Primary Lamellar Macular Holes: To Vit or Not to Vit
Abstract
1. Introduction
2. Historical Background
3. Epidemiology
4. Multimodal Imaging
5. Epiretinal Proliferation (ERP)
6. Pathogenesis
7. Natural History
8. Treatment
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, N.E.; Wendel, R.T. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch. Ophthalmol. 1991, 109, 654–659. [Google Scholar] [CrossRef]
- Duker., J.S.; Kaiser, P.K.; Binder, S.; de Smet, M.D.; Gaudric, A.; Reichel, E.; Sadda, S.R.; Sebag, J.; Spaide, R.F.; Stalmans, P. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 2013, 120, 2611–2619. [Google Scholar] [CrossRef]
- Gass, J.D. Lamellar macular hole: A complication of cystoid macular edema after cataract extraction: A clinicopathologic case report. Trans. Am. Ophthalmol. Soc. 1975, 73, 231–250. [Google Scholar]
- Gass, J.D. Lamellar macular hole: A complication of cystoid macular edema after cataract extraction. Arch. Ophthalmol. 1976, 94, 793–800. [Google Scholar] [CrossRef]
- Allen, A.W.; Gass, J.D.M. Contraction of a Perifoveal Epiretinal Membrane Simulating a Macular Hole. Am. J. Ophthalmol. 1976, 82, 684–691. [Google Scholar] [CrossRef]
- Barak, Y.; Ihnen, M.A.; Schaal, S. Spectral domain optical coherence tomography in the diagnosis and management of vitreoretinal interface pathologies. J. Ophthalmol. 2012, 2012, 876472. [Google Scholar] [CrossRef]
- Mirza, R.G.; Johnson, M.W.; Jampol, L.M. Optical Coherence Tomography Use in Evaluation of the Vitreoretinal Interface: A Review. Surv. Ophthalmol. 2007, 52, 397–421. [Google Scholar] [CrossRef]
- Haouchine, B.; Massin, P.; Tadayoni, R.; Erginay, A.; Gaudric, A. Diagnosis of macular pseudoholes and lamellar macular holes by optical coherence tomography. Am. J. Ophthalmol. 2004, 138, 732–739. [Google Scholar] [CrossRef]
- Witkin, A.J.; Ko, T.H.; Fujimoto, J.G.; Schuman, J.S.; Baumal, C.R.; Rogers, A.H.; Reichel, E.; Duker, J.S. Redefining Lamellar Holes and the Vitreomacular Interface: An Ultrahigh-Resolution Optical Coherence Tomography Study. Ophthalmology 2006, 113, 388–397. [Google Scholar] [CrossRef]
- Kokame, G.T.; Tokuhara, K.G. Surgical Management of Inner Lamellar Macular Hole. Ophthalmic Surg. Lasers Imaging Retin. 2007, 38, 61–63. [Google Scholar] [CrossRef]
- Witkin, A.J.; Castro, L.C.; Reichel, E.; Rogers, A.H.; Baumal, C.R.; Duker, J.S. Anatomic and Visual Outcomes of Vitrectomy for Lamellar Macular Holes. Ophthalmic Surg. Lasers Imaging Retin. 2010, 41, 418–424. [Google Scholar] [CrossRef]
- Garretson, B.R.; Pollack, J.S.; Ruby, A.J.; Drenser, K.A.; Williams, G.A.; Sarrafizadeh, R. Vitrectomy for a Symptomatic Lamellar Macular Hole. Ophthalmology 2008, 115, 884–886.e1. [Google Scholar] [CrossRef]
- Hirakawa, M.; Uemura, A.; Nakano, T.; Sakamoto, T. Pars Plana Vitrectomy With Gas Tamponade for Lamellar Macular Holes. Am. J. Ophthalmol. 2005, 140, 1154–1155. [Google Scholar] [CrossRef]
- Klein, B.R.; Hiner, C.J.; Glaser, B.M.; Murphy, R.P.; Sjaarda, R.N.; Thompson, J.T. Fundus Photographic and Fluorescein Angiographic Characteristics of Pseudoholes of the Macula in Eyes with Epiretinal Membranes. Ophthalmology 1995, 102, 768–774. [Google Scholar] [CrossRef]
- Zampedri, E.; Romanelli, F.; Semeraro, F.; Parolini, B.; Frisina, R. Spectral-domain optical coherence tomography findings in idiopathic lamellar macular hole. Graefes. Arch. Clin. Exp. Ophthalmol. 2017, 255, 699–707. [Google Scholar] [CrossRef]
- Govetto, A.; Dacquay, Y.; Farajzadeh, M.; Platner, E.; Hirabayashi, K.; Hosseini, H.; Schwartz, S.D.; Hubschman, J.P. Lamellar Macular Hole: Two Distinct Clinical Entities? Am. J. Ophthalmol. 2016, 164, 99–109. [Google Scholar] [CrossRef]
- Hubschman, J.P.; Govetto, A.; Spaide, R.F.; Schumann, R.; Steel, D.; Figueroa, M.S.; Sebag, J.; Gaudric, A.; Staurenghi, G.; Haritoglou, C.; et al. Optical coherence tomography-based consensus definition for lamellar macular hole. Br. J. Ophthalmol. 2020, 104, 1741–1747. [Google Scholar] [CrossRef]
