Infective Endocarditis by Pasteurella Species: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Search
2.2. Study Selection
2.3. Outcomes of Interest
2.4. Data Extraction and Definitions
2.5. Statistical Analysis
3. Results
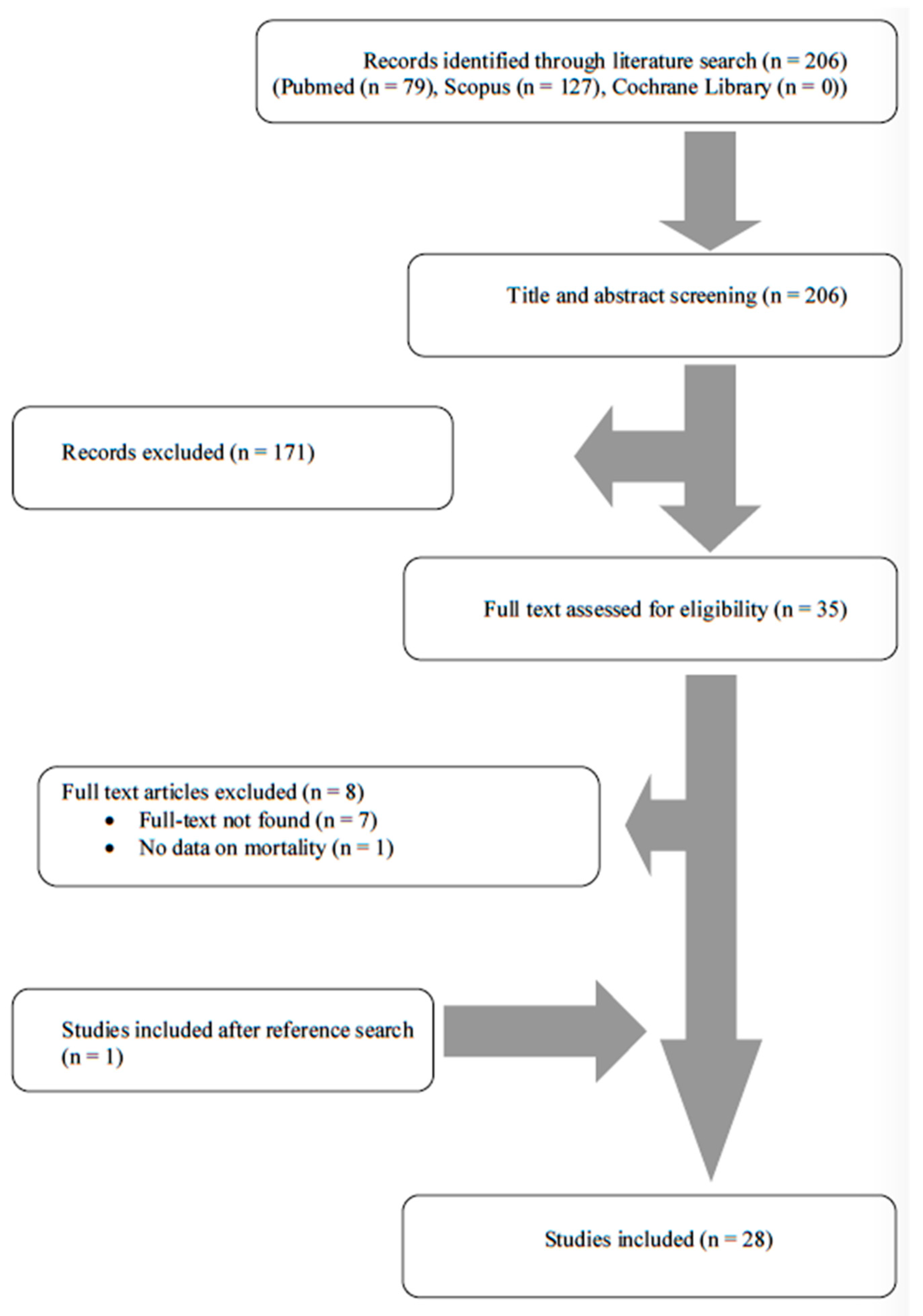
3.1. Literature Search
3.2. Included Studies’ Characteristics
3.3. Epidemiology of IE by Pasteurella Spp.
3.4. Microbiology of IE by Pasteurella Spp.
3.5. Diagnosis of IE by Pasteurella Spp.
3.6. Clinical Characteristics of IE by Pasteurella Spp.
3.7. Treatment and Outcomes of IE by Pasteurella Spp.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, J.E.; Dolin, E.; Blaser, M.J. Mandell, Douglas, And Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Elsevier: Philadelphia, PA, USA, 2019. [Google Scholar]
- Zbinden, R. Aggregatibacter, Capnocytophaga, Eikenella, Kingella, Pasteurella, and Other Fastidious or Rarely Encountered Gram-Negative Rods. In Manual of Clinical Microbiology; Jorgensen, J.H., Carroll, K.C., Funke, G., Pfaller, M.A., Landry, M.L., Richter, S.S., Warnock, D.W., Eds.; ASM Press: Washington, DC, USA, 2015; pp. 652–666. ISBN 978-1-68367-280-7. [Google Scholar]
- Townsend, K.M.; Boyce, J.D.; Chung, J.Y.; Frost, A.J.; Adler, B. Genetic Organization of Pasteurella multocida Cap Loci and Development of a Multiplex Capsular PCR Typing System. J. Clin. Microbiol. 2001, 39, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.D.; Ajam, N.; Blackall, P.J.; Asiah, N.M.; Ramlan, M.; Maria, J.; Yuslan, S.; Thong, K.L. Capsular Serotyping of Pasteurella multocida from Various Animal Hosts—A Comparison of Phenotypic and Genotypic Methods. Trop. Biomed. 2011, 28, 55–63. [Google Scholar] [PubMed]
- Wilson, B.A.; Ho, M. Pasteurella multocida: From Zoonosis to Cellular Microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Gaca, J.G.; Chu, V.H. Management Considerations in Infective Endocarditis: A Review. JAMA 2018, 320, 72–83. [Google Scholar] [CrossRef]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical Presentation, Aetiology and Outcome of Infective Endocarditis. Results of the ESC-EORP EURO-ENDO (European Infective Endocarditis) Registry: A Prospective Cohort Study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef]
- Shah, A.S.V.; McAllister, D.A.; Gallacher, P.; Astengo, F.; Rodríguez Pérez, J.A.; Hall, J.; Lee, K.K.; Bing, R.; Anand, A.; Nathwani, D.; et al. Incidence, Microbiology, and Outcomes in Patients Hospitalized with Infective Endocarditis. Circulation 2020, 141, 2067–2077. [Google Scholar] [CrossRef]
- Cresti, A.; Chiavarelli, M.; Scalese, M.; Nencioni, C.; Valentini, S.; Guerrini, F.; D’Aiello, I.; Picchi, A.; De Sensi, F.; Habib, G. Epidemiological and Mortality Trends in Infective Endocarditis, a 17-Year Population-Based Prospective Study. Cardiovasc. Diagn. Ther. 2017, 7, 27–35. [Google Scholar] [CrossRef]
- Papakonstantinou, P.E.; Samonis, G.; Andrianaki, A.M.; Christofaki, M.; Dimopoulou, D.; Papadakis, J.; Gikas, A.; Kofteridis, D.P. Epidemiology, Microbiological and Clinical Features, Treatment, and Outcomes of Infective Endocarditis in Crete, Greece. Infect. Chemother. 2018, 50, 21–28. [Google Scholar] [CrossRef]
