Consistency among Office, Home, and Ambulatory Blood Pressure Values in Women with Chronic Hypertension and History of Eclampsia or Preeclampsia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Office Blood Pressure Measurement
2.3. Ambulatory Blood Pressure Measurement
2.4. Home Blood Pressure Measurement
2.5. Treatment
2.6. Study Outcomes
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Study Group
3.2. Achieved Blood Pressure
3.3. Systolic Blood Pressure
3.4. Diastolic Blood Pressure
3.5. Pregnancy Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Jameil, N.; Aziz Khan, F.; Fareed Khan, M.; Tabassum, H. A brief overview of preeclampsia. J. Clin. Med. Res. 2014, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Le Ray, I.; Zhu, J.; Zhang, J.; Hua, J.; Reilly, M. Preeclampsia prevalence, risk factors, and pregnancy outcomes in Sweden and China. JAMA Netw. Open 2021, 4, e218401. [Google Scholar] [CrossRef] [PubMed]
- Duley, L. Pre-eclampsia, eclampsia, and hypertension. BMJ Clin. Evid. 2011, 2011, 3275298. [Google Scholar]
- Hernández-Díaz, S.; Toh, S.; Cnattingius, S. Risk of pre-eclampsia in first and subsequent pregnancies: Prospective cohort study. BMJ 2009, 338, b2255. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar]
- Cífková, R.; Johnson, M.R.; Kahan, T.; Brguljan, J.; Williams, B.; Coca, A.; Manolis, A.; Thomopoulos, C.; Borghi, C.; Tsioufis, C.; et al. Peripartum management of hypertension: A position paper of the ESC Council on Hypertension and the European Society of Hypertension. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 384–393. [Google Scholar] [CrossRef]
- Brown, M.A.; Buddle, M.L.; Cario, G.M.; Whitworth, J.A. Ambulatory blood pressure monitoring during pregnancy comparison with mercury sphygmomanometry. Am. J. Hypertens. 1993, 6, 745–749. [Google Scholar]
- Salazar, M.R.; Espeche, W.G.; Leiva Sisnieguez, B.C.; Balbín, E.; Leiva Sisnieguez, C.E.; Stavile, R.N.; March, C.E.; Grassi, F.; Santillan, C.; Cor, S.; et al. Significance of masked and nocturnal hypertension in normotensive women coursing a high-risk pregnancy. J. Hypertens. 2016, 34, 2248–2252. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Giannubilo, S.R.; Dell’Uomo, B.; Corradetti, A. Prediction of gestational hypertension or intrauterine fetal growth restriction by mid-trimester 24-h ambulatory blood pressure monitoring. Int. J. Gynaecol. Obstet. 2004, 85, 126–131. [Google Scholar] [CrossRef]