- Meuer, S.M.; Myers, C.E.; Klein, B.E.; Swift, M.K.; Huang, Y.; Gangaputra, S.; Pak, J.W.; Danis, R.P.; Klein, R. The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: The beaver dam eye study. Ophthalmology 2015, 122, 787–795. [Google Scholar] [CrossRef]
- Liesenborghs, I.; De Clerck, E.E.B.; Berendschot, T.T.; Goezinne, F.; Schram, M.; Henry, R.M.; Stehouwer, C.D.; Webers, C.A.; Schouten, J.S. Prevalence of optical coherence tomography detected vitreomacular interface disorders: The Maastricht Study. Acta Ophthalmol. 2018, 96, 729–736. [Google Scholar] [CrossRef]
- Ben Ghezala, I.; Seydou, A.; Gabrielle, P.H.; Bouche-Pillon, J.; Binquet, C.; Delcourt, C.; Daien, V.; Bron, A.M.; Creuzot-Garcher, C. Epidemiology of Vitreomacular Interface Abnormalities Using Macular Spectral-Domain Optical Coherence Tomography in an Elderly Population (the Montrachet Study). Retina 2021, 41, 60–67. [Google Scholar] [CrossRef]
- Zapata, M.A.; Figueroa, M.S.; González, E.E.; Huguet, C.; Giralt, J.; Pinazo, R.G.; Abecia, E.; Donate, J.; Vigo, J.F.; Palomero, P.A.; et al. Prevalence of Vitreoretinal Interface Abnormalities on Spectral-Domain OCT in Healthy Participants over 45 Years of Age. Ophthalmol. Retin. 2016, 1, 249–254. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Kim, S.; Chung, S.-H. Prevalence of Macular Abnormalities Identified Only on Optical Coherence Tomography in Korean Patients Scheduled for Cataract Surgery. Korean J. Ophthalmol. 2021, 35, 153–158. [Google Scholar] [CrossRef]
- Nava, U.; Cereda, M.G.; Bottoni, F.; Preziosa, C.; Pellegrini, M.; Giani, A.; Staurenghi, G. Long-term follow-up of fellow eye in patients with lamellar macular hole. Graefes. Arch. Clin. Exp. Ophthalmol. 2017, 255, 1485–1492. [Google Scholar] [CrossRef]
- Haouchine, B.; Massin, P.; Gaudric, A. Foveal pseudocyst as the first step in macular hole formation: A prospective study by optical coherence tomography. Ophthalmology 2001, 108, 15–22. [Google Scholar] [CrossRef]
- Takahashi, H.; Kishi, S. Tomographic features of a lamellar macular hole formation and a lamellar hole that progressed to a full-thickness macular hole. Am. J. Ophthalmol. 2000, 130, 677–679. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Ohji, M.; Fujikado, T.; Saito, Y.; Motokura, M.; Ishimoto, I.; Tano, Y. Differentiating full thickness macular holes from impending macular holes and macular pseudoholes. Br. J. Ophthalmol. 1997, 81, 117–122. [Google Scholar] [CrossRef][Green Version]
- Parolini, B.; Schumann, R.G.; Cereda, M.G.; Haritoglou, C.; Pertile, G. Lamellar Macular Hole: A Clinicopathologic Correlation of Surgically Excised Epiretinal Membranes. Investig. Opthalmology Vis. Sci. 2011, 52, 9074–9083. [Google Scholar] [CrossRef]
- Michalewska, Z.; Michalewski, J.; Odrobina, D.; Nawrocki, J. Non-full-thickness macular holes reassessed with spectral domain optical coherence tomography. Retina 2012, 32, 922–929. [Google Scholar] [CrossRef]
- Clamp, M.F.; Wilkes, G.; Leis, L.S.; McDonald, H.R.; Johnson, R.N.; Jumper, J.M.; Fu, A.D.; Cunningham, E.T., Jr.; Stewart, P.J.; Haug, S.J.; et al. En face spectral domain optical coherence tomography analysis of lamellar macular holes. Retina 2014, 34, 1360–1366. [Google Scholar] [CrossRef]
- Acquistapace, A.; Cereda, M.G.; Cigada, M.; Staurenghi, G.; Bottoni, F. Imaging of tangential traction types in lamellar macular holes. Graefes. Arch. Clin. Exp. Ophthalmol. 2017, 255, 2331–2336. [Google Scholar] [CrossRef]
- Gaudric, A.; Aloulou, Y.; Tadayoni, R.; Massin, P. Macular Pseudoholes With Lamellar Cleavage of Their Edge Remain Pseudoholes. Am. J. Ophthalmol. 2013, 155, 733–742.e1-4. [Google Scholar] [CrossRef]
- Chen, J.C.; Lee, L.R. Clinical spectrum of lamellar macular defects including pseudoholes and pseudocysts defined by optical coherence tomography. Br. J. Ophthalmol. 2008, 92, 1342–1346. [Google Scholar] [CrossRef]
- Michalewski, J.; Michalewska, Z.; Dziegielewski, K.; Nawrocki, J. Evolution from macular pseudohole to lamellar macular hole-spectral domain OCT study. Graefes. Arch. Clin. Exp. Ophthalmol. 2011, 249, 175–178. [Google Scholar] [CrossRef]