- Porter, R.S.; Hay, C.M. Pasteurella Endocarditis: A Case Report and Statistical Analysis of the Literature. Case Rep. Infect. Dis. 2020, 2020, 8890211. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.C.; Small, K.; Brodley, C.E.; Lau, J.; Trikalinos, T.A. Deploying an Interactive Machine Learning System in an Evidence-Based Practice Center: Abstrackr. In Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium, Miami, FL, USA, 28–30 January 2012; pp. 819–824. [Google Scholar]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE Working Group GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Doty, G.L.; Loomus, G.N.; Wolf, P.L. Pasteurella Endocarditis. N. Engl. J. Med. 1963, 268, 830–832. [Google Scholar] [CrossRef]
- Gump, D.W.; Holden, R.A. Endocarditis Caused by a New Species of Pasteurella. Ann. Intern. Med. 1972, 76, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, V.; Knutsen, S.B.; Ragnhildstveit, E.; Skagseth, E.; Solberg, C.O. Endocarditis Caused by Pasteurella multocida. Scand. J. Infect. Dis. 1977, 9, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.P.; Spurrell, J.R. Pasteurella multocida Endocarditis. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 1862–1863. [Google Scholar] [CrossRef][Green Version]
- Salmon, D.; Fantin, B.; Bricaire, F.; Vilde, J.L.; Pangon, B.; Ferand, D. Endocarditis Due to Pasteurella multocida with Glomerulonephritis. Am. J. Med. 1989, 86, 493. [Google Scholar] [CrossRef]
- Yaneza, A.L.; Jivan, H.; Kumari, P.; Togoo, M.S. Pasteurella Haemolytica Endocarditis. J. Infect. 1991, 23, 65–67. [Google Scholar] [CrossRef]
- Hombal, S.M.; Dincsoy, H.P. Pasteurella multocida Endocarditis. Am. J. Clin. Pathol. 1992, 98, 565–568. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, K.; Ikeda, U.; Ogawa, C.; Fukazawa, H.; Eto, M.; Shimada, K. Pasteurella Ureae Endocarditis. Intern. Med. 1993, 32, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Sorbello, A.F.; O’Donnell, J.; Kaiser-Smith, J.; Fitzharris, J.; Shinkarow, J.; Doneson, S. Infective Endocarditis Due to Pasteurella Dagmatis: Case Report and Review. Clin. Infect. Dis. 1994, 18, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Genne, D.; Siegrist, H.H.; Monnier, P.; Nobel, M.; Humair, L.; de Torrente, A. Pasteurella multocida Endocarditis: Report of a Case and Review of the Literature. Scand. J. Infect. Dis. 1996, 28, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Nettles, R.E.; Sexton, D.J. Pasteurella multocida Prosthetic Valve Endocarditis: Case Report and Review. Clin. Infect. Dis. 1997, 25, 920–921. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vasquez, J.E.; Ferguson, D.A.; Bin-Sagheer, S.; Myers, J.W.; Ramsak, A.; Wilson, M.A.; Sarubbi, F.A. Pasteurella multocida Endocarditis: A Molecular Epidemiological Study. Clin. Infect. Dis. 1998, 26, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Rosenbach, K.A.; Poblete, J.; Larkin, I. Prosthetic Valve Endocarditis Caused by Pasteurella Dagmatis. South Med. J. 2001, 94, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, Y.; Moriyama, Y.; Iguro, Y.; Toda, R.; Taira, A. Pasteurella multocida Endocarditis: Report of a Case. Surg. Today 2002, 32, 513–515. [Google Scholar] [CrossRef]
- Al-Ghonaim, M.A.; Abba, A.A.; Al-Nozha, M. Endocarditis Caused by Pasteurella multocida. Ann. Saudi Med. 2006, 26, 147–149. [Google Scholar] [CrossRef]
