Tube–Iris Distance and Corneal Endothelial Cell Damage Following Ahmed Glaucoma Valve Implantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgical Techniques
2.3. Examinations
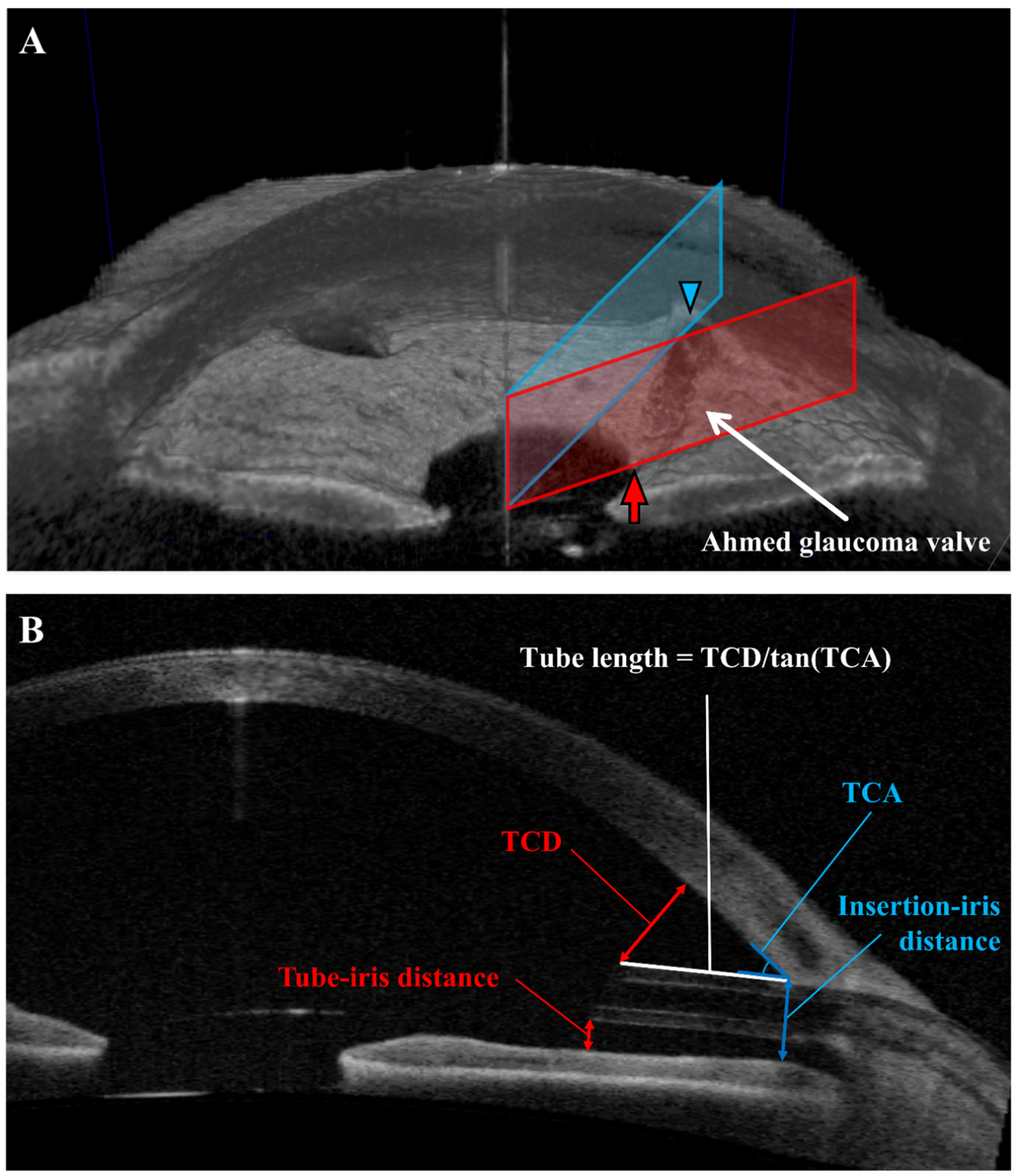
2.4. Anterior Segment Optical Coherence Tomography (AS-OCT)
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Comparison between Patients Separated by Low and High ECD Change Rates
3.3. Tube–Iris Distance Was the Only Statistically Significant Factor Predicting the ECD Change Rate
3.4. Determination of the Optimal Cut-Off Value for TID
3.5. Comparison between Patients with Short and Long TID
3.6. IID/TCA Was Significantly Associated with ECD Change Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arora, K.S.; Robin, A.L.; Corcoran, K.J.; Corcoran, S.L.; Ramulu, P.Y. Use of Various Glaucoma Surgeries and Procedures in Medicare Beneficiaries from 1994 to 2012. Ophthalmology 2015, 122, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L. Treatment Outcomes in the Tube Versus Trabeculectomy (TVT) Study After Five Years of Follow-up. Am. J. Ophthalmol. 2012, 153, 789–803.e782. [Google Scholar] [CrossRef] [PubMed]
- Vinod, K.; Gedde, S.J.; Feuer, W.J.; Panarelli, J.F.; Chang, T.C.; Chen, P.P.; Parrish, R.K., 2nd. Practice Preferences for Glaucoma Surgery: A Survey of the American Glaucoma Society. J. Glaucoma 2017, 26, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Topouzis, F.; Coleman, A.L.; Choplin, N.; Bethlem, M.M.; Hill, R.; Yu, F.; Panek, W.C.; Wilson, M.R. Follow-up of the original cohort with the Ahmed glaucoma valve implant. Am. J. Ophthalmol. 1999, 128, 198–204. [Google Scholar] [CrossRef]
- Minckler, D.S.; Francis, B.A.; Hodapp, E.A.; Jampel, H.D.; Lin, S.C.; Samples, J.R.; Smith, S.D.; Singh, K. Aqueous shunts in glaucoma: A report by the American Academy of Ophthalmology. Ophthalmology 2008, 115, 1089–1098. [Google Scholar] [CrossRef]
- Lee, E.-K.; Yun, Y.-J.; Lee, J.-E.; Yim, J.-H.; Kim, C.-S. Changes in Corneal Endothelial Cells after Ahmed Glaucoma Valve Implantation: 2-Year Follow-up. Am. J. Ophthalmol. 2009, 148, 361–367. [Google Scholar] [CrossRef]
- Mendrinos, E.; Dosso, A.; Sommerhalder, J.; Shaarawy, T. Coupling of HRT II and AS-OCT to evaluate corneal endothelial cell loss and in vivo visualization of the Ahmed glaucoma valve implant. Eye 2009, 23, 1836–1844. [Google Scholar] [CrossRef]
- Bailey, A.K.; Sarkisian, S.R., Jr. Complications of tube implants and their management. Curr. Opin. Ophthalmol. 2014, 25, 148–153. [Google Scholar] [CrossRef]