- Bottoni, F. Fundus Autofluorescence in Lamellar Macular Holes and Pseudoholes: A Review. J. Ophthalmol. 2019, 2019, 4948212. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Holz, F.G.; Bird, A.C.; Spaide, R.F. Fundus autofluorescence imaging: Review and perspectives. Retina 2008, 28, 385–409. [Google Scholar] [CrossRef]
- dell’Omo, R.; Filippelli, M.; De Turris, S.; Govetto, A.; Napolitano, P.; dell’Omo, E.; Costagliola, C. Multimodal Imaging of Lamellar Macular Holes. J. Ophthalmol. 2021, 2021, 8820444. [Google Scholar] [CrossRef]
- Bottoni, F.; Carmassi, L.; Cigada, M.; Staurenghi, G. Diagnosis of macular pseudoholes and lamellar macular holes: Is optical coherence tomography the “gold standard”? Br. J. Ophthalmol. 2008, 92, 635–639. [Google Scholar] [CrossRef]
- Bottoni, F.; Zanzottera, E.; Carini, E.; Cereda, M.; Cigada, M.; Staurenghi, G. Re-accumulation of macular pigment after successful macular hole surgery. Br. J. Ophthalmol. 2016, 100, 693–698. [Google Scholar] [CrossRef]
- dell’Omo, R.; Vogt, D.; Schumann, R.G.; Turris, S.; Virgili, G.; Staurenghi, G.; Cereda, M.; Costagliola, C.; Priglinger, S.G.; Bottoni, F. The Relationship Between Blue-Fundus Autofluorescence and Optical Coherence Tomography in Eyes With Lamellar Macular Holes. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3079–3087. [Google Scholar] [CrossRef]
- Hirano, M.; Morizane, Y.; Kimura, S.; Hosokawa, M.; Shiode, Y.; Doi, S.; Toshima, S.; Takahashi, K.; Hosogi, M.; Fujiwara, A.; et al. Assessment of Lamellar Macular Hole and Macular Pseudohole With a Combination of En Face and Radial B-scan Optical Coherence Tomography Imaging. Am. J. Ophthalmol. 2018, 188, 29–40. [Google Scholar] [CrossRef]
- Reibaldi, M.; Parravano, M.; Varano, M.; Longo, A.; Avitabile, T.; Uva, M.G.; Zagari, M.; Toro, M.; Boscia, F.; Boccassini, B.; et al. Foveal Microstructure and Functional Parameters in Lamellar Macular Hole. Am. J. Ophthalmol. 2012, 154, 974–980.e1. [Google Scholar] [CrossRef]
- Yeo, J.H.; Oh, R.; Lee, J.Y.; Kim, J.-G.; Yoon, Y.H.; Kim, Y.J. Optical coherence tomography angiographic findings of lamellar macular hole: Comparisons between tractional and degenerative subtypes. Sci. Rep. 2020, 10, 13331. [Google Scholar] [CrossRef]
- Catania, F.; Allegrini, D.; Nembri, A.; Confalonieri, F.; Zollet, P.; Crincoli, E.; Romano, M.R. Macular Microvascular Modifications in Progressive Lamellar Macular Holes. Diagnostics 2021, 11, 1717. [Google Scholar] [CrossRef]
- Bottoni, F.; Deiro, A.P.; Giani, A.; Orini, C.; Cigada, M.; Staurenghi, G. The natural history of lamellar macular holes: A spectral domain optical coherence tomography study. Graefes. Arch. Clin. Exp. Ophthalmol. 2013, 251, 467–475. [Google Scholar] [CrossRef]
- Shiraga, F.; Takasu, I.; Fukuda, K.; Fujita, T.; Yamashita, A.; Hirooka, K.; Shirakata, Y.; Morizane, Y.; Fujiwara, A. Modified Vitreous Surgery for Symptomatic Lamellar Macular Hole With Epiretinal Membrane Containing Macular Pigment. Retina 2013, 33, 1263–1269. [Google Scholar] [CrossRef]
- Pang, C.E.; Spaide, R.F.; Freund, K.B. Epiretinal proliferation seen in association with lamellar macular holes: A distinct clinical entity. Retina 2014, 34, 1513–1523. [Google Scholar] [CrossRef]
- Pang, C.E.; Spaide, R.F.; Freund, K.B. Comparing functional and morphologic characteristics of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Retina 2015, 35, 720–726. [Google Scholar] [CrossRef]
- Itoh, Y.; Levison, A.L.; Kaiser, P.K.; Srivastava, S.K.; Singh, R.P.; Ehlers, J.P. Prevalence and characteristics of hyporeflective preretinal tissue in vitreomacular interface disorders. Br. J. Ophthalmol. 2016, 100, 399–404. [Google Scholar] [CrossRef]
- dell’Omo, R.; Virgili, G.; Rizzo, S.; De Turris, S.; Coclite, G.; Giorgio, D.; dell’Omo, E.; Costagliola, C. Role of Lamellar Hole-Associated Epiretinal Proliferation in Lamellar Macular Holes. Am. J. Ophthalmol. 2017, 175, 16–29. [Google Scholar] [CrossRef]