- Graf, S.; Binder, T.; Heger, M.; Apfalter, P.; Simon, N.; Winkler, S. Isolated Endocarditis of the Pulmonary Valve Caused by Pasteurella multocida. Infection 2007, 35, 43–45. [Google Scholar] [CrossRef]
- Reinsch, N.; Plicht, B.; Lind, A.; Jánosi, R.A.; Buck, T.; Kamler, M.; Jakob, H.; Naber, C.K.; Erbel, R. Recurrent Infective Endocarditis with Uncommon Gram-Negative Pasteurella multocida and Pseudomonas Aeruginosa: A Case Report. J. Heart Valve Dis. 2008, 17, 710–713. [Google Scholar]
- Naba, M.R.; Araj, G.F.; Kanafani, Z.A.; Kanj, S.S. First Case of Pasteurella multocida Endocarditis of the Tricuspid Valve: A Favorable Outcome Following Medical Treatment. Int. J. Infect. Dis. 2009, 13, e267–e269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strahm, C.; Goldenberger, D.; Gutmann, M.; Kuhnert, P.; Graber, P. Prosthetic Valve Endocarditis Caused by a Pasteurella Dagmatis-like Isolate Originating from a Patient’s Cat. J. Clin. Microbiol. 2012, 50, 2818–2819. [Google Scholar] [CrossRef]
- Tirmizi, A.; Butt, S.; Molitorisz, S. First Reported Case of Pasteurella Pneumotropica Tricuspid Valve Endocarditis. Int. J. Cardiol. 2012, 161, e44–e45. [Google Scholar] [CrossRef]
- Satta, G.; Gorton, R.L.; Kandil, H. Prosthetic Valve Endocarditis Caused by Pasteurella in a Penicillin Allergic Patient: Challenges in Diagnosis and Treatment. Infect. Dis. Rep. 2012, 4, e32. [Google Scholar] [CrossRef] [PubMed]
- Mikaberidz, N.; Li, E.Y.; Taub, C.C. Pasteurella multocida Infective Endocarditis in an Immunocompetent Patient Complicated by Rhabdomyolysis and Permanent Hearing Loss. J. Cardiovasc. Dis. Res. 2013, 4, 55–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Branch, J.; Kakutani, T.; Kuroda, S.; Shiba, Y.; Kitagawa, I. Pasteurella multocida Infective Endocarditis: A Possible Link with Primary Upper Respiratory Tract Infection. Intern. Med. 2015, 54, 3225–3231. [Google Scholar] [CrossRef]
- Guilbart, M.; Zogheib, E.; Hchikat, A.H.; Kirat, K.; Ferraz, L.; Guerin-Robardey, A.-M.; Trojette, F.; Moubarak-Daher, M.; Dupont, H. Fatal Multifocal Pasteurella multocida Infection: A Case Report. BMC Res. Notes 2015, 8, 287. [Google Scholar] [CrossRef]
- Ahlsson, A.; Friberg, Ö.; Källman, J. An Angry Cat Causing Pasteurella multocida Endocarditis and Aortic Valve Replacement-A Case Report. Int. J. Surg. Case Rep. 2016, 24, 91–93. [Google Scholar] [CrossRef]
- Ghanem, H.; Martin, C.; Farrer, W.; Sivasubramanian, G. Eustachian Valve Endocarditis Due to Pasturella Multocida—A Novel Case. Clin. Infect. Pract. 2021, 12, 100076. [Google Scholar] [CrossRef]
- Hung, W.-S.; Wu, M.; Jung, S.-M.; Chu, P.-H. Infective Endocarditis Caused by Pasteurella Aerogenes Possibly from a Dog. Clin. Infect. Pract. 2021, 12, 100107. [Google Scholar] [CrossRef]
- Wilkie, I.W.; Harper, M.; Boyce, J.D.; Adler, B. Pasteurella multocida: Diseases and Pathogenesis. Curr. Top. Microbiol. Immunol. 