- Kim, K.N.; Lee, S.B.; Lee, Y.H.; Lee, J.J.; Lim, H.B.; Kim, C.S. Changes in corneal endothelial cell density and the cumulative risk of corneal decompensation after Ahmed glaucoma valve implantation. Br. J. Ophthalmol. 2016, 100, 933–938. [Google Scholar] [CrossRef]
- Qin, V.L.; Kaleem, M.; Conti, F.F.; Rockwood, E.J.; Singh, A.; Sood-Mendiratta, S.; Sears, J.E.; Silva, F.Q.; Eisengart, J.; Singh, R.P. Long-term clinical outcomes of pars Plana versus anterior chamber placement of Glaucoma implant tubes. J. Glaucoma 2018, 27, 440–444. [Google Scholar] [CrossRef]
- Rososinski, A.; Wechsler, D.; Grigg, J. Retrospective review of pars plana versus anterior chamber placement of Baerveldt glaucoma drainage device. J. Glaucoma 2015, 24, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Weiner, A.; Cohn, A.D.; Balasubramaniam, M.; Weiner, A.J. Glaucoma tube shunt implantation through the ciliary sulcus in pseudophakic eyes with high risk of corneal decompensation. J. Glaucoma 2010, 19, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Maris, P.J., Jr.; Tsai, J.C.; Khatib, N.; Bansal, R.; Al-Aswad, L.A. Clinical outcomes of Ahmed Glaucoma valve in posterior segment versus anterior chamber. J. Glaucoma 2013, 22, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.S.; Lee, T.; Seo, D.; Choi, W.; Bae, H.W.; Kim, C.Y. Corneal endothelial cell changes and surgical results after Ahmed glaucoma valve implantation: Ciliary sulcus versus anterior chamber tube placement. Sci. Rep. 2021, 11, 12986. [Google Scholar] [CrossRef]
- Moon, K.; Kim, Y.C.; Kim, K.S. Ciliary Sulcus Ahmed Valve Implantation. KJO 2007, 21, 127–130. [Google Scholar] [CrossRef]
- Tan, A.N.; Webers, C.A.; Berendschot, T.T.; de Brabander, J.; de Witte, P.M.; Nuijts, R.M.; Schouten, J.S.; Beckers, H.J. Corneal endothelial cell loss after Baerveldt glaucoma drainage device implantation in the anterior chamber. Acta Ophthalmol. 2017, 95, 91–96. [Google Scholar] [CrossRef]
- Koo, E.B.; Hou, J.; Han, Y.; Keenan, J.D.; Stamper, R.L.; Jeng, B.H. Effect of glaucoma tube shunt parameters on cornea endothelial cells in patients with Ahmed valve implants. Cornea 2015, 34, 37–41. [Google Scholar] [CrossRef]
- Lee, H.M.; Kim, K.N.; Park, K.S.; Lee, N.H.; Lee, S.B.; Kim, C.-S. Relationship between tube parameters and corneal endothelial cell damage after Ahmed glaucoma valve implantation: A comparative study. J. Clin. Med. 2020, 9, 2546. [Google Scholar] [CrossRef]