- Chehaibou, I.; Pettenkofer, M.; Govetto, A.; Rabina, G.; Sadda, S.R.; Hubschman, J.P. Identification of epiretinal proliferation in various retinal diseases and vitreoretinal interface disorders. Int. J. Retin. Vitr. 2020, 6, 31. [Google Scholar] [CrossRef]
- Lee Kim, E.; Weiner, A.J.; Ung, C.; Roh, M.; Wang, J.; Lee, I.J.; Huang, N.T.; Stem, M.; Dahrouj, M.; Eliott, D.; et al. Characterization of Epiretinal Proliferation in Full-Thickness Macular Holes and Effects on Surgical Outcomes. Ophthalmol. Retin. 2019, 3, 694–702. [Google Scholar] [CrossRef]
- Chehaibou, I.; Hubschman, J.-P.; Kasi, S.; Su, D.; Joseph, A.; Prasad, P.; Abbey, A.M.; Gaudric, A.; Tadayoni, R.; Rahimy, E. Spontaneous Conversion of Lamellar Macular Holes to Full-Thickness Macular Holes: Clinical Features and Surgical Outcomes. Ophthalmol. Retin. 2021, 5, 1009–1016. [Google Scholar] [CrossRef]
- Marques, M.F.; Rodrigues, S.; Raimundo, M.; Costa, J.; Marques, J.P.; Alfaiate, M.; Figueira, J. Epiretinal Proliferations Associated with Lamellar Macular Holes: Clinical and Surgical Implications. Ophthalmologica 2018, 240, 8–13. [Google Scholar] [CrossRef]
- Compera, D.; Entchev, E.; Haritoglou, C.; Scheler, R.; Mayer, W.J.; Wolf, A.; Kampik, A.; Schumann, R.G. Lamellar Hole–Associated Epiretinal Proliferation in Comparison to Epiretinal Membranes of Macular Pseudoholes. Am. J. Ophthalmol. 2015, 160, 373–384.e1. [Google Scholar] [CrossRef]
- Lai, T.T.; Chen, S.N.; Yang, C.M. Epiretinal proliferation in lamellar macular holes and full-thickness macular holes: Clinical and surgical findings. Graefes. Arch. Clin. Exp. Ophthalmol. 2016, 254, 629–638. [Google Scholar] [CrossRef]
- Dell’Omo, R.; De Turris, S.; Dell’Omo, E.; Costagliola, C. Visualization of Lamellar Hole-Associated Epiretinal Proliferation With Blue-Reflectance Imaging. Retina 2018, 38, e34–e35. [Google Scholar] [CrossRef]
- Son, G.; Lee, J.S.; Lee, S.; Sohn, J. Epiretinal Proliferation Associated with Macular Hole and Intraoperative Perifoveal Crown Phenomenon. Korean J. Ophthalmol. 2016, 30, 399–409. [Google Scholar] [CrossRef]
- Obana, A.; Sasano, H.; Okazaki, S.; Otsuki, Y.; Seto, T.; Gohto, Y. Evidence of Carotenoid in Surgically Removed Lamellar Hole-Associated Epiretinal Proliferation. Investig. Opthalmology Vis. Sci. 2017, 58, 5157–5163. [Google Scholar] [CrossRef]
- Schumann, R.G.; Compera, D.; Schaumberger, M.M.; Wolf, A.; Fazekas, C.; Mayer, W.J.; Kampik, A.; Haritoglou, C. piretinal membrane characteristics correlate with photoreceptor layer defects in lamellar macular holes and macular pseudoholes. Retina 2015, 35, 727–735. [Google Scholar] [CrossRef]
- Doshi, R.R.; Lowrance, M.D.; Kim, B.T.; Davis, J.L.; Rosenfeld, P.J. Epiretinal Macular Edema Associated With Thick Epiretinal Membranes. Ophthalmic Surg. Lasers Imaging Retin. 2013, 44, 508–512. [Google Scholar] [CrossRef]
- Choi, W.S.; Merlau, D.J.; Chang, S. Vitrectomy for Macular Disorders Associated with Lamellar Macular Hole Epiretinal Proliferation. Retina 2018, 38, 664–669. [Google Scholar] [CrossRef]
- Ko, J.; Kim, G.A.; Lee, S.C.; Lee, J.H.; Koh, H.J.; Kim, S.S.; Byeon, S.H.; Lee, C. Surgical outcomes of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Acta Ophthalmol. 2016, 95, e221–e226. [Google Scholar] [CrossRef]
- Pang, C.E.; Maberley, D.A.; Freund, K.B.; White, V.A.; Rasmussen, S.; To, E.; Matsubara, J.A. Lamellar Hole-Associated Epiretinal Proliferation: A Clinicopathologic Correlation. Retina 2016, 36, 1408–1412. [Google Scholar] [CrossRef]
- Bringmann, A.; Unterlauft, J.D.; Wiedemann, R.; Rehak, M.; Wiedemann, P. Morphology of partial-thickness macular defects: Presumed roles of Müller cells and tissue layer interfaces of low mechanical stability. Int. J. Retin. Vitr. 2020, 6, 28. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Rochon-Duvigneaud, A. Recherches sur la fovea de la retine humaine et particulie rement sur le bouquet des cones centraux. Arch. Anat. Microsc. 1907, 9, 315–342. [Google Scholar]
