Reassessment of Surgical Procedures for Complex Obstructive Genital Malformations: A Case Series on Different Surgical Approaches
Abstract
:1. Introduction
2. Materials and Methods
- Diagnostic and surgical strategy:
- Definition of outcome parameters:
- 1.
- Surgical outcome parameters:
- 1.1
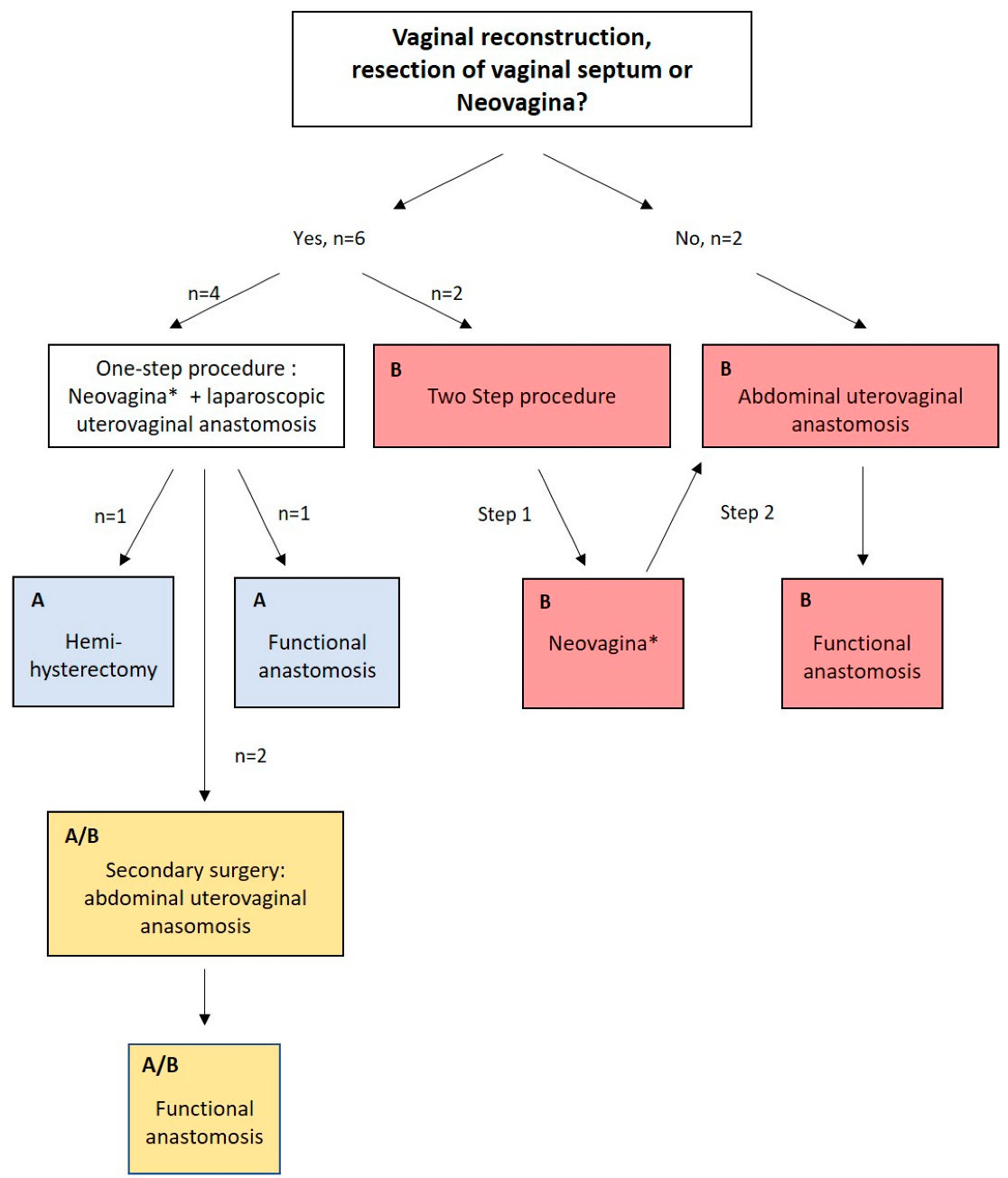
- Primary surgical strategy
- 1.2
- Number of surgeries in total
- 2.