- Hau, S.; Scott, A.; Bunce, C.; Barton, K. Corneal endothelial morphology in eyes implanted with anterior chamber aqueous shunts. Cornea 2011, 30, 50–55. [Google Scholar] [CrossRef]
- Lee, C.K.; Ma, K.T.; Hong, Y.J.; Kim, C.Y. Long-term clinical outcomes of Ahmed valve implantation in patients with refractory glaucoma. PLoS ONE 2017, 12, e0187533. [Google Scholar] [CrossRef] [Green Version]
- Donath, C.; Grässel, E.; Baier, D.; Pfeiffer, C.; Bleich, S.; Hillemacher, T. Predictors of binge drinking in adolescents: Ultimate and distal factors—A representative study. BMC Public Health 2012, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, M.; Rad, S.S.; Yazdani, S.; Ghahari, E.; Yaseri, M. Effect of early treatment with aqueous suppressants on Ahmed glaucoma valve implantation outcomes. Ophthalmology 2014, 121, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Oddone, F.; Roberti, G.; Posarelli, C.; Agnifili, L.; Mastropasqua, L.; Carnevale, C.; Micelli Ferrari, T.; Pace, V.; Sacchi, M.; Cremonesi, E.; et al. Endothelial Cell Density after XEN Implant Surgery: Short-term Data from the Italian XEN Glaucoma Treatment Registry (XEN-GTR). J. Glaucoma 2021, 30, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Ibarz-Barberá, M.; Morales-Fernández, L.; Corroto-Cuadrado, A.; Martinez-Galdón, F.; Tañá-Rivero, P.; Gómez de Liaño, R.; Teus, M.A. Corneal Endothelial Cell Loss After PRESERFLO™ MicroShunt Implantation in the Anterior Chamber: Anterior Segment OCT Tube Location as a Risk Factor. Ophthalmol. Ther. 2022, 11, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Vatcheva, K.P.; Lee, M.; McCormick, J.B.; Rahbar, M.H. Multicollinearity in Regression Analyses Conducted in Epidemiologic Studies. Epidemiology (Sunnyvale) 2016, 6, 227. [Google Scholar] [CrossRef] [PubMed]
- Lopilly Park, H.; Jung, K.; Park, C. Serial intracameral visualization of the Ahmed glaucoma valve tube by anterior segment optical coherence tomography. Eye 2012, 26, 1256–1262. [Google Scholar] [CrossRef]
- Joyce, N.C. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003, 22, 359–389. [Google Scholar] [CrossRef]


| Baseline | Overall | Patients without an ECD Decline (Group A, n = 65) | Patients with an ECD Decline (Group B, n = 38) | p-Value |
|---|---|---|---|---|
| Age | 64.20 ± 13.85 | 63.95 ± 13.79 | 64.63 ± 14.12 | 0.812 |
| Sex (M/F) | 64/39 | 40/25 | 24/14 | 0.963 * |
| Laterality (R/L) | 56/47 | 38/27 | 18/20 | 0.376 * |