- Yamada, E. Some Structural Features of the Fovea Centralis in the Human Retina. Arch. Ophthalmol. 1969, 82, 151–159. [Google Scholar] [CrossRef]
- Gass, J.D. Muller cell cone, an overlooked part of the anatomy of the fovea centralis: Hypotheses concerning its role in the pathogenesis of macular hole and foveomacualr retinoschisis. Arch. Ophthalmol. 1999, 117, 821–823. [Google Scholar] [CrossRef]
- Bringmann, A.; Syrbe, S.; Görner, K.; Kacza, J.; Francke, M.; Wiedemann, P.; Reichenbach, A. The primate fovea: Structure, function and development. Prog. Retin. Eye Res. 2018, 66, 49–84. [Google Scholar] [CrossRef]
- Chung, H.; Byeon, S.H. New insights into the pathoanatomy of macular holes based on features of optical coherence tomography. Surv. Ophthalmol. 2017, 62, 506–521. [Google Scholar] [CrossRef]
- Spaide, R.F. Closure of an outer lamellar macular hole by vitrectomy: Hypothesis for one mechanism of macular hole formation. Retina 2000, 20, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Unterlauft, J.D.; Wiedemann, R.; Barth, T.; Rehak, M.; Wiedemann, P. Degenerative lamellar macular holes: Tractional development and morphological alterations. Int. Ophthalmol. 2021, 41, 1203–1221. [Google Scholar] [CrossRef] [PubMed]
- Scharf, J.M.; Hilely, A.; Preti, R.C.; Grondin, C.; Chehaibou, I.; Greaves, G.; Tran, K.; Wang, D.; Ip, M.S.; Hubschman, J.P.; et al. Hyperreflective Stress Lines and Macular Holes. Investig. Opthalmology Vis. Sci. 2020, 61, 50. [Google Scholar] [CrossRef]
- Gass, J.D. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch. Ophthalmol. 1988, 106, 629–639. [Google Scholar] [CrossRef]
- Gaudric, A.; Haouchine, B.; Massin, P.; Paques, M.; Blain, P.; Erginay, A. Macular hole formation: New data provided by optical coherence tomography. Arch. Ophthalmol. 1999, 117, 744–751. [Google Scholar] [CrossRef]
- Kishi, S.; Takahashi, H. Three-dimensional observations of developing macular holes. Am. J. Ophthalmol. 2000, 130, 65–75. [Google Scholar] [CrossRef]
- eh, P.-T.; Chen, T.-C.; Yang, C.-H.; Ho, T.-C.; Chen, M.-S.; Huang, J.-S.; Yang, C.-M. Formation of idiopathic macular hole-reappraisal. Graefes. Arch. Clin. Exp. Ophthalmol. 2010, 248, 793–798. [Google Scholar]
- Unoki, N.; Nishijima, K.; Kita, M.; Oh, H.; Sakamoto, A.; Kameda, T.; Hayashi, H.; Yoshimura, N. Lamellar macular hole formation in patients with diabetic cystoid macular edema. Retina 2009, 29, 1128–1133. [Google Scholar] [CrossRef]
- Tsukada, K.; Tsujikawa, A.; Murakami, T.; Ogino, K.; Yoshimura, N. Lamellar macular hole formation in chronic cystoid macular edema associated with retinal vein occlusion. Jpn. J. Ophthalmol. 2011, 55, 506–513. [Google Scholar] [CrossRef]
- Patel, B.; Duvall, J.; Tullo, A.B. Lamellar Macular Hole Associated with Idiopathic Juxtafoveolar Telangiectasia. Br. J. Ophthalmol. 1988, 72, 1. [Google Scholar] [CrossRef]
- Charbel Issa, P.; Scholl, H.P.; Gaudric, A.; Massin, P.; Kreiger, A.E.; Schwartz, S.; Holz, F.G. Macular full-thickness and lamellar holes in association with type 2 idiopathic macular telangiectasia. Eye 2009, 23, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Rishi, P.K.A. Lamellar Macular Hole in a Case of Type-2 Macular Telangiectasia. Case Report. Retin. 2008, 28, 1. [Google Scholar]
- Rodriguez, A.; Valencia, M.; Gomez, F.E. Vitreoretinal traction and lamellar macular holes associated with cicatricial toxoplasmic retinochoroiditis: Case series report. Eur. J. Ophthalmol. 2016, 26, e128-e133. [Google Scholar] [CrossRef]
- Falcone, M.M.; Patel, N.A.; Yannuzzi, N.A.; Acon, D.; Negron, C.I.; McKeown, C.; Berrocal, A.M. Bilateral atypical lamellar holes in a patient with oculocutaneous albinism. Ophthalmic Genet. 2020, 41, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Segal, O.; Ferencz, J.M.; Mimouni, M.; Nesher, R.; Cohen, P.; Nemet, A.Y. Lamellar Macular Hole Associated with End Stage Exudative Age-Related Macular Degeneration. IMAJ 2015, 17, 750–754. [Google Scholar]