- Clinical outcome parameters concerning vagina and anastomosis
- 2.1
- Vaginal length and sexual intercourse;
- 2.2
- Orthograde menstruation;
- 2.3
- Sonographic exclusion of hematometra/-kolpos;
- 2.4
- Restenosis or reoccurring hematometra; and
- 2.5
- Complications.
3. Results and Presentation of Cases
3.1. Patient Characteristics
3.2. Surgical Outcome Parameters
3.3. Clinical Outcome Parameters
3.4. Presentation of Cases
3.4.1. One-Step Approach, Method A
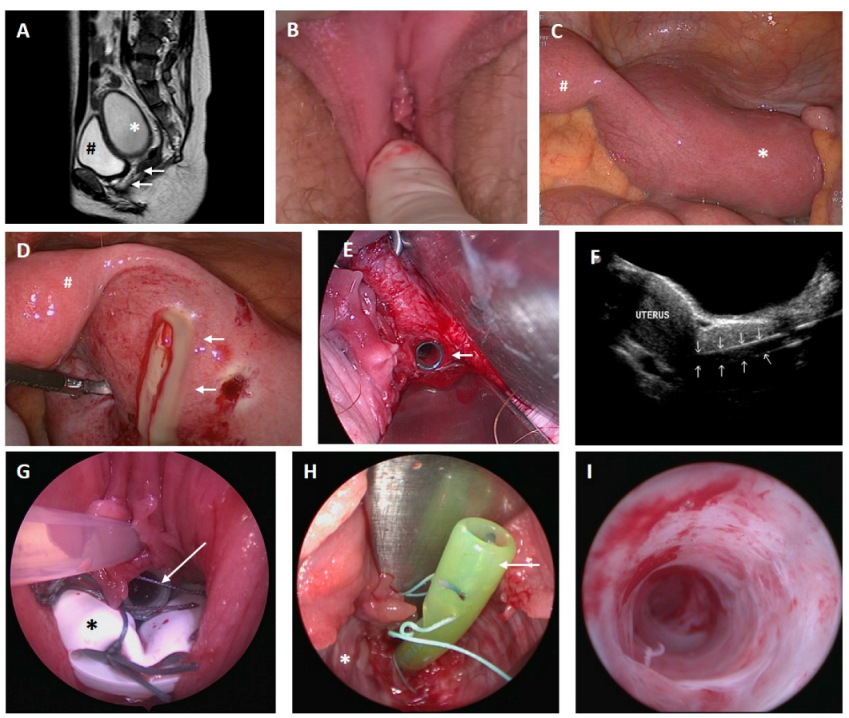
- Case 1
- Case 2
3.4.2. One-Step Approach with Secondary Abdominal Uterovaginal Anastomosis Due to Reobstruction, Method A/B
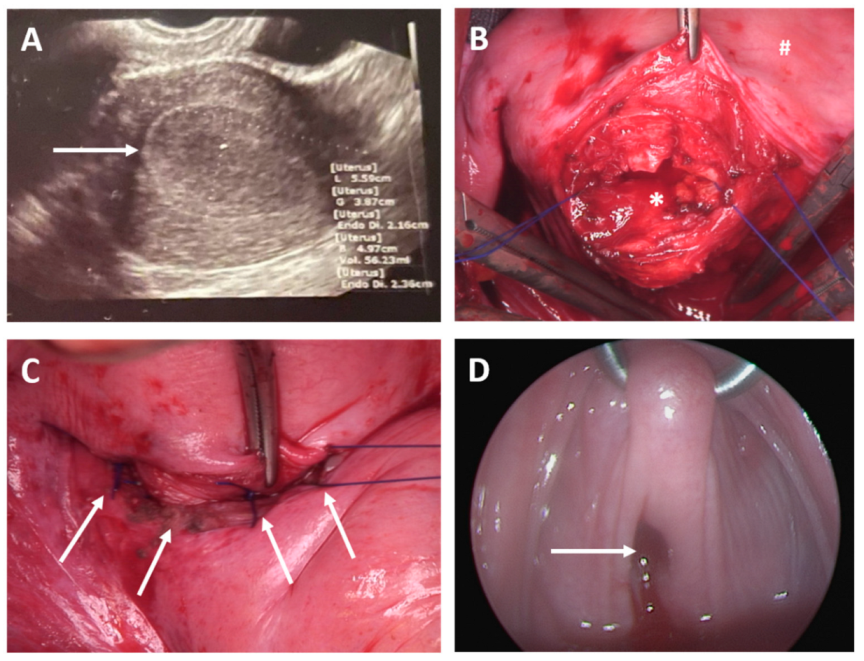
- Case 3
- Case 4
3.4.3. Two-Step/Open Abdominal Approach, Method B
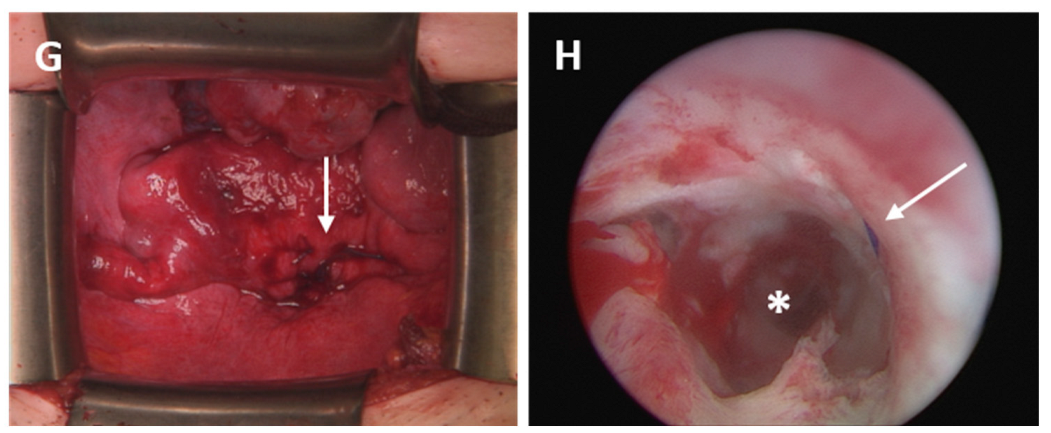
- Case 5
- Case 6
- Case 7
- Case 8
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Reichman, D.E.; Laufer, M.R. Congenital uterine anomalies affecting reproduction. Best Pract. Res. Clin. Obstet. Gynaecol. 2010, 24, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Leitlinienprogramm der DGGG, OEGGG und SGGG 2018, Weibliche Genitale Fehlbildungen; AWMF Registernummer 015/052, Leitlinienklasse S2k, Stand 02/2020, Version 1.0. 2020. Available online: https://www.awmf.org/uploads/tx_szleitlinien/015-052l_S1_Weibliche_genitale_Fehlbildungen_2020-06.pdf (accessed on 19 June 2022).
- Sadler, T.W. Medizinische Embryologie; Georg Thieme Verlag KG: Stuttgart, Germany, 2008; Volume 11, p. 529. [Google Scholar]
- Santana Gonzalez, L.; Artibani, M.; Ahmed, A.A. Studying Mullerian duct anomalies—From cataloguing phenotypes to discovering causation. Dis. Model Mech. 2021, 14, dmm047977. [Google Scholar] [CrossRef] [PubMed]
- Grimbizis, G.F.; Gordts, S.; Sardo, A.D.S.; Brucker, S.; De Angelis, C.; Gergolet, M.; Li, T.-C.; Tanos, V.; Brölmann, H.; Gianaroli, L.; et al. The ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies. Hum. Reprod. 2013, 28, 2032–2044. [Google Scholar] [CrossRef]
- Fujino, K.; Ikemoto, Y.; Kitade, M.; Takeda, S. Novel Method of Cervicoplasty Using Autologous Peritoneum for Cervicovaginal Atresia. Surg. J. 2020, 6, e28–e32. [Google Scholar] [CrossRef]
- Roberts, C.P.; Rock, J.A. Surgical methods in the treatment of congenital anomalies of the uterine cervix. Curr. Opin. Obstet. Gynecol. 2011, 23, 251–257. [Google Scholar] [CrossRef]
- Acien, P.; Acien, M.I. The history of female genital tract malformation classifications and proposal of an updated system. Hum. Reprod. Update 2011, 17, 693–705. [Google Scholar] [CrossRef]
- Mikos, T.; Gordts, S.; Grimbizis, G.F. Current knowledge about the management of congenital cervical malformations: A literature review. Fertil. Steril. 2020, 113, 723–732. [Google Scholar] [CrossRef]