| AXL (mm) | 24.80 ± 1.89 | 24.78 ± 1.86 | 24.82 ± 1.96 | 0.919 |
| CCT (μm) | 521.37 ± 51.27 | 518.60 ± 51.91 | 526.11 ± 50.49 | 0.476 |
| Follow-up (years) | 4.09 ± 2.20 | 4.02 ± 2.32 | 4.22 ± 2.01 | 0.663 |
| Preoperative IOP (mmHg) | 26.02 ± 8.56 | 25.56 ± 8.68 | 26.82 ± 8.41 | 0.475 |
| Final IOP (mmHg) | 14.17 ± 4.51 | 14.68 ± 4.56 | 13.30 ± 4.36 | 0.135 |
| Preoperative ECD (cells/mm2) | 2183.42 ± 527.69 | 2215.87 ± 473.24 | 2127.90 ± 612.57 | 0.417 |
| Final ECD (cells/mm2) | 1765.49 ± 663.50 | 2119.09 ± 494.94 | 1160.66 ± 441.07 | <0.001 |
| ECD change rate (%/year) | −4.26 ± 9.49 | 0.72 ± 5.42 | −12.78 ± 8.9 | <0.001 |
| Systemic disease | ||||
| Hypertension | 43 (41.75) | 30 (46.15) | 13 (34.21) | 0.328 * |
| Tuberculosis | 5 (4.85) | 3 (4.62) | 2 (5.26) | 1.000 † |
| Diabetes mellitus | 38 (36.89) | 29 (44.62) | 9 (23.68) | 0.056 * |
| Hepatitis | 2 (1.94) | 2 (3.08) | 0 (0.0) | 0.530 † |
| Cerebrovascular accident | 5 (4.85) | 4 (6.15) | 1 (2.63) | 0.649 † |
| Glaucoma | ||||
| POAG | 57 (55.34) | 37 (56.92) | 20 (52.63) | 0.828 * |
| Chronic angle-closure glaucoma | 3 (2.91) | 2 (3.08) | 1 (2.63) | 1.000 † |
| Neovascular glaucoma | 11 (10.68) | 8 (12.31) | 3 (7.89) | 0.742 † |
| Pigmentary glaucoma | 1 (0.97) | 1 (1.54) | 0 (0.0) | 1.000 † |
| Pseudoexfoliation glaucoma | 3 (2.91) | 2 (3.08) | 1 (2.63) | 1.000 † |
| Secondary (d/t uveitis) | 12 (11.65) | 6 (9.23) | 6 (15.79) | 0.495 * |
| Secondary other | 16 (15.53) | 9 (13.85) | 7 (18.42) | 0.736 * |
| Tube Parameters | Overall | Patients without an ECD Decline (Group A, n = 65) | Patients with an ECD Decline (Group B, n = 38) | p-Value |
|---|---|---|---|---|
| Tube length (mm) | 1.15 ± 0.44 | 1.09 ± 0.37 | 1.27 ± 0.53 | 0.044 * |
| Tube–corneal distance (mm) | 0.77 ± 0.47 | 0.77 ± 0.40 | 0.76 ± 0.57 | 0.932 |
| Tube–iris distance (mm) | 0.32 ± 0.36 | 0.23 ± 0.25 | 0.47 ± 0.46 | 0.004 * |
| Tube–corneal angle (°) | 31.85 ± 11.58 | 33.67 ± 11.24 | 28.73 ± 11.62 | 0.036 * |
| Insertion–iris distance (mm) | 0.68 ± 0.27 | 0.66 ± 0.24 | 0.71 ± 0.32 | 0.372 |
| Tube location (ST/SN) † | 101/2 | 63/2 | 38/0 | 0.530 |
| Variables | Univariable Analysis | ||
|---|---|---|---|
| β | 95% Confidence Interval | p-Value | |
| Age (years) | 1.10 × 10−5 | [−1.34 × 10−3, 1.36 × 10−3] | 0.987 |
| Sex (M/F) | −4.30 × 10−3 | [−4.27 × 10−2, 3.41 × 10−2] | 0.825 |
| Laterality (R/L) | 3.40 × 10−5 | [−3.74 × 10−2, 3.74 × 10−2] | 0.999 |
| AXL (mm) | 1.70 × 10−4 | [−9.74 × 10−3, 1.01 × 10−2] | 0.973 |