- Francone, A.; Yun, L.; Kothari, N.; Cheng, I.; Farajzadeh, M.; Govetto, A.; Hubschman, J.-P. Lamellar Macular Holes in the Presence of Age-Related Macular Degeneration. Retina 2020, 40, 1079–1086. [Google Scholar] [CrossRef]
- Rush, R.B.; Rush, S.W. Bilateral Lamellar Macular Hole Surgery in Retinitis Pigmentosa. Retin. Cases Brief Rep. 2016, 10, 2. [Google Scholar] [CrossRef]
- Kumar, V.; Goel, N. Lamellar macular hole in X linked retinoschisis. BMJ Case Rep. 2016, 2016, bcr2016215287. [Google Scholar] [CrossRef]
- Scassa, C.; Cupo, G.; Bruno, M.; Iervolino, R.; Scarinci, F.; Giusti, C. Early Lamellar Macular Hole in Alport Syndrome: Case report and review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 122–125. [Google Scholar]
- Ioannidis, A.S.; Liasis, A.; Sheldrick, J.; Snead, M.; Nischal, K.K. Lamellar macular hole as the presenting feature in a child with Coats’ disease. J. Pediatr. Ophthalmol. Strabismus 2005, 42, 1. [Google Scholar] [CrossRef]
- Lai, T.T.; Yang, C.M. Lamellar Hole-Associated Epiretina Proliferation in Lamellar Macular Hole and Full Thickness Macular Hole in High Myopia. Retina 2018, 38, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Shimada, N.; Ohno-Matsui, K.; Yoshida, T.; Sugamoto, Y.; Tokoro, T.; Mochizuki, M. Progression from macular retinoschisis to retinal detachment in highly myopic eyes is associated with outer lamellar hole formation. Br. J. Ophthalmol. 2008, 92, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Shimada, N.; Moriyama, M.; Hayashi, K.; Yoshida, T.; Tokoro, T.; Ohno-Matsui, K. Natural History of Lamellar Macular Holes in Highly Myopic Eyes. Am. J. Ophthalmol. 2011, 152, 96–99.e1. [Google Scholar] [CrossRef]
- Rino, F.; Elena, Z.; Ivan, M.; Paolo, B.; Barbara, P.; Federica, R. Lamellar macular hole in high myopic eyes with posterior staphyloma: Morphological and functional characteristics. Graefes. Arch. Clin. Exp. Ophthalmol. 2016, 254, 2141–2150. [Google Scholar] [CrossRef]
- Ghoraba, H. Types of macular holes encountered during diabetic vitrectomy. Retina 2002, 22, 176–182. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, P. Lamellar Macular Hole with Lamellar Hole-Associated Epiretinal Proliferation in Familial Exudative Vitreoretinopathy. Retin. Cases Brief Rep. 2021, 15, 365–368. [Google Scholar] [CrossRef]
- Weichel, E.D.; Colyer, M.H. Traumatic Macular Holes Secondary to Combat Ocular Trauma. Retina 2009, 29, 5. [Google Scholar] [CrossRef]
- Tsui, I.; Campolattaro, B.N.; Lopez, R. Progression of Traumatic Lamellar Macular Hole to Full Thickness Macular Hole and Retinal Detachment in a 3-year-old Child. Retin. Cases Brief Rep. 2010, 4, 2. [Google Scholar] [CrossRef]
- Brazitikos, P.D.; Stangos, N.T. Macular hole formation in diabetic retinopathy: The role of coexisting macular edema. Doc. Ophthalmol. 1999, 97, 273–278. [Google Scholar] [CrossRef]
- Kakehashi, A.; Trempe, C.L.; Fujio, N.; McMeel, J.W.; Schepens, C.L. Retinal breaks in diabetic retinopathy: Vitreoretinal relationships. Ophthalmic Surg. 1994, 25, 695–699. [Google Scholar] [CrossRef]
- Wu, L.; Evans, T.; Arevalo, J.F. Idiopathic macular telangiectasia type 2 (idiopathic juxtafoveolar retinal telangiectasis type 2A, Mac Tel 2). Surv. Ophthalmol. 2013, 58, 536–559. [Google Scholar] [CrossRef]
- Kal, M.; Winiarczyk, M.; Gluszek, S.; Mackiewicz, J. Choroidal thickness in lamellar macular holes. Graefes. Arch. Clin. Exp. Ophthalmol. 2021, 259, 653–659. [Google Scholar] [CrossRef]
- Theodossiadis, P.G.; Grigoropoulos, V.G.; Emfietzoglou, I.; Nikolaidis, P.; Papathanasiou, M.; Theodossiadis, G.P. Spontaneous closure of lamellar macular holes studied by optical coherence tomography. Acta Ophthalmol. 2009, 90, 96–98. [Google Scholar] [CrossRef]
- Cutler, N.E.; Singh, R.P. Spontaneous Closure of Lamellar Macular Hole with Epiretinal Proliferation. Ophthalmol. Retin. 2019, 3, 997. [Google Scholar] [CrossRef]
