs. 1R ** | 0R vs. 2R–3R ** | 1R vs. 2R–3R ** |
| TT-ESALSg (%) | 17.5 ± 6.0 | 13.0 ± 5.2 | 13.5 ± 5.4 | <0.0005 | <0.0005 | 0.037 | 0.94 |
| TT-PALS (%) | 19.1 ± 6.2 | 14.2 ± 5.4 | 13.9 ± 5.1 | <0.0005 | <0.0005 | 0.006 | 0.98 |
| TT-ESALS (%) | 17.5 ± 6.0 | 13.0 ± 5.1 | 13.3 ± 5.4 | <0.0005 | <0.0005 | 0.025 | 0.98 |
| TT-PALSR (1/s) | 1.0 ± 0.4 | 0.8 ± 0.3 | 0.7 ± 0.2 | 0.001 | 0.004 | 0.012 | 0.70 |
| S-PALS (%) | 19.4 ± 7.5 | 15.9 ± 6.4 | 14.6 ± 6.2 | 0.013 | 0.040 | 0.031 | 0.75 |
| S-PALSR (1/s) | 1.5 ± 0.4 | 1.3 ± 0.5 | 1.3 ± 0.4 | 0.018 | 0.018 | 0.15 | 0.97 |
| Software-Variable | 0R | Any Grade Rejection | p a | 0R–1R | TR-ACR | p b |
|---|---|---|---|---|---|---|
| TT-ESALSg (%) | 17.5 ± 6.0 | 13.1 ± 5.2 | <0.0005 | 15.3 ± 6.1 | 13.5 ± 5.4 | 0.27 |
| TT-PALS (%) | 19.1 ± 6.2 | 14.1 ± 5.4 | <0.0005 | 16.7 ± 6.3 | 13.9 ± 5.7 | 0.10 |
| TT-ESALS (%) | 17.5 ± 6.0 | 13.1 ± 5.1 | <0.0005 | 15.3 ± 6.0 | 13.3 ± 5.4 | 0.21 |
| TT-PALSR (1/s) | 1.0 ± 0.4 | 0.7 [0.6–1.0] | <0.0005 | 0.8 [0.6–1.1] | 0.7 ± 0.2 | 0.053 |
| S-PALS (%) | 19.4 ± 7.4 | 14.4 [11.6–7.2] | 0.006 | 15.6 [12.5–21.9] | 14.6 ± 6.2 | 0.07 |
| S-PALSR (1/s) | 1.5 ± 0.4 | 1.3 ± 0.5 | 0.005 | 1.4 ± 0.5 | 1.3 ± 0.4 | 0.44 |
| Software | Variability | Parameter | ICC (95% CI) | p-Value | Bias (%) | Bland–Altman Limits of Agreement (%) |
|---|---|---|---|---|---|---|
| Siemens | Intra-observer | PALS | 0.94 (0.77 to 0.98) | <0.0005 | 1.76 | −4.42 to 7.95 |
| PALSR | 0.84 (0.47 to 0.95) | 0.003 | −0.13 | −1.14 to 0.89 | ||
| Inter-observer | PALS | 0.80 (0.30 to 0.94) | 0.007 | 0.70 | −12.16 to 13.56 | |
| PALSR | 0.77 (0.17 to 0.94) | 0.01 | 0.01 | −0.97 to 0.99 | ||
| TomTec | Intra-observer | PALS | 0.88 (0.50 to 0.97) | 0.003 | −0.23 | −8.49 to 8.03 |
| PALSR | 0.89 (0.58 to 0.97) | 0.002 | −0.06 | −0.46 to 0.34 | ||
| Inter-observer | PALS | 0.87 (0.51 to 0.97) | 0.003 | 1.58 | −7.26 to 10.42 | |
| PALSR | 0.74 (−0.14 to 0.94) | 0.04 | −0.01 | −0.59 to 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Diego, S.; Ruiz-Ortiz, M.; Delgado-Ortega, M.; Kim, J.; Weinsaft, J.W.; Sánchez-Fernández, J.J.; Ortega-Salas, R.; Carnero-Montoro, L.; Carrasco-Ávalos, F.; López-Aguilera, J.; et al. The Role of Left Atrial Longitudinal Strain in the Diagnosis of Acute Cellular Rejection in Heart Transplant Recipients. J. Clin. Med. 2022, 11, 4987. https://doi.org/10.3390/jcm11174987
Rodríguez-Diego S, Ruiz-Ortiz M, Delgado-Ortega M, Kim J, Weinsaft JW, Sánchez-Fernández JJ, Ortega-Salas R, Carnero-Montoro L, Carrasco-Ávalos F, López-Aguilera J, et al. The Role of Left Atrial Longitudinal Strain in the Diagnosis of Acute Cellular Rejection in Heart Transplant Recipients. Journal of Clinical Medicine. 2022; 11(17):4987. https://doi.org/10.3390/jcm11174987
Chicago/Turabian StyleRodríguez-Diego, Sara, Martín Ruiz-Ortiz, Mónica Delgado-Ortega, Jiwon Kim, Jonathan W. Weinsaft, José J. Sánchez-Fernández, Rosa Ortega-Salas, Lucía Carnero-Montoro, Francisco Carrasco-Ávalos, José López-Aguilera, and et al. 2022. "The Role of Left Atrial Longitudinal Strain in the Diagnosis of Acute Cellular Rejection in Heart Transplant Recipients" Journal of Clinical Medicine 11, no. 17: 4987. https://doi.org/10.3390/jcm11174987