2012, 361, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Muntaner, L.; Suriñach, J.M.; Zuñiga, D.; De Sevilla, T.F.; Ferrer, A. Respiratory Pasteurellosis: Infection or Colonization? Scand. J. Infect. Dis. 2008, 40, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Guillard, T.; Martin, M.; Duval, V.; Brasme, L.; David, C.; Vernet-Garnier, V.; Lebargy, F.; de Champs, C. Respiratory Tract Colonization by Pasteurella Pneumotropica in a Patient with an Alpha1-Antitrypsin Deficiency Unexpectedly Well Identified by Automated System Vitek 2. Diagn. Microbiol. Infect. Dis. 2010, 68, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Wolfson, J.S.; Swartz, M.N.; Hooper, D.C. Pasteurella multocida Infections. Report of 34 Cases and Review of the Literature. Medicine 1984, 63, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Holst, E.; Rollof, J.; Larsson, L.; Nielsen, J.P. Characterization and Distribution of Pasteurella Species Recovered from Infected Humans. J. Clin. Microbiol. 1992, 30, 2984–2987. [Google Scholar] [CrossRef]
- Abrahamian, F.M.; Goldstein, E.J.C. Microbiology of Animal Bite Wound Infections. Clin. Microbiol. Rev. 2011, 24, 231–246. [Google Scholar] [CrossRef]
- Brook, I. Microbiology of Human and Animal Bite Wounds in Children. Pediatr. Infect. Dis. J. 1987, 6, 29–32. [Google Scholar] [CrossRef]
- Francis, D.P.; Holmes, M.A.; Brandon, G. Pasteurella multocida. Infections after Domestic Animal Bites and Scratches. JAMA 1975, 233, 42–45. [Google Scholar] [CrossRef]
- Dendle, C.; Looke, D. Review Article: Animal Bites: An Update for Management with a Focus on Infections. Emerg. Med. Australas. 2008, 20, 458–467. [Google Scholar] [CrossRef]
- Talan, D.A.; Citron, D.M.; Abrahamian, F.M.; Moran, G.J.; Goldstein, E.J. Bacteriologic Analysis of Infected Dog and Cat Bites. Emergency Medicine Animal Bite Infection Study Group. N. Engl. J. Med. 1999, 340, 85–92. [Google Scholar] [CrossRef]
- Westling, K.; Farra, A.; Cars, B.; Ekblom, A.G.; Sandstedt, K.; Settergren, B.; Wretlind, B.; Jorup, C. Cat Bite Wound Infections: A Prospective Clinical and Microbiological Study at Three Emergency Wards in Stockholm, Sweden. J. Infect. 2006, 53, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Boyanton, B.L.; Freij, B.J.; Robinson-Dunn, B.; Makin, J.; Runge, J.K.; Luna, R.A. Neonatal Pasteurella multocida Subsp. Septica Meningitis Traced to Household Cats: Molecular Linkage Analysis Using Repetitive-Sequence-Based PCR. J. Clin. Microbiol. 2016, 54, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Hubbert, W.T.; Rosen, M.N. Pasteurella multocida Infections. II. Pasteurella multocida Infection in Man Unrelated to Animal Bite. Am. J. Public Health Nations Health 1970, 60, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Vondra, M.S.; Myers, J.P. Pasteurella multocida Bacteremia: Report of 12 Cases in the 21st Century and Comprehensive Review of the Adult Literature. Infect. Dis. Clin. Pract. 2011, 19, 197–203. [Google Scholar] [CrossRef]
- Cahill, T.J.; Prendergast, B.D. Infective Endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef]
- Morpeth, S.; Murdoch, D.; Cabell, C.H.; Karchmer, A.W.; Pappas, P.; Levine, D.; Nacinovich, F.; Tattevin, P.; Fernández-Hidalgo, N.; Dickerman, S.; et al. Non-HACEK Gram-Negative Bacillus Endocarditis. Ann. Intern. Med. 2007, 147, 829–835. [Google Scholar] [CrossRef]
- Bouza, E.; Muñoz, P.; Burillo, A. Gram-Negative Endocarditis: Disease Presentation, Diagnosis and Treatment. Curr. Opin. Infect. Dis. 2021, 34, 672–680. [Google Scholar] [CrossRef]