- Liro, M.; Gasowski, J.; Wydra, D.; Grodzicki, T.; Emerich, J.; Narkiewicz, K. Twenty-four-hour and conventional blood pressure components and risk of preterm delivery or neonatal complications in gestational hypertension. Blood Press. 2009, 18, 36–43. [Google Scholar] [CrossRef]
- Eguchi, K.; Ohmaru, T.; Ohkuchi, A.; Hirashima, C.; Takahashi, K.; Suzuki, H.; Kario, K.; Matsubara, S.; Suzuki, M. Ambulatory BP monitoring and clinic BP in predicting small-for-gestational-age infants during pregnancy. J. Hum. Hypertens. 2016, 30, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.S.; Palatini, P.; Parati, G.; O’Brien, E.; Januszewicz, A.; Lurbe, E.; Persu, A.; Mancia, G.; Kreutz, R.; European Society of Hypertension Council and the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J. Hypertens. 2021, 39, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Robinson, A.; Bowyer, L.; Buddle, M.L.; Martin, A.; Hargood, J.L.; Cario, G.M. Ambulatory blood pressure monitoring in pregnancy: What is normal? Am. J. Obstet. Gynecol. 1998, 178, 836–842. [Google Scholar] [CrossRef]
- Churchill, D.; Beevers, D.G. Differences between office and 24-hour ambulatory blood pressure measurement during pregnancy. Obstet. Gynecol. 1996, 88, 455–461. [Google Scholar] [CrossRef]
- Brown, M.A.; McHugh, L.; Mangos, G.; Davis, G. Automated self-initiated blood pressure or 24-hour ambulatory blood pressure monitoring in pregnancy? BJOG 2004, 111, 38–41. [Google Scholar] [CrossRef]
- Olofsson, P.; Persson, K. A comparison between conventional and 24-hour automatic blood pressure monitoring in hypertensive pregnancy. Acta Obstet. Gynecol. Scand. 1995, 74, 429–433. [Google Scholar] [CrossRef]
- Townsend, R.R. Out-of-office blood pressure monitoring: A comparison of ambulatory blood pressure monitoring and home (self) monitoring of blood pressure. Hypertension 2020, 76, 1667–1673. [Google Scholar] [CrossRef]
- Chrubasik, S.; Droste, C.; Glimm, E.; Black, A. Comparison of different methods of blood pressure measurements. Blood Press. Monit. 2007, 12, 157–166. [Google Scholar] [CrossRef]
- Zeniodi, M.E.; Ntineri, A.; Kollias, A.; Servos, G.; Moyssakis, I.; Destounis, A.; Harokopakis, A.; Vazeou, A.; Stergiou, G.S. Home and ambulatory blood pressure monitoring in children, adolescents and young adults: Comparison, diagnostic agreement and association with preclinical organ damage. J. Hypertens. 2020, 38, 1047–1055. [Google Scholar] [CrossRef]