- Preti, R.C.; Zacharias, L.C.; Cunha, L.P.; Monteiro, M.L.R.; Sarraf, D. Spontaneous closure of degenerative lamellar macular hole with epiretinal membrane proliferation. Int. J. Retin. Vitr. 2021, 7, 64. [Google Scholar] [CrossRef]
- Chehaibou, I.; Manoharan, N.; Govetto, A.; Tsui, I.; Hubschman, J.-P. Spontaneous Lamellar Macular Holes Closure. Retin. Cases Brief Rep. 2022, 16, 397–400. [Google Scholar] [CrossRef]
- Asaad, S.Z. Full-Thickness Macular Hole Progressing from Lamellar Macular Hole with Epiretinal Proliferation. Case Rep. Ophthalmol. 2021, 12, 134–141. [Google Scholar] [CrossRef]
- Theodossiadis, P.G.; Grigoropoulos, V.G.; Emfietzoglou, I.; Nikolaidis, P.; Vergados, I.; Apostolopoulos, M.; Theodossiadis, G.P. Evolution of lamellar macular hole studied by optical coherence tomography. Graefes. Arch. Clin. Exp. Ophthalmol. 2009, 247, 13–20. [Google Scholar] [CrossRef]
- Stino, H.; Wassermann, L.; Ristl, R.; Abela-Formanek, C.; Georgopoulos, M.; Sacu, S.; Schmidt-Erfurth, U.; Pollreisz, A. Evaluation of neuroretinal integrity in optical coherence tomography-graded eyes with partial-thickness macular holes. Acta Ophthalmol. 2022, 100, e1280–e1286. [Google Scholar] [CrossRef]
- Purtskhvanidze, K.; Balken, L.; Hamann, T.; Wöster, L.; von der Burchard, C.; Roider, J.; Treumer, F. Long-term follow-up of lamellar macular holes and pseudoholes over at least 5 years. Graefes. Arch. Clin. Exp. Ophthalmol. 2018, 256, 1067–1078. [Google Scholar] [CrossRef]
- Chung, H.; Lee, K.; Hwang, D.J.; Park, Y.; Sohn, J. Prediction of Morphologic Deterioration in Patients with Lamellar Macular Holes. Retina 2016, 36, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Have, H.; Petrovski, B.E.; Zajac, M.; Lumi, X.; Melekidou, W.; Lytvynchuk, L.; Ruban, A.; Znaor, L.; Nawrocki, J.; Nawrocka, Z.A.; et al. Outcomes from the Retrospective Multicenter Cross-Sectional Study on Lamellar Macular Hole Surgery. Clin. Ophthalmol. 2022, 16, 1847–1860. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-P.; Chen, S.-N.; Chuang, C.-C.; Lin, C.-W.; Lin, C.-J.; Huang, J.-Y.; Yang, C.-M.; Chen, M.-S.; Yang, C.-H. Surgical treatment of lamellar macular hole secondary to epiretinal membrane. Graefes. Arch. Clin. Exp. Ophthalmol. 2013, 251, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Casparis, H.; Bovey, E.H. Surgical treatment of lamellar macular hole associated with epimacular membrane. Retina 2011, 31, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.S.; Noval, S.; Contreras, I. Macular structure on optical coherence tomography after lamellar macular hole surgery and its correlation with visual outcome. Can. J. Ophthalmol. 2011, 46, 491–497. [Google Scholar] [CrossRef]
- Michalewska, Z.; Michalewski, J.; Odrobina, D.; Pikulski, Z.; Cisiecki, S.; Dzięgielewski, K.; Nawrocki, J. Surgical treatment of lamellar macular holes. Graefes. Arch. Clin. Exp. Ophthalmol. 2010, 248, 1395–1400. [Google Scholar] [CrossRef]
- Androudi, S.; Stangos, A.; Brazitikos, P.D. Lamellar Macular Holes: Tomographic Features and Surgical Outcome. Am. J. Ophthalmol. 2009, 148, 420–426. [Google Scholar] [CrossRef]
- Michalewska, Z. Non-Full-Thickness Macular Holes: A Closer Look. Ophthalmic Surg. Lasers Imaging Retin. 2012, 43, 152–161. [Google Scholar] [CrossRef]
- Lee, S.J.; Jang, S.Y.; Moon, D.; Choi, K.S.; Jung, G.Y. Long-term surgical outcomes after vitrectomy for symptomatic lamellar macular holes. Retina 2012, 32, 1743–1748. [Google Scholar] [CrossRef]
- Parisi, G.; Fallico, M.; Maugeri, A.; Barchitta, M.; Agodi, A.; Russo, A.; Longo, A.; Avitabile, T.; Castellino, N.; Bonfiglio, V.; et al. Primary vitrectomy for degenerative and tractional lamellar macular holes: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0246667. [Google Scholar] [CrossRef]
- Obata, S.; Ichiyama, Y.; Kakinoki, M.; Sawada, O.; Saishin, Y.; Ohji, M. Comparison of Surgical Outcomes Between Two Types of Lamellar Macular Holes. Clin. Ophthalmol. 2019, 13, 2541–2546. [Google Scholar] [CrossRef] [PubMed]