- Loubet, P.; Lescure, F.-X.; Lepage, L.; Kirsch, M.; Armand-Lefevre, L.; Bouadma, L.; Lariven, S.; Duval, X.; Yazdanpanah, Y.; Joly, V. Endocarditis Due to Gram-Negative Bacilli at a French Teaching Hospital over a 6-Year Period: Clinical Characteristics and Outcome. Infect. Dis. 2015, 47, 889–895. [Google Scholar] [CrossRef]
- Veve, M.P.; McCurry, E.D.; Cooksey, G.E.; Shorman, M.A. Epidemiology and Outcomes of Non-HACEK Infective Endocarditis in the Southeast United States. PLoS ONE 2020, 15, e0230199. [Google Scholar] [CrossRef]
- Ioannou, P.; Vougiouklakis, G. Infective Endocarditis by Proteus Species: A Systematic Review. Germs 2020, 10, 229–239. [Google Scholar] [CrossRef]
- Ioannou, P.; Mavrikaki, V.; Kofteridis, D.P. Infective Endocarditis by Acinetobacter Species: A Systematic Review. J. Chemother. 2021, 33, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, P.; Vamvoukaki, R.; Kofteridis, D.P. Infective Endocarditis by Enterobacter cloacae: A Systematic Review and Meta-Analysis. J. Chemother. 2021, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, P.; Miliara, E.; Baliou, S.; Kofteridis, D.P. Infective Endocarditis by Klebsiella Species: A Systematic Review. J. Chemother. 2021, 33, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, P.; Vougiouklakis, G.; Baliou, S.; Miliara, E.; Kofteridis, D.P. Infective Endocarditis by Yersinia Species: A Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 19. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Durante-Mangoni, E.; Ravasio, V.; Barbaro, F.; Ursi, M.P.; Pasticci, M.B.; Bassetti, M.; Grossi, P.; Venditti, M.; et al. Risk Factors and Outcomes of Endocarditis Due to Non-HACEK Gram-Negative Bacilli: Data from the Prospective Multicenter Italian Endocarditis Study Cohort. Antimicrob. Agents Chemother. 2018, 62, e02208-17. [Google Scholar] [CrossRef]
- Ioannou, P.; Alexakis, K.; Spentzouri, D.; Kofteridis, D.P. Infective Endocarditis by Serratia Species: A Systematic Review. J. Chemother. 2022, 1–13. [Google Scholar] [CrossRef]
- Giannitsioti, E.; Skiadas, I.; Antoniadou, A.; Tsiodras, S.; Kanavos, K.; Triantafyllidi, H.; Giamarellou, H.; Hellenic Endocarditis Study Group Nosocomial, vs. Community-Acquired Infective Endocarditis in Greece: Changing Epidemiological Profile and Mortality Risk. Clin. Microbiol. Infect. 2007, 13, 763–769. [Google Scholar] [CrossRef]
- Chatelier, E.; Mahieu, R.; Hamel, J.-F.; Chenouard, R.; Lozac’h, P.; Sallé, A.; Kouatchet, A.; Martin, L.; Lavigne, C.; Pailhoriès, H.; et al. Pasteurella Bacteraemia: Impact of Comorbidities on Outcome, Based on a Case Series and Literature Review. Int. J. Infect. Dis. 2020, 92, 89–96. [Google Scholar] [CrossRef]
- Vesza, Z.; Boattini, M.; Pinto, M.; Marques da Silva, P. Pasteurella Infections in a Tertiary Centre—From Cellulitis to Multiple-Organ Failure: Retrospective Case Series. SAGE Open Med. Case Rep. 2017, 5, 2050313X17748286. [Google Scholar] [CrossRef]
- Orsini, J.; Perez, R.; Llosa, A.; Araguez, N. Non-Zoonotic Pasteurella multocida Infection as a Cause of Septic Shock in a Patient with Liver Cirrhosis: A Case Report and Review of the Literature. J. Glob. Infect. Dis. 2013, 5, 176–178. [Google Scholar] [CrossRef]