- Eguchi, K.; Kuruvilla, S.; Ishikawa, J.; Ogedegbe, G.; Gerin, W.; Schwartz, J.E.; Pickering, T.G. Pickering, Correlations between different measures of clinic, home, and ambulatory blood pressure in hypertensive patients. Blood Press. Monit. 2011, 16, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Ntineri, A.; Niiranen, T.J.; McManus, R.J.; Lindroos, A.; Jula, A.; Schwartz, C.; Kollias, A.; Andreadis, E.A.; Stergiou, G.S. Ambulatory versus home blood pressure monitoring: Frequency and determinants of blood pressure difference and diagnostic disagreement. J. Hypertens. 2019, 37, 1974–1981. [Google Scholar] [PubMed]
- Stergiou, G.S.; Palatini, P.; Asmar, R.; Ioannidis, J.P.; Kollias, A.; Lacy, P.; McManus, R.J.; Myers, M.G.; Parati, G.; Shennan, A.; et al. Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J. Hypertens. 2019, 37, 459–466. [Google Scholar] [PubMed]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar]
- Sobieraj, P.; Gaciong, Z. How to assess the agreement between different methods of blood pressure measurement? J. Clin. Hypertens. 2020, 22, 690. [Google Scholar] [CrossRef]
- Seidlerová, J.; Gelžinský, J.; Mateřánková, M.; Ceral, J.; König, P.; Filipovský, J. In the aftermath of Sprint: Further comparison of unattended automated office blood pressure measurement and 24-hour blood pressure monitoring. Blood Press. 2018, 27, 256–261. [Google Scholar]


| Parameter | Value (N = 79) |
|---|---|
| Age (years) | 34 ± 4.7 |
| Baseline blood pressure (mm Hg): | |
| 24 h ABPM SBP/DBP | 122.9 ± 13.1/77.0 ± 9.9 |
| Day ABPM SBP/DBP | 126.9 ± 13.6/80.9 ± 10.4 |
| Night ABPM SBP/DBP | 113.7 ± 13.8/67.9 ± 9.9 |
| OBPM SBP/DBP | 134.0 ± 15.9/83.7 ± 11.4 |
| HBPM SBP/DBP | 128.1 ± 16.5/79.9 ± 11.3 |
| Pre-pregnancy nutritional status: | |
| BMI (kg/m2) | 24.1 ± 3.2 |
| Underweight (n, %) | 1 (1.3) |
| Normal weight (n, %) | 50 (63.3) |
| Overweight (n, %) | 28 (35.4) |
| Obese (n, %) | 1 (1.3) |
| History of pre-eclampsia (n, %) | 71 (89.9) |
| History of eclampsia (n, %) | 8 (10.1) |
| History of stillbirth (n, %) | 10 (12.7) |
| History of miscarriage (n, %) | 72 (91.1) |
| Obstetric history: | |
| 2nd pregnancy (n, %) | 59 (74.7) |
| 3rd pregnancy (n, %) | 11 (13.9) |
| 4th pregnancy (n, %) | 4 (5.1) |
| 5th pregnancy (n, %) | 3 (3.8) |