- Coassin, M.; Mastrofilippo, V.; Stewart, J.M.; Fanti, A.; Belpoliti, M.; Cimino, L.; Iovieno, A.; Fontana, L. Lamellar macular holes: Surgical outcome of 106 patients with long-term follow-up. Graefes. Arch. Clin. Exp. Ophthalmol. 2018, 256, 1265–1273. [Google Scholar] [CrossRef]
- Murphy, D.C.; Rees, J.; Steel, D.H. Surgical interventions for lamellar macular holes. Cochrane Database Syst. Rev. 2021, 11, CD013678. [Google Scholar] [PubMed]
- Morescalchi, F.; Russo, A.; Gambicorti, E.; Cancarini, A.; Scaroni, N.; Bahja, H.; Costagliola, C.; Semeraro, F. Peeling of the Internal Limiting Membrane with Foveal Sparing for Treatment of Degenerative Lamellar Macular Hole. Retina 2020, 40, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- risina, R.; Parrozzani, R.; Pilotto, E.; Midena, E. A Double Inverted Flap Surgical Technique for the Treatment of Idiopathic Lamellar Macular Hole Associated with Atypical Epiretinal Membrane. Ophthalmologica 2019, 242, 49–58. [Google Scholar] [CrossRef]
- Takahashi, K.; Morizane, Y.; Kimura, S.; Shiode, Y.; Doi, S.; Okanouchi, T.; Takasu, I.; Inoue, Y.; Shiraga, F. Results of lamellar macular hole-associated epiretinal proliferation embedding technique for the treatment of degenerative lamellar macular hole. Graefes. Arch. Clin. Exp. Ophthalmol. 2019, 257, 2147–2154. [Google Scholar] [CrossRef]
- Ho, T.C.; Ho, A.Y.; Chen, M.S. Reconstructing Foveola by Foveolar Internal Limiting Membrane Non-Peeling and Tissue Repositioning for Lamellar Hole-Related Epiretinal Proliferation. Sci. Rep. 2019, 9, 16030. [Google Scholar] [CrossRef]
- Hagenau, F.; Luft, N.; Nobl, M.; Vogt, D.; Klaas, J.E.; Schworm, B.; Siedlecki, J.; Kreutzer, T.C.; Priglinger, S.G. Improving morphological outcome in lamellar macular hole surgery by using highly concentrated autologous platelet-rich plasma. Graefes. Arch. Clin. Exp. Ophthalmol. 2021, 260, 1517–1524. [Google Scholar] [CrossRef]
- Gonzalez, A.; Amin, S.; Iqbal, O.; Potter, S.M.; Khurshid, S.G. Use of Autologous Platelets for Lamellar Macular Hole Repair. Case Rep. Ophthalmol. Med. 2019, 2019, 1471754. [Google Scholar] [CrossRef]
- Lee, C.; Koh, H.J.; Lim, H.T.; Lee, K.S.; Lee, S.C. Prognostic factors in vitrectomy for lamellar macular hole assessed by spectral-domain optical coherence tomography. Acta Ophthalmol. 2012, 90, e597–e602. [Google Scholar] [CrossRef]
- Omoto, T.; Asahina, Y.; Zhou, H.P.; Fujino, R.; Takao, M.; Obata, R.; Inoue, T.; Asaoka, R.; Maruyama-Inoue, M.; Yanagi, Y.; et al. Visual outcomes and prognostic factors of vitrectomy for lamellar macular holes and epiretinal membrane foveoschisis. PLoS ONE 2021, 16, e0247509. [Google Scholar] [CrossRef]
- Romano, M.R.; Vallejo-Garcia, J.L.; Camesasca, F.I.; Vinciguerra, P.; Costagliola, C. Vitreo-papillary adhesion as a prognostic factor in pseudo- and lamellar macular holes. Eye 2012, 26, 810–815. [Google Scholar] [CrossRef]
- Xu, H.; Qin, L.; Zhang, Y.; Xiao, Y.; Zhang, M. Surgery outcomes of lamellar macular eyes with or without lamellar hole-associated epiretinal proliferation: A meta-analysis. BMC Ophthalmol. 2020, 20, 345. [Google Scholar] [CrossRef]
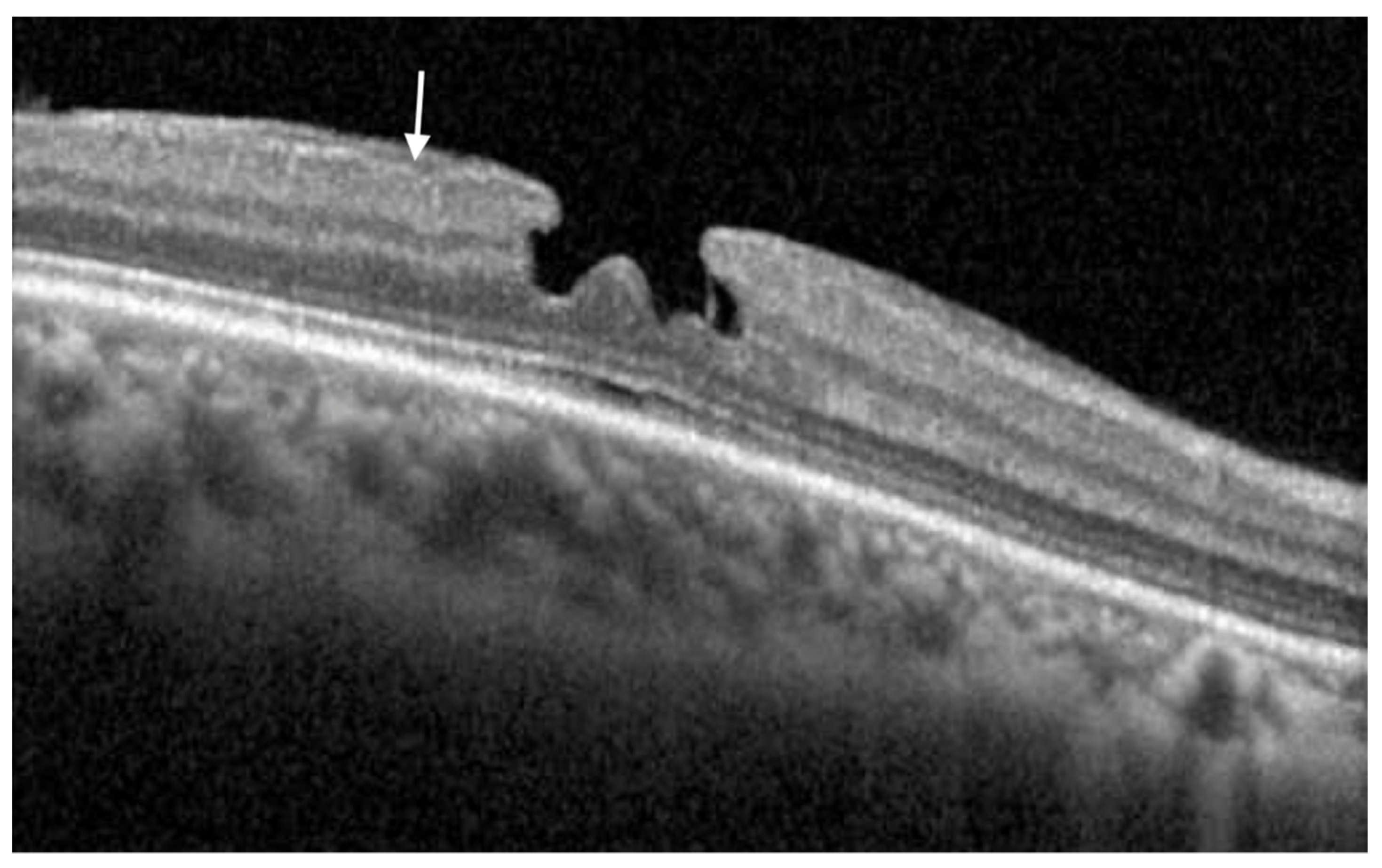
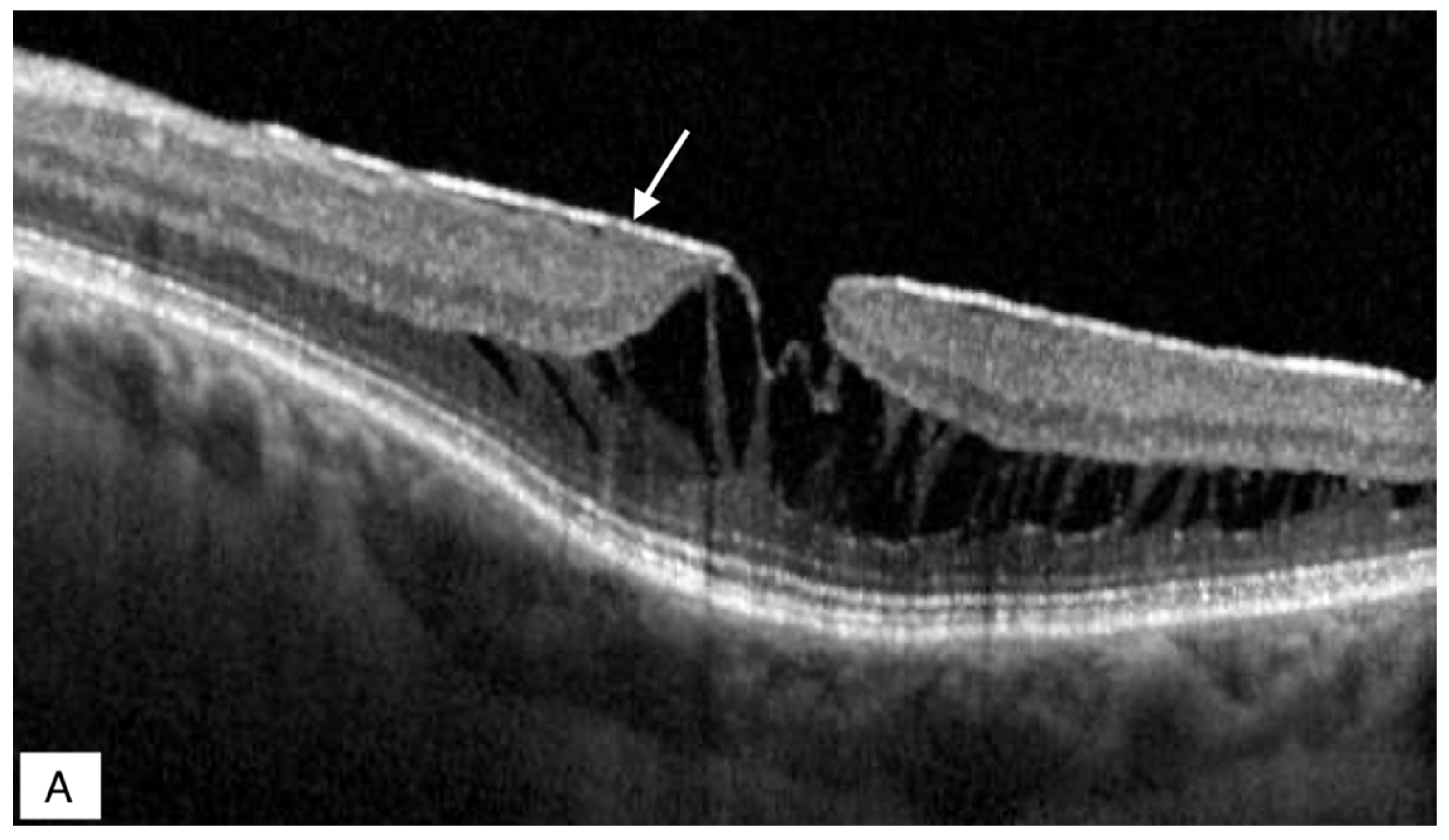
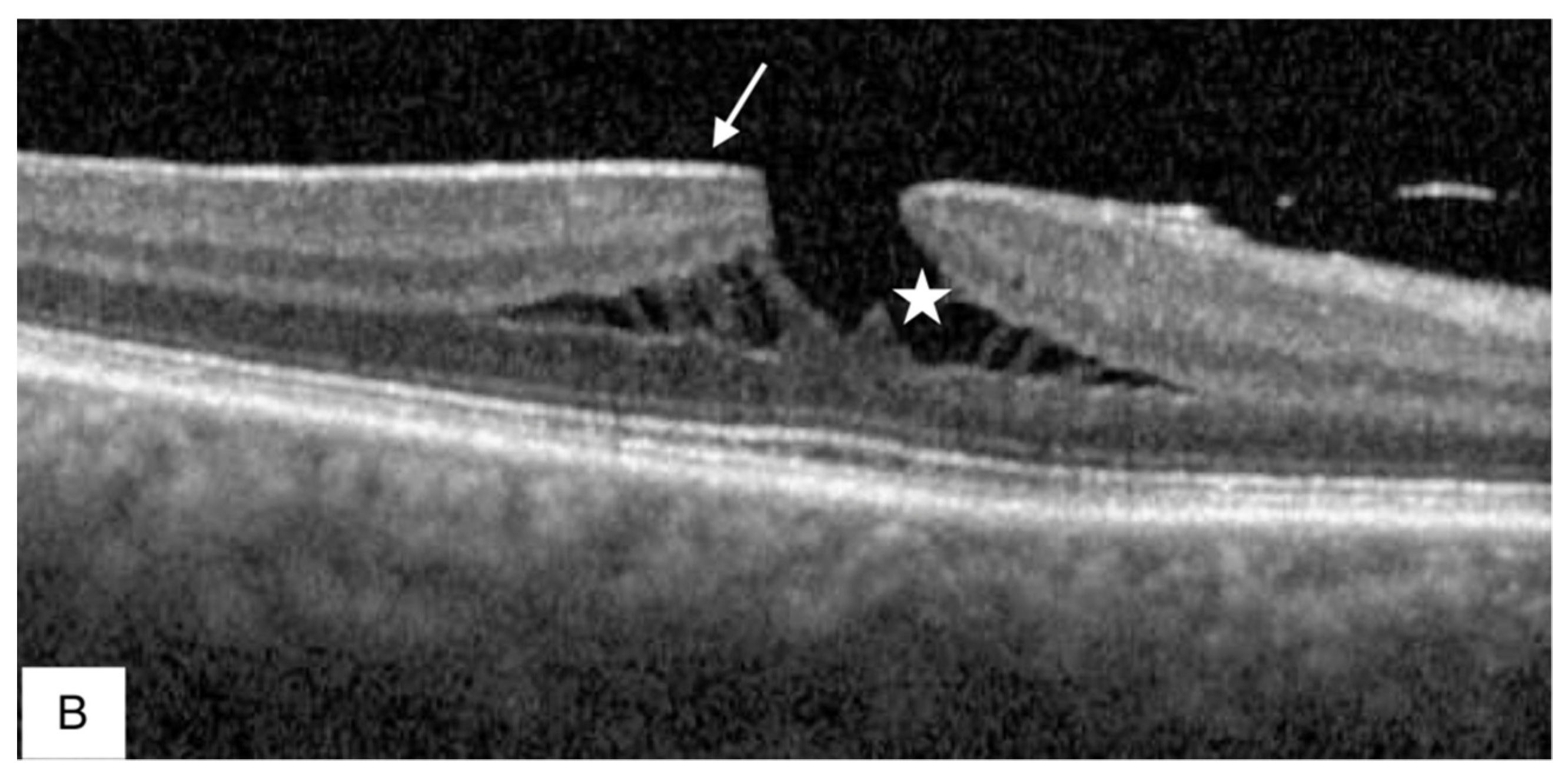
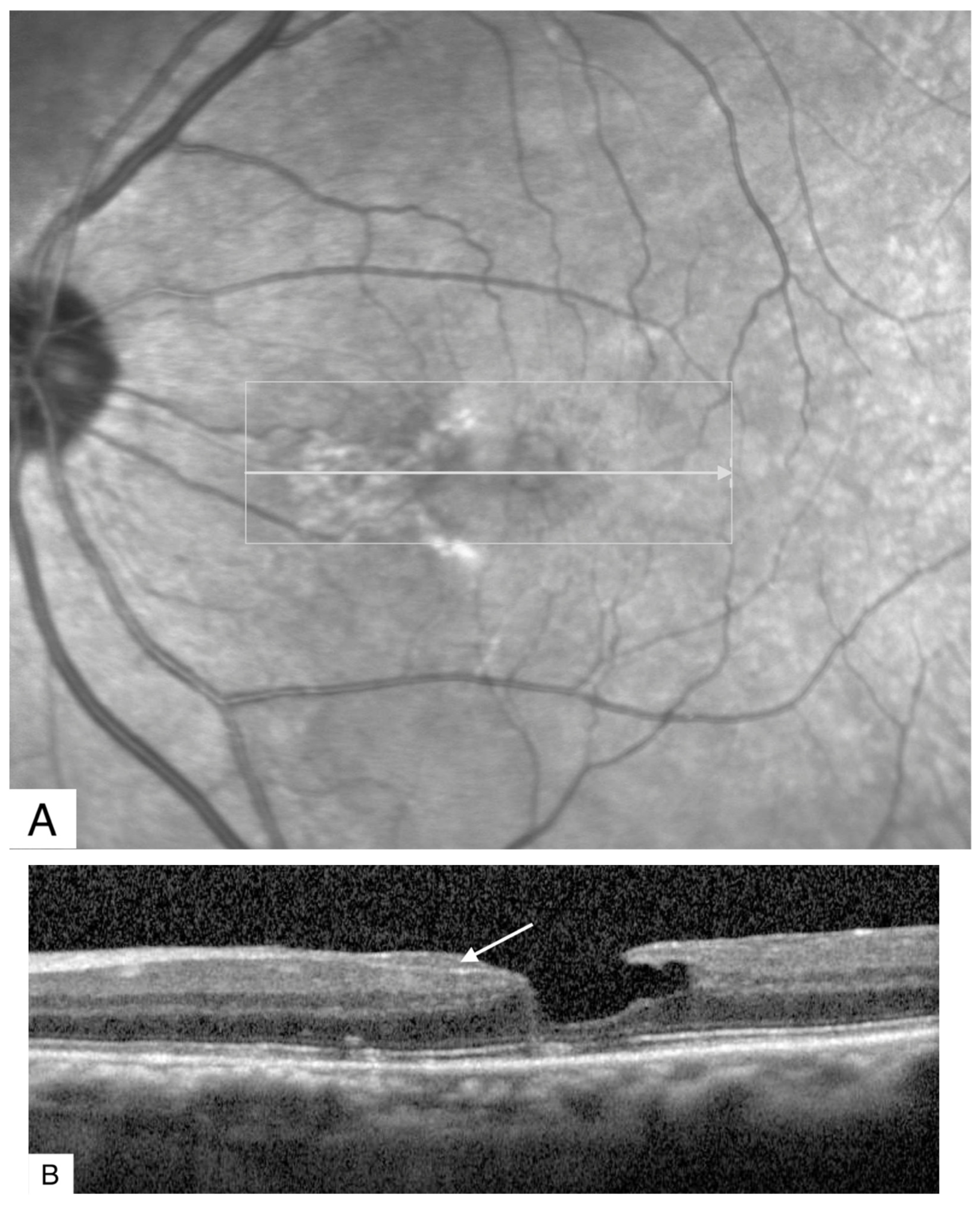
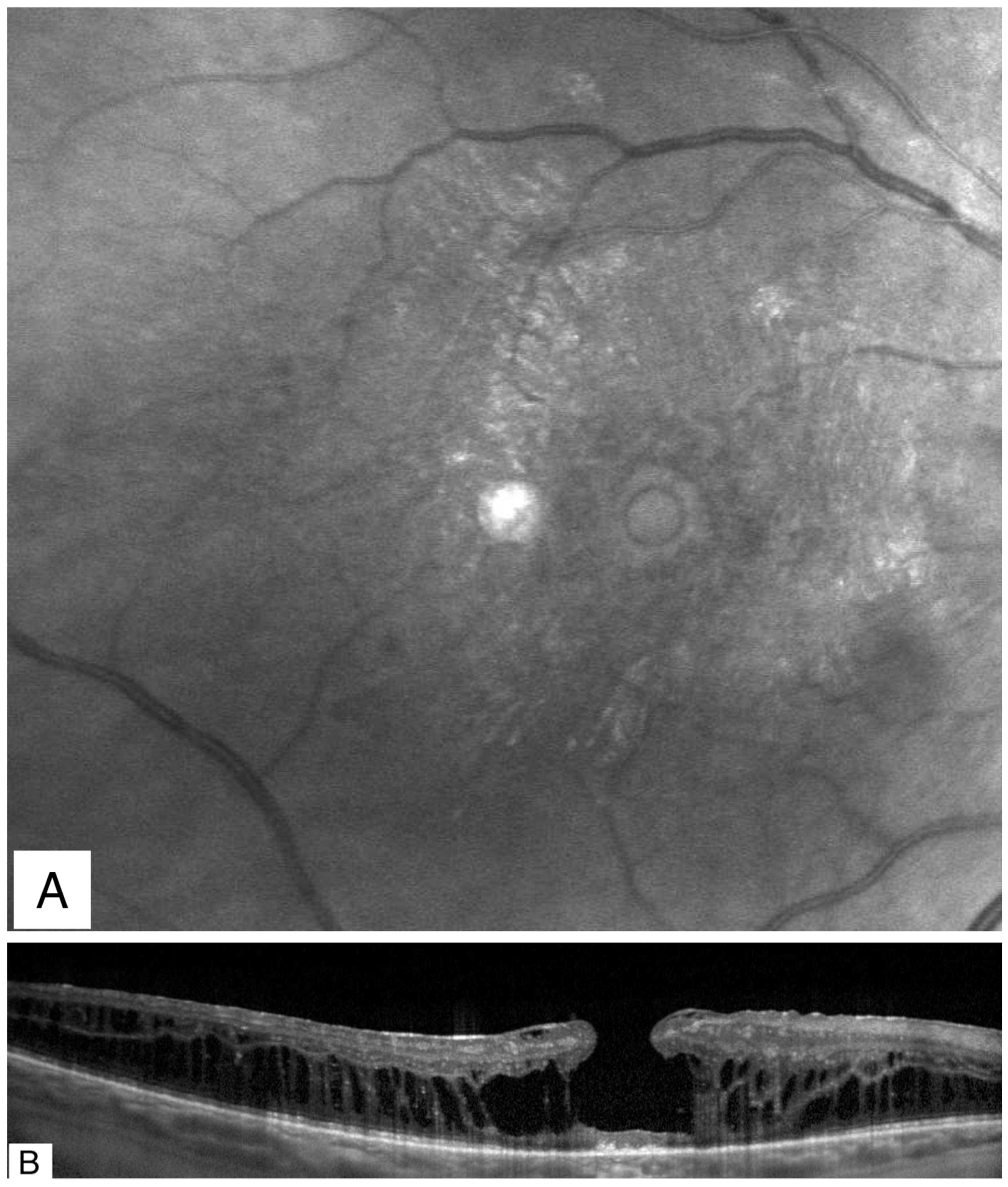

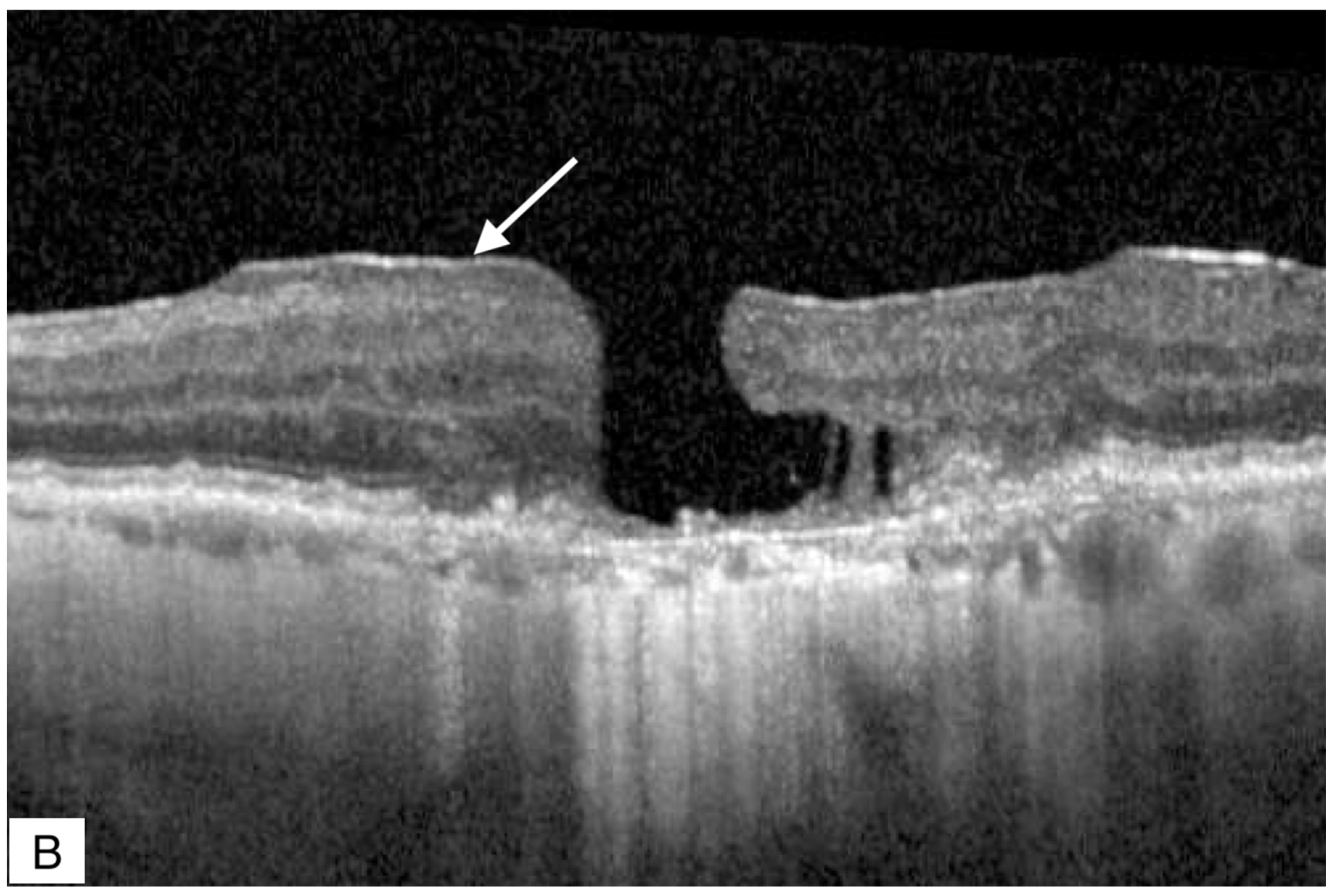
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Bradshaw, R. Primary Lamellar Macular Holes: To Vit or Not to Vit. J. Clin. Med. 2022, 11, 5046. https://doi.org/10.3390/jcm11175046
Wu L, Bradshaw R. Primary Lamellar Macular Holes: To Vit or Not to Vit. Journal of Clinical Medicine. 2022; 11(17):5046. https://doi.org/10.3390/jcm11175046
Chicago/Turabian StyleWu, Lihteh, and Ryan Bradshaw. 2022. "Primary Lamellar Macular Holes: To Vit or Not to Vit" Journal of Clinical Medicine 11, no. 17: 5046. https://doi.org/10.3390/jcm11175046
APA StyleWu, L., & Bradshaw, R. (2022). Primary Lamellar Macular Holes: To Vit or Not to Vit. Journal of Clinical Medicine, 11(17), 5046. https://doi.org/10.3390/jcm11175046