- Alexopoulou, A.; Agiasotelli, D.; Vasilieva, L.E.; Dourakis, S.P. Bacterial Translocation Markers in Liver Cirrhosis. Ann. Gastroenterol. 2017, 30, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Bourély, C.; Cazeau, G.; Jouy, E.; Haenni, M.; Madec, J.-Y.; Jarrige, N.; Leblond, A.; Gay, E. Antimicrobial Resistance of Pasteurella multocida Isolated from Diseased Food-Producing Animals and Pets. Vet. Microbiol. 2019, 235, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Kehrenberg, C.; Schulze-Tanzil, G.; Martel, J.L.; Chaslus-Dancla, E.; Schwarz, S. Antimicrobial Resistance in Pasteurella and Mannheimia: Epidemiology and Genetic Basis. Vet. Res. 2001, 32, 323–339. [Google Scholar] [CrossRef]
- Nakwan, N.; Nakwan, N.; Atta, T.; Chokephaibulkit, K. Neonatal Pasteurellosis: A Review of Reported Cases. Arch. Dis. Child. Fetal Neonatal. Ed. 2009, 94, F373–F376. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Dincman, T.; Clyburn, B.E.; Steed, L.L.; Rockey, D.C. Clinical Features and Outcomes of Pasteurella multocida Infection. Medicine 2015, 94, e1285. [Google Scholar] [CrossRef] [PubMed]
- Rhea, S.; Weber, D.J.; Poole, C.; Cairns, C. Risk Factors for Hospitalization after Dog Bite Injury: A Case-Cohort Study of Emergency Department Visits. Acad Emerg. Med. 2014, 21, 196–203. [Google Scholar] [CrossRef]
- Christenson, E.S.; Ahmed, H.M.; Durand, C.M. Pasteurella multocida Infection in Solid Organ Transplantation. Lancet Infect. Dis. 2015, 15, 235–240. [Google Scholar] [CrossRef]
- Klein, N.C.; Cunha, B.A. Pasteurella multocida Pneumonia. Semin. Respir. Infect. 1997, 12, 54–56. [Google Scholar]
- Byrd, R.P.; Roy, T.M. Pasteurella multocida Respiratory Infection: An Important Cat-Associated Zoonosis. Arch. Intern. Med. 2003, 163, 1239. [Google Scholar] [CrossRef]
- Marinella, M.A. Community-Acquired Pneumonia Due to Pasteurella multocida. Respir. Care 2004, 49, 1528–1529. [Google Scholar]
- Harris, P.J.; Osswald, M.B. Pasteurella multocida Epiglottitis: A Review and Report of a New Case with Associated Chronic Lymphocytic Leukemia. Ear. Nose Throat J. 2010, 89, E4–E7. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Sakata, T.; Toyokawa, M.; Nishi, I.; Tomono, K. A Chronic Respiratory Pasteurella multocida Infection Is Well-Controlled by Long-Term Macrolide Therapy. Intern. Med. 2016, 55, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Kopita, J.M.; Handshoe, D.; Kussin, P.S.; Kelemen, M. Cat Germs! Pleuropulmonary Pasteurella Infection in an Old Man. North Carol. Med. J. 1993, 54, 308–311. [Google Scholar]
- Ferreira, J.; Treger, K.; Busey, K. Pneumonia and Disseminated Bacteremia with Pasteurella multocida in the Immune Competent Host: A Case Report and a Review of the Literature. Respir. Med. Case Rep. 2015, 15, 54–56. [Google Scholar] [CrossRef]
- Green, B.T.; Ramsey, K.M.; Nolan, P.E. Pasteurella multocida Meningitis: Case Report and Review of the Last 11 y. Scand. J. Infect. Dis. 2002, 34, 213–217. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Moloney, A.; Hickey, M. Pasteurella multocida Meningitis: Case Report and Review of the Literature. J. Infect. 2005, 50, 344–345. [Google Scholar] [CrossRef]
- Kimura, R.; Hayashi, Y.; Takeuchi, T.; Shimizu, M.; Iwata, M.; Tanahashi, J.; Ito, M. Pasteurella multocida Septicemia Caused by Close Contact with a Domestic Cat: Case Report and Literature Review. J. Infect. Chemother. 2004, 10, 250–252. [Google Scholar] [CrossRef]