| 6th pregnancy (n, %) | 1 (1.3) |
| 8th pregnancy (n, %) | 1 (1.3) |
| Pregnancy Week | Method of Measurement | SBP (mm Hg) | p-Value | DBP (mm Hg) | p-Value |
|---|---|---|---|---|---|
| 10th | ABPM 24 h | 123.5 ± 10.7 | - | 77.6 ± 8 | - |
| ABPM day | 126.9 ± 10.1 | <0.001 | 81.2 ± 7.8 | <0.001 | |
| ABPM night | 114.7 ± 12.3 | <0.001 | 69.6 ± 9.3 | <0.001 | |
| HBPM | 130.6 ± 13.5 | <0.001 | 79.4 ± 9.1 | 0.057 | |
| OBPM | 135.2 ± 13.6 | <0.001 | 83.4 ± 9.3 | <0.001 | |
| 25th | ABPM 24 h | 122.4 ± 10 | - | 76.3 ± 7.6 | - |
| ABPM day | 125.8 ± 10.3 | <0.001 | 79.6 ± 7.9 | <0.001 | |
| ABPM night | 114.4 ± 11.5 | <0.001 | 68.3 ± 8.8 | <0.001 | |
| HBPM | 127.3 ± 10.1 | <0.001 | 75.5 ± 7.8 | 0.728 | |
| OBPM | 133.1 ± 10.5 | <0.001 | 81 ± 8.1 | <0.001 | |
| 37th | ABPM 24 h | 129.3 ± 9.4 | - | 79.9 ± 7.6 | - |
| ABPM day | 132.7 ± 9.9 | <0.001 | 83.3 ± 8.2 | <0.001 | |
| ABPM night | 122.6 ± 13.8 | <0.001 | 72.1 ± 7.9 | <0.001 | |
| HBPM | 134.8 ± 9.6 | <0.001 | 80.7 ± 7.3 | 1.0 | |
| OBPM | 141.5 ± 10.5 | <0.001 | 86.5 ± 7.9 | <0.001 |
| Week | Method of Blood Pressure Measurement | Bias (mm Hg) | Lower Limit of Agreement (mm Hg) | Upper Limit of Agreement (mm Hg) |
|---|---|---|---|---|
| 10th | SBP: 24 h ABPM vs. HBPM | −7 (−8.6–−5.5) | −20.4 (−23.1–−17.7) | 6.3 (3.6–9) |
| SBP: 24 h ABPM vs. OBPM | −11.6 (−13.2–−10.1) | −24.9 (−27.6–−22.3) | 1.7 (−1–4.4) | |
| DBP: 24 h ABPM vs. HBPM | −1.8 (−3.2–−0.4) | −14 (−16.4–−11.5) | 10.4 (7.9–12.8) | |
| DBP: 24 h ABPM vs. OBPM | −5.8 (−7.2–−4.4) | −17.9 (−20.4–−15.5) | 6.3 (3.8–8.7) | |
| SBP: day ABPM vs. HBPM | −3.7 (−5.1–−2.2) | −15.8 (−18.3–−13.4) | 8.5 (6.1–11) | |
| SBP: day ABPM vs. OBPM | −8.2 (−9.7–−6.8) | −20.5 (−22.9–−18) | 4 (1.5–6.5) | |
| DBP: day ABPM vs. HBPM | 1.8 (0.4–3.2) | −10.3 (−12.7–−7.8) | 13.9 (11.4–16.3) | |
| DBP: day ABPM vs. OBPM | −2.2 (−3.7–−0.8) | −14.4 (−16.8–−11.9) | 9.9 (7.5–12.3) | |
| SBP: OBPM vs. HBPM | 4.6 (4.3–4.9) | 2.2 (1.7–2.6) | 7 (6.5–7.5) | |
| DBP: OBPM vs. HBPM | 4 (3.6–4.4) | 0.7 (0–1.4) | 7.3 (6.7–8) | |
| 25th | SBP: 24 h ABPM vs. HBPM | −4.9 (−6.1–−3.8) | −14.6 (−16.5–−12.7) | 4.7 (2.8–6.6) |
| SBP: 24 h ABPM vs. OBPM | −10.7 (−11.7–−9.6) | −19.9 (−21.7–−18) | −1.5 (−3.3–0.4) | |
| DBP: 24 h ABPM vs. HBPM | 0.8 (−0.4–2.1) | −9.8 (−11.9–−7.7) | 11.4 (9.3–13.5) | |
| DBP: 24 h ABPM vs. OBPM | −4.7 (−5.9–−3.5) | −15 (−17.1–−13) | 5.7 (3.6–7.8) | |