| CCT (μm) | −7.70 × 10−5 | [−4.42 × 10−4, 2.88 × 10−4] | 0.676 |
| Follow-up (years) | −3.26 × 10−3 | [−1.17 × 10−2, 5.21 × 10−3] | 0.447 |
| Preoperative IOP (mmHg) | −2.52 × 10−4 | [−2.44 × 10−3, 1.93 × 10−3] | 0.819 |
| Final IOP (mmHg) | 2.89 × 10−3 | [−1.22 × 10−3, 7.00 × 10−3] | 0.166 |
| Preoperative ECD(cells/mm2) | −2.10 × 10−5 | [−5.70 × 10−5, 1.40 × 10−5] | 0.232 |
| Final ECD (cells/mm2) | 8.70 × 10−5 | [6.40 × 10−5, 1.09 × 10−4] | <0.001 * |
| Systemic disease | |||
| Hypertension | 2.01 × 10−2 | [−1.75 × 10−2, 5.77 × 10−2] | 0.292 |
| Tuberculosis | −6.50 × 10−3 | [−9.32 × 10−2, 8.02 × 10−2] | 0.882 |
| Diabetes mellitus | 2.64 × 10−2 | [−1.19 × 10−2, 6.47 × 10−2] | 0.174 |
| Hepatitis | 9.97 × 10−2 | [−3.39 × 10−2, 2.33 × 10−1] | 0.142 |
| Cerebrovascular accident | 5.60 × 10−2 | [−3.00 × 10−2, 1.42 × 10−1] | 0.199 |
| Glaucoma | |||
| POAG | 7.25 × 10−3 | [−3.02 × 10−2, 4.47 × 10−2] | 0.702 |
| Chronic angle-closure glaucoma | 9.86 × 10−3 | [−1.01 × 10−1, 1.21 × 10−1] | 0.860 |
| Neovascular glaucoma | 2.99 × 10−2 | [−3.02 × 10−2, 8.99 × 10−2] | 0.326 |
| Pigmentary glaucoma | 7.35 × 10−2 | [−1.16 × 10−1, 2.63 × 10−1] | 0.443 |
| Pseudoexfoliation glaucoma | −3.57 × 10−2 | [−1.46 × 10−1, 7.49 × 10−2] | 0.524 |
| Secondary (d/t uveitis) | −2.87 × 10−2 | [−8.65 × 10−2, 2.91 × 10−2] | 0.326 |
| Secondary other | −1.27 × 10−2 | [−6.40 × 10−2, 3.87 × 10−2] | 0.626 |
| Tube parameters | |||
| Tube length (mm) | −4.22 × 10−2 | [−8.38 × 10−2, −5.64 × 10−4] | 0.047 * |
| Tube–corneal distance (mm) | −4.04 × 10−3 | [−4.41 × 10−2, 3.60 × 10−2] | 0.842 |
| Tube–iris distance (mm) | −7.22 × 10−2 | [−1.22 × 10−1, −2.23 × 10−2] | 0.005 * |
| Tube–corneal angle (°) | 1.11 × 10−3 | [−4.94 × 10−4, 2.71 × 10−3] | 0.173 |
| Insertion–iris distance (mm) | −6.73 × 10−2 | [−1.35 × 10−1, 5.87 × 10−4] | 0.052 |
| Tube location (ST/SN) | −6.53 × 10−2 | [−2.00 × 10−1, 6.91 × 10−2] | 0.337 |
| Anterior chamber parameters | |||
| ACD (mm) | −2.37 × 10−2 | [−4.87 × 10−2, 1.33 × 10−3] | 0.063 |
| ITC (%) | −9.30 × 10−5 | [−7.19 × 10−4, 5.34 × 10−4] | 0.770 |
| ACW (mm) | 1.82 × 10−2 | [−2.27 × 10−2, 5.91 × 10−2] | 0.379 |
| nasal AOD500 (μm) | 3.00 × 10−5 | [−4.10 × 10−5, 1.01 × 10−4] | 0.405 |
| nasal AOD750 (μm) | 1.20 × 10−5 | [−4.10 × 10−5, 6.60 × 10−5] | 0.650 |
| nasal ARA500 (μm) | 4.70 × 10−5 | [−1.20 × 10−4, 2.15 × 10−4] | 0.576 |
| nasal ARA750 (μm) | 2.90 × 10−5 | [−7.20 × 10−5, 1.30 × 10−4] | 0.575 |