| Characteristic | Value (n Out of 28, Unless Otherwise Mentioned) |
|---|---|
| Male, n (%) | 20 (71.4%) |
| Age, mean (SD) in years | 56.4 (15.7) |
| Predisposing factors | |
| Animal bite or close contact, n (%) | 21 (75%) |
| Prosthetic valve, n (%) | 6 (21.4%) |
| Bad teeth hygiene or recent dental work, n (%) | 3 (10.7%) |
| Previous IE, n (%) | 3 (10.7%) |
| Liver cirrhosis, n (%) | 3 (10.7%) |
| IVDU, n (%) | 2 (7.1%) |
| Rheumatic fever, n (%) | 1 (3.6%) |
| ESRD on hemodialysis, n (%) | 1 (3.6%) |
| Immunosuppression, n (%) | 1 (3.6%) |
| Recent cardiac surgery (within three months), n (%) | 0 (0%) |
| Congenital heart disease, n (%) | 0 (0%) |
| Valve localization | |
| Aortic valve, n (%) | 13 out of 26 (50%) |
| Mitral valve, n (%) | 8 out of 26 (30.8%) |
| Tricuspid valve, n (%) | 3 out of 26 (11.5%) |
| Pulmonary valve, n (%) | 1 out of 26 (3.8%) |
| Eustachian valve, n (%) | 1 out of 26 (3.8%) |
| Mural endocardium, n (%) | 1 out of 26 (3.8%) |
| Multiple valves, n (%) | 1 out of 26 (3.8%) |
| Microbiological data | |
| Pasteurella multocida, n (%) | 20 (71.4%) |
| Pasteurella dagmatis, n (%) | 3 (10.7%) |
| Pasteurellapneumotropica, n (%) | 2 (7.1%) |
| Pasteurellahaemolytica, n (%) | 2 (7.1%) |
| Pasteurellaaerogenes, n (%) | 1 (3.6%) |
| Antimicrobial resistance | |
| Penicillin, n (%) | 0 out of 11 (0%) |
| Aminoglycosides, n (%) | 1 out of 9 (11.1%) |
| Cephalosporins, n (%) | 0 out of 8 (0%) |
| Ampicillin, n (%) | 0 out of 8 (0%) |
| Tetracyclines, n (%) | 0 out of 7 (0%) |
| Method of diagnosis | |
| Transthoracic echocardiography, n (%) | 12 out of 25 (48%) |
| Transesophageal echocardiography, n (%) | 7 out of 25 (28%) |
| Valve culture, n (%) | 7 out of 25 (28%) |
| Autopsy, n (%) | 3 (10.7%) |
| Empirical diagnosis, n (%) | 1 (3.6%) |
| Clinical characteristics | |
| Fever, n (%) | 26 out of 27 (96.3%) |
| Sepsis, n (%) | 22 out of 27 (81.5%) |
| Septic shock, n (%) | 6 (21.4%) |
| Heart failure, n (%) | 6 (21.4%) |
| Immunologic phenomena, n (%) | 5 (17.9%) |
| Embolic phenomena, n (%) | 4 (14.3%) |
| Paravalvular abscess, n (%) | 4 out of 27 (14.8%) |
| Treatment | |
| Duration of treatment in weeks, median (IQR) | 6 (4.5–6.5) |
| Cephalosporin, n (%) | 12 out of 27 (44.4%) |
| Aminopenicillins, n (%) | 10 out of 27 (37%) |
| Penicillin, n (%) | 8 out of 27 (29.6%) |
| Piperacillin and tazobactam, n (%) | 5 out of 27 (18.5%) |
| Aminoglycoside, n (%) | 6 out of 27 (22.2%) |
| Quinolone, n (%) | 5 out of 27 (18.5%) |
| Carbapenem, n (%) | 2 out of 27 (7.4%) |
| Tetracycline, n (%) | 2 out of 27 (7.4%) |
| Surgical management, n (%) | 12 (42.9%) |
| Outcomes | |
| Clinical cure, n (%) | 23 (82.1%) |
| Deaths due to infection, n (%) | 5 (17.9%) |
| Deaths overall, n (%) | 5 (17.9%) |
| Characteristic | Patients Who Survived (n Out of 23, Unless Otherwise Mentioned) | Patients Who Died (n Out of 5, Unless Otherwise Mentioned) |
|---|---|---|
| Male, n (%) | 16 (69.6%) | 4 (80%) |
| Age, mean (SD) in years | 55.9 (16.5) | 58.8 (12.8) |
| Predisposing factors | ||
| Animal bite or close contact, n (%) | 18 (78.3%) | 3 (60%) |
| Prosthetic valve, n (%) | 6 (26.1%) | 0 (0%) |
| Bad teeth hygiene or recent dental work, n (%) | 3 (13%) | 0 (0%) |
| Previous IE, n (%) | 3 (13%) | 0 (0%) |
| Liver cirrhosis, n (%) | 2 (8.7%) | 1 (20%) |