- Rock, J.A.; Roberts, C.P.; Jones, H.W., Jr. Congenital anomalies of the uterine cervix: Lessons from 30 cases managed clinically by a common protocol. Fertil. Steril. 2010, 94, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Minami, C.; Tsunematsu, R.; Hiasa, K.; Egashira, K.; Kato, K. Successful Surgical Treatment for Congenital Vaginal Agenesis Accompanied by Functional Uterus: A Report of Two Cases. Gynecol. Minim. Invasive Ther. 2019, 8, 76–79. [Google Scholar]
- Schöller, D.; Hölting, M.; Stefanescu, D.; Burow, H.; Schönfisch, B.; Rall, K.; Taran, F.-A.; Grimbizis, G.F.; Sardo, A.D.S.; Brucker, S.Y. Female genital tract congenital malformations and the applicability of the ESHRE/ESGE classification: A systematic retrospective analysis of 920 patients. Arch. Gynecol. Obstet. 2018, 297, 1473–1481. [Google Scholar] [CrossRef]
- Marzieh, G.; Soodabeh, D.; Narges, I.-M.; Saghar, S.-S.; Sara, E. Vaginal reconstruction using no grafts with evidence of squamous epithelialization in neovaginal vault: A simple approach. J. Obstet. Gynaecol. Res. 2011, 37, 195–201. [Google Scholar] [CrossRef]
- Banister, J.B.; McIndoe, A.H. Congenital Absence of the Vagina, treated by Means of an Indwelling Skin-Graft. Proc. R. Soc. Med. 1938, 31, 1055–1056. [Google Scholar] [CrossRef] [PubMed]
- Wallwiener, D.J.W.; Kreienberg, R.; Friese, K.; Didrich, K.; Beckmann, M.W. Atlas der Gynäkologischen Operationen; Georg Thieme Verlag KG: Stuttgart, Germany, 2009; Volume 7. [Google Scholar]
- van Bijsterveldt, C.; Willemsen, W. Treatment of patients with a congenital transversal vaginal septum or a partial aplasia of the vagina. The vaginal pull-through versus the push-through technique. J. Pediatric Adolesc. Gynecol. 2009, 22, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.; Crouch, N.S.; Minto, C.L.; Liao, L.M.; Creighton, S.M. Female genital appearance: “normality” unfolds. BJOG 2005, 112, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Grimbizis, G.F.; Tsalikis, T.; Mikos, T.; Papadopoulos, N.; Tarlatzis, B.C.; Bontis, J.N. Successful end-to-end cervico-cervical anastomosis in a patient with congenital cervical fragmentation: Case report. Hum. Reprod. 2004, 19, 1204–1210. [Google Scholar] [CrossRef]
- Rall, K.; Schenk, B.; Schäffeler, N.; Schöller, D.; Kölle, A.; Schönfisch, B.; Brucker, S.Y. Long Term Findings Concerning the Mental and Physical Condition, Quality of Life and Sexuality after Laparoscopically Assisted Creation of a Neovagina (Modified Vecchietti Technique) in Young MRKHS (Mayer-Rokitansky-Kuster-Hauser-Syndrome) Patients. J. Clin. Med. 2021, 10, 1269. [Google Scholar] [CrossRef]