| nasal TISA500 (μm) | 7.00 × 10−5 | [−1.18 × 10−4, 2.59 × 10−4] | 0.461 |
| nasal TISA750 (μm) | 3.60 × 10−5 | [−7.20 × 10−5, 1.44 × 10−4] | 0.510 |
| nasal TIA500 (°) | 3.25 × 10−4 | [−7.94 × 10−4, 1.45 × 10−3] | 0.565 |
| nasal TIA750 (°) | 2.26 × 10−4 | [−1.02 × 10−3, 1.47 × 10−3] | 0.719 |
| temporal AOD500 (μm) | 3.10 × 10−5 | [−3.50 × 10−5, 9.80 × 10−5] | 0.350 |
| temporal AOD750 (μm) | 1.50 × 10−5 | [−3.40 × 10−5, 6.50 × 10−5] | 0.540 |
| temporal ARA500 (μm) | 9.70 × 10−5 | [−5.00 × 10−5, 2.43 × 10−4] | 0.194 |
| temporal ARA750 (μm) | 4.60 × 10−5 | [−4.50 × 10−5, 1.37 × 10−4] | 0.317 |
| temporal TISA500 (μm) | 9.50 × 10−5 | [−7.30 × 10−5, 2.62 × 10−4] | 0.264 |
| temp_TISA_750 (μm) | 4.70 × 10−5 | [−5.00 × 10−5, 1.45 × 10−4] | 0.337 |
| temp_TIA_500 (°) | 3.73 × 10−4 | [−6.68 × 10−4, 1.41 × 10−3] | 0.479 |
| temp_TIA_750 (°) | 4.02 × 10−4 | [−7.94 × 10−4, 1.60 × 10−3] | 0.506 |
| Variables | Multivariable Analysis (TCD Excluded) | Multivariable Analysis (TCA Excluded) | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | |
| Tube parameters | ||||||
| Tube length (mm) | −3.71 × 10−2 | [−7.80 × 10−2, 4.00 × 10−3] | 0.076 | −3.00 × 10−2 | [−8.40 × 10−2, 2.40 × 10−2] | 0.269 |
| Tube–corneal distance (mm) | −1.13 × 10−2 | [−6.60 × 10−2, 4.40 × 10−2] | 0.683 | |||
| Tube–iris distance (mm) | −6.84 × 10−2 | [−1.28 × 10−1, −9.00 × 10−3] | 0.025 * | −7.43 × 10−2 | [−1.34 × 10−1, −1.50 × 10−2] | 0.015 * |
| Tube–corneal angle (°) | −3.52 × 10−5 | [−2.00 × 10−3, 2.00 × 10−3] | 0.970 | |||
| Anterior chamber parameters | ||||||
| ITC (%) | −6.25 × 10−5 | [−1.00 × 10−3, 1.00 × 10−3] | 0.838 | −7.03 × 10−5 | [−1.00 × 10−3, 1.00 × 10−3] | 0.818 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Cho, W.J.; Kim, J.D.; Cho, H.; Bae, H.W.; Kim, C.Y.; Choi, W. Tube–Iris Distance and Corneal Endothelial Cell Damage Following Ahmed Glaucoma Valve Implantation. J. Clin. Med. 2022, 11, 5057. https://doi.org/10.3390/jcm11175057
Kim Y, Cho WJ, Kim JD, Cho H, Bae HW, Kim CY, Choi W. Tube–Iris Distance and Corneal Endothelial Cell Damage Following Ahmed Glaucoma Valve Implantation. Journal of Clinical Medicine. 2022; 11(17):5057. https://doi.org/10.3390/jcm11175057
Chicago/Turabian StyleKim, Yitak, Won Jeong Cho, Jung Dong Kim, Hyuna Cho, Hyoung Won Bae, Chan Yun Kim, and Wungrak Choi. 2022. "Tube–Iris Distance and Corneal Endothelial Cell Damage Following Ahmed Glaucoma Valve Implantation" Journal of Clinical Medicine 11, no. 17: 5057. https://doi.org/10.3390/jcm11175057