| IVDU, n (%) | 2 (8.7%) | 0 (0%) |
| Rheumatic fever, n (%) | 0 (0%) | 1 (20%) |
| ESRD on hemodialysis, n (%) | 0 (0%) | 1 (20%) |
| Immunosuppression, n (%) | 1 (4.3%) | 0 (0%) |
| Valve localization | ||
| Aortic valve, n (%) | 11 out of 22 (50%) | 2 out of 4 (50%) |
| Mitral valve, n (%) | 6 out of 22 (27.3%) | 2 out of 4 (50%) |
| Tricuspid valve, n (%) | 3 out of 22 (13.6%) | 0 out of 4 (0%) |
| Pulmonary valve, n (%) | 1 out of 22 (4.5%) | 0 out of 4 (0%) |
| Eustachian valve, n (%) | 1 out of 22 (4.5%) | 0 out of 4 (0%) |
| Mural endocardium, n (%) | 1 out of 22 (4.5%) | 0 out of 4 (0%) |
| Multiple valves, n (%) | 1 out of 22 (4.5%) | 0 out of 4 (0%) |
| Microbiological data | ||
| Pasteurella multocida, n (%) | 17 (73.9%) | 3 (60%) |
| Pasteurella dagmatis, n (%) | 3 (13%) | 0 (0%) |
| Pasteurellapneumotropica, n (%) | 2 (8.7%) | 0 (0%) |
| Pasteurellahaemolytica, n (%) | 0 (0) | 2 (40%) |
| Pasteurellaaerogenes, n (%) | 1 (4.3%) | 0 (0%) |
| Method of diagnosis | ||
| Transthoracic echocardiography, n (%) | 10 out of 20 (50%) | 2 (40%) |
| Transesophageal echocardiography, n (%) | 7 out of 20 (35%) | 0 (0%) |
| Valve culture, n (%) | 6 out of 20 (30%) | 1 (20%) |
| Autopsy, n (%) | 0 (0%) | 3 (60%) |
| Empirical diagnosis, n (%) | 1 (4.3%) | 0 (0%) |
| Clinical characteristics | ||
| Fever, n (%) | 21 out of 22 (95.5%) | 5 (100%) |
| Sepsis, n (%) | 17 out of 22 (77.3%) | 5 (100%) |
| Septic shock, n (%) | 3 (13%) | 3 (60%) |
| Heart failure, n (%) | 5 (21.7%) | 1 (20%) |
| Immunologic phenomena, n (%) | 4 (17.4%) | 1 (20%) |
| Embolic phenomena, n (%) | 2 (8.7%) | 2 (40%) |
| Paravalvular abscess, n (%) | 4 out of 22 (18.2%) | 0 (0%) |
| Treatment | ||
| Duration of treatment in weeks, median (IQR) | 6 (4.5–6.5) | NA |
| Cephalosporin, n (%) | 11 out of 22 (50%) | 1 (20%) |
| Aminopenicillins, n (%) | 7 out of 22 (31.8%) | 3 (60%) |
| Penicillin, n (%) | 7 out of 22 (31.8%) | 1 (20%) |
| Piperacillin and tazobactam, n (%) | 4 out of 22 (18.2%) | 1 (20%) |
| Aminoglycoside, n (%) | 3 out of 22 (13.6%) | 3 (60%) |
| Quinolone, n (%) | 4 out of 22 (18.2%) | 1 (20%) |
| Carbapenem, n (%) | 2 out of 22 (9.1%) | 0 (0%) |
| Tetracycline, n (%) | 1 out of 22 (4.5%) | 1 (20%) |
| Surgical management, n (%) | 12 (52.2%) | 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alifragki, A.; Kontogianni, A.; Protopapa, I.; Baliou, S.; Ioannou, P. Infective Endocarditis by Pasteurella Species: A Systematic Review. J. Clin. Med. 2022, 11, 5037. https://doi.org/10.3390/jcm11175037
Alifragki A, Kontogianni A, Protopapa I, Baliou S, Ioannou P. Infective Endocarditis by Pasteurella Species: A Systematic Review. Journal of Clinical Medicine. 2022; 11(17):5037. https://doi.org/10.3390/jcm11175037
Chicago/Turabian StyleAlifragki, Angeliki, Argyro Kontogianni, Ioanna Protopapa, Stella Baliou, and Petros Ioannou. 2022. "Infective Endocarditis by Pasteurella Species: A Systematic Review" Journal of Clinical Medicine 11, no. 17: 5037. https://doi.org/10.3390/jcm11175037
APA StyleAlifragki, A., Kontogianni, A., Protopapa, I., Baliou, S., & Ioannou, P. (2022). Infective Endocarditis by Pasteurella Species: A Systematic Review. Journal of Clinical Medicine, 11(17), 5037. https://doi.org/10.3390/jcm11175037