- Rall, K.; Schickner, M.C.; Barresi, G.; Schönfisch, B.; Wallwiener, M.; Wallwiener, C.W.; Wallwiener, D.; Brucker, S.Y. Laparoscopically assisted neovaginoplasty in vaginal agenesis: A long-term outcome study in 240 patients. J. Pediatric Adolesc. Gynecol. 2014, 27, 379–385. [Google Scholar] [CrossRef]
- Deffarges, J.V.; Haddad, B.; Musset, R.; Paniel, B.J. Utero-vaginal anastomosis in women with uterine cervix atresia: Long-term follow-up and reproductive performance. A study of 18 cases. Hum. Reprod. 2001, 16, 1722–1725. [Google Scholar] [CrossRef]
- Brucker, S.Y.; Gegusch, M.; Zubke, W.; Rall, K.; Gauwerky, J.F.; Wallwiener, D. Neovagina creation in vaginal agenesis: Development of a new laparoscopic Vecchietti-based procedure and optimized instruments in a prospective comparative interventional study in 101 patients. Fertil. Steril. 2008, 90, 1940–1952. [Google Scholar] [CrossRef]
- Ellerkamp, V.; Rall, K.K.; Schaefer, J.; Stefanescu, D.; Schoeller, D.; Brucker, S.; Fuchs, J. Surgical Therapy After Failed Feminizing Genitoplasty in Young Adults With Disorders of Sex Development: Retrospective Analysis and Review of the Literature. J. Sex. Med. 2021, 18, 1797–1806. [Google Scholar] [CrossRef]
- Kraiem, S.; Zoukar, O.; Hnayin, A.; Zouari, A.; Faleh, R.; Haddad, A. Complete cervical agenesis: Successful surgical treatment: One case report. Pan Afr. Med J. 2020, 36, 211. [Google Scholar] [CrossRef] [PubMed]
- Jasonni, V.M.; La Marca, A.; Matonti, G. Utero-vaginal anastomosis in the treatment of cervical atresia. Acta Obstet. Gynecol. Scand. 2007, 86, 1517–1518. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Fouad, R.; Elsetohy, K.A.; Hashem, A.T.; AbdAllah, A.A.; Fathi, A.I. Uterovaginal Anastomosis for Cases of Cryptomenorrhea Due to Cervical Atresia with Vaginal Aplasia: Benefits and Risks. J. Pediatric Adolesc. Gynecol. 2017, 30, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, A.; Karateke, A.; Haliloglu, B. Abdominal surgical approach to a case of complete cervical and partial vaginal agenesis. Fertil. Steril. 2005, 84, 217. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.R.; Mohapatra, I. Cervical Dysgenesis: A Rare Mullerian Duct Anomaly. Cureus 2021, 13, e18279. [Google Scholar] [CrossRef] [PubMed]
- Romanski, P.A.; Bortoletto, P.; Pfeifer, S.M. Unilateral Obstructed Mullerian Anomalies: A Series of Unusual Variants of Known Anomalies. J. Pediatric Adolesc. Gynecol. 2021, 34, 749–757. [Google Scholar] [CrossRef]
- Kudela, G.; Wiernik, A.; Drosdzol-Cop, A.; Machnikowska-Sokołowska, M.; Gawlik, A.; Hyla-Klekot, L.; Gruszczyńska, K.; Koszutski, T. Multiple variants of obstructed hemivagina and ipsilateral renal anomaly (OHVIRA) syndrome—One clinical center case series and the systematic review of 734 cases. J. Pediatric Urol. 2021, 17, 653.e1–653.e9. [Google Scholar] [CrossRef]