| SBP: day ABPM vs. HBPM | −1.5 (−3–−0.1) | −13.9 (−16.4–−11.4) | 10.8 (8.4–13.3) | |
| SBP: day ABPM vs. OBPM | −7.3 (−8.7–−5.8) | −19.5 (−21.9–−17) | 5 (2.5–7.4) | |
| DBP: day ABPM vs. HBPM | 4.1 (2.8–5.4) | −7.1 (−9.3–−4.9) | 15.4 (13.1–17.6) | |
| DBP: day ABPM vs. OBPM | −1.4 (−2.6–−0.1) | −12.5 (−14.8–−10.3) | 9.8 (7.6–12.1) | |
| SBP: OBPM vs. HBPM | 5.7 (5.4–6.1) | 2.9 (2.3–3.5) | 8.5 (8–9.1) | |
| DBP: OBPM vs. HBPM | 5.5 (5–6) | 1.5 (0.7–2.3) | 9.5 (8.7–10.3) | |
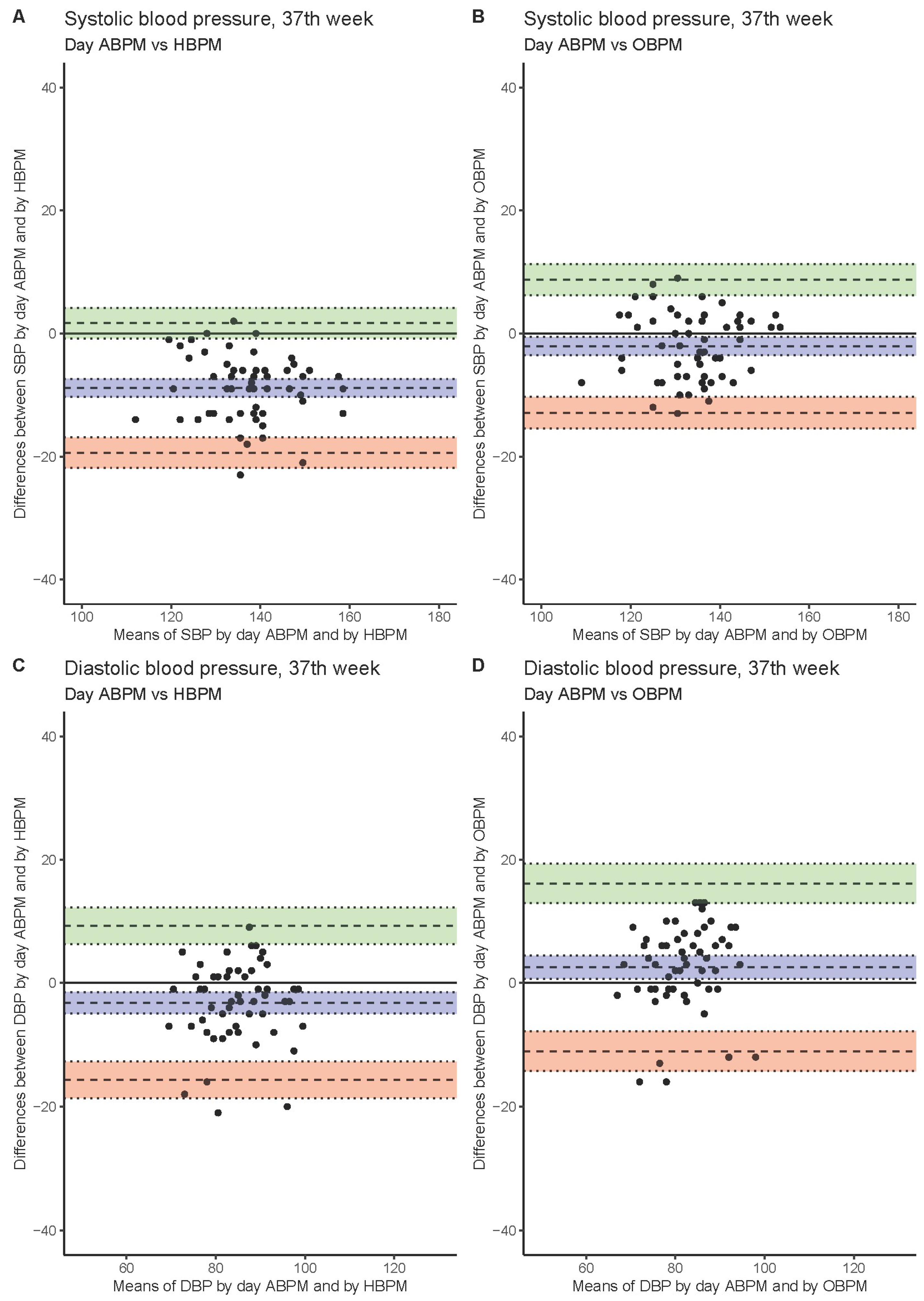
| 37th | SBP: 24 h ABPM vs. HBPM | −5.5 (−6.9–−4) | −15.9 (−18.4–−13.5) | 5 (2.5–7.4) |
| SBP: 24 h ABPM vs. OBPM | −12.3 (−13.8–−10.8) | −23.1 (−25.6–−20.5) | −1.5 (−4.1–1.1) | |
| DBP: 24 h ABPM vs. HBPM | −0.9 (−2.7–1) | −14.2 (−17.3–−11) | 12.4 (9.3–15.6) | |
| DBP: 24 h ABPM vs. OBPM | −6.6 (−8.3–−4.9) | −18.9 (−21.8–−16) | 5.7 (2.7–8.6) | |
| SBP: day ABPM vs. HBPM | −2.1 (−3.6–−0.6) | −12.9 (−15.5–−10.3) | 8.7 (6.2–11.3) | |
| SBP: day ABPM vs. OBPM | −8.9 (−10.3–−7.4) | −19.4 (−21.9–−16.9) | 1.7 (−0.8–4.2) | |
| DBP: day ABPM vs. HBPM | 2.5 (0.7–4.4) | −11 (−14.3–−7.8) | 16.1 (12.9–19.3) | |
| DBP: day ABPM vs. OBPM | −3.2 (−4.9–−1.5) | −15.7 (−18.6–−12.7) | 9.2 (6.3–12.2) | |
| SBP: OBPM vs. HBPM | 6.8 (6–7.6) | 0.8 (−0.6–2.2) | 12.8 (11.3–14.2) | |
| DBP: OBPM vs. HBPM | 5.8 (5–6.5) | 0.5 (−0.7–1.8) | 11 (9.8–12.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojciechowska, E.; Sobieraj, P.; Siński, M.; Zaborska-Dworak, M.A.; Gryglas, P.; Lewandowski, J. Consistency among Office, Home, and Ambulatory Blood Pressure Values in Women with Chronic Hypertension and History of Eclampsia or Preeclampsia. J. Clin. Med. 2022, 11, 5065. https://doi.org/10.3390/jcm11175065
Wojciechowska E, Sobieraj P, Siński M, Zaborska-Dworak MA, Gryglas P, Lewandowski J. Consistency among Office, Home, and Ambulatory Blood Pressure Values in Women with Chronic Hypertension and History of Eclampsia or Preeclampsia. Journal of Clinical Medicine. 2022; 11(17):5065. https://doi.org/10.3390/jcm11175065
Chicago/Turabian StyleWojciechowska, Ewa, Piotr Sobieraj, Maciej Siński, Maria Anna Zaborska-Dworak, Piotr Gryglas, and Jacek Lewandowski. 2022. "Consistency among Office, Home, and Ambulatory Blood Pressure Values in Women with Chronic Hypertension and History of Eclampsia or Preeclampsia" Journal of Clinical Medicine 11, no. 17: 5065. https://doi.org/10.3390/jcm11175065