| Case Number | Age at Initial Presentation | ESHRE/ESGE | ASRM | Associated Malformations | Clinical Presentation | Endometriosis at Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 17 | U3b C3 V3 / OHVIRA | Uterus didelphis R unilateral cervical agenesis Mid vaginal septum/OHVIRA | Unilateral renal aplasia right side | Hematometra | no |
| 2 | 13 | U4a/C4/V4 | R unicornuate uterus with L associated atrophic uterine remnant Cervical agenesis Distal vaginal agenesis | Multiple malformations: double kidney on both sides, skeletal malformations, anal atresia, tetralogy of fallot | Acute abdominal pain, pyometra | no |
| 3 | 12 | U4b C0 V3/4 | L unicornuate uterus Normal cervix Distal vaginal septum | Renal agenesis right side | Abdominal pain | no |
| 4 | 14 | U4a C4 V3 | R unicornuate with L associated atrophic uterine remnant Cervical agenesis Distal vaginal agenesis | Arterial septum defect and venticel septum defect, Arteria lusoria, konnatal hypothyroidism | Recurrent hematometra after surgery in other clinic | no |
| 5 | 16 | U0 C0 V4 | Normal uterus Normal cervix Distal vaginal agenesis | - | Assumed vaginal septum, primary amenorrhea | yes |
| 6 | 20 | U0 C4, V0 | Normal Uterus Cervical agenesis Normal vagina | - | Request for treatment, diagnosis at other clinic | yes |
| 7 | 17 | U1a C4 V3/4 | T-shaped uterus Cervical agenesis Distal vaginal agenesis | - | Primary amenorrhea | yes |
| 8 | 11 | U0 C4 V0 | Normal Uterus Cervical agenesis Normal vagina | - | Primary amenorrhea, pelvic pain | yes |
| Case Number | ESHRE/ESGE | Treatment Algorithm | Diagnostic Surgery | Prior Surgery for Neovagina * | Date of Uterovaginal Anastomosis | Number of Surgeries for Uterovaginal Anastomosis | Planned Follow-Up Hysteroscopy And Cervical Dilation | Unplanned Hysteroscopy and Cervical Dilation | Secondary Surgery | Unplanned Hysteroscopy and Cervical Dilation | Total Number of Surgeries |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | U3b C3 V3/OHVIRA | A | 0 | no | 28 April 2015 | 1 | 2 | 0 | 3 | 1 | 7 |
| 2 | U4a/C4/V4 | A | 1 | no | 1 February 2012 | 1 | 0 | 0 | 1 | 3 | 6 |
| 3 | U4b C0 V3/4 | A/B | 2 ** | no | 15 January 2015 15 September 2020 | 2 | 1 | 0 | 1 | 5 | 11 |
| 4 | U4a C4 V3 | A/B | 0 | no | July 2017 9 July 2019 | 2 | 1 | 1 | 2 | 1 | 7 |
| 5 | U0 C0 V4 | B | 0 | yes | 2 March 2021 | 1 | 1 | 3 | 0 | 0 | 5 |
| 6 | U0, C4, V0 | B | 1 ** | no | 2 February 2022 | 1 | 1 | 0 | 0 | 0 | 3 |
| 7 | U1a C4 V3/4 | B | 2 *** | yes ** | 16 February 2022 | 1 | 1 | 0 | 0 | 0 | 4 |
| 8 | U0 C4 V0 | B | 2 | no | 27 September 2017 | 1 | 0 | 0 | 0 | 0 | 3 |
| Case Number | Date of Uterovaginal Anastomosis | Latest Examination | Vaginal Lenght | Sexual Intercourse | Orthograde Menstruation | Absence of Hematometra | Re-Stenosis and/ or Hematometra | Hysterectomy | Complications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 April 2015 | 7 January 2019 | n * | lost to follow up | normal menstruation normal left sided hemi uterus and cervix | yes, but pyometra right uterus | recurrent pyometra right hemiuterus | right sided hemi-hysterectomy, preserving the left-sided functional hemiuterus | - |
| 2 | 26 January 2012 | 29 October 2018 | n * | satisfying | yes, regular cycle | yes | yes, 3 × surgery for vaginal and/or cervical dilation, pyokolpos, sactosalpinx | - | - |
| 3 | 15 January 2015 15 September 2020 | 11 April 2021 | n * | satisfying | no, hormonal therapy | yes | recurrent vaginal discharge | - | - |
| 4 | July 2017 9 July 2019 | 14 March 2022 | n * | not to date | yes | yes | - | - | - |
| 5 | 2 March 2021 | 3 January 2022 | n * | not to date | pending | yes | yes, 3 × dilation | - | - |
| 6 | 2 February 2022 | 15 March 2022 | n ** | satisfying | pending | yes | pelvic pain and hematormetra after weaning from hormonal contraception | - | - |
| 7 | 16 February 2022 | 22 March 2022 | n ** | not to date | pending | yes | - | - | |
| 8 | 27 September 2017 | 30 October 2017 | n ** | lost to follow up | lost to follow-up | yes | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoeller, A.; Steinmacher, S.; Schlammerl, K.; Hoopmann, M.; Reisenauer, C.; Hattermann, V.; Brucker, S.Y.; Rall, K. Reassessment of Surgical Procedures for Complex Obstructive Genital Malformations: A Case Series on Different Surgical Approaches. J. Clin. Med. 2022, 11, 5026. https://doi.org/10.3390/jcm11175026
Hoeller A, Steinmacher S, Schlammerl K, Hoopmann M, Reisenauer C, Hattermann V, Brucker SY, Rall K. Reassessment of Surgical Procedures for Complex Obstructive Genital Malformations: A Case Series on Different Surgical Approaches. Journal of Clinical Medicine. 2022; 11(17):5026. https://doi.org/10.3390/jcm11175026
Chicago/Turabian StyleHoeller, Alice, Sahra Steinmacher, Katharina Schlammerl, Markus Hoopmann, Christl Reisenauer, Valerie Hattermann, Sara Y. Brucker, and Katharina Rall. 2022. "Reassessment of Surgical Procedures for Complex Obstructive Genital Malformations: A Case Series on Different Surgical Approaches" Journal of Clinical Medicine 11, no. 17: 5026. https://doi.org/10.3390/jcm11175026
APA StyleHoeller, A., Steinmacher, S., Schlammerl, K., Hoopmann, M., Reisenauer, C., Hattermann, V., Brucker, S. Y., & Rall, K. (2022). Reassessment of Surgical Procedures for Complex Obstructive Genital Malformations: A Case Series on Different Surgical Approaches. Journal of Clinical Medicine, 11(17), 5026. https://doi.org/10.3390/jcm11175026







