Unresolved Systemic Inflammation, Long COVID, and the Common Pathomechanisms of Somatic and Psychiatric Comorbidity
1. Introduction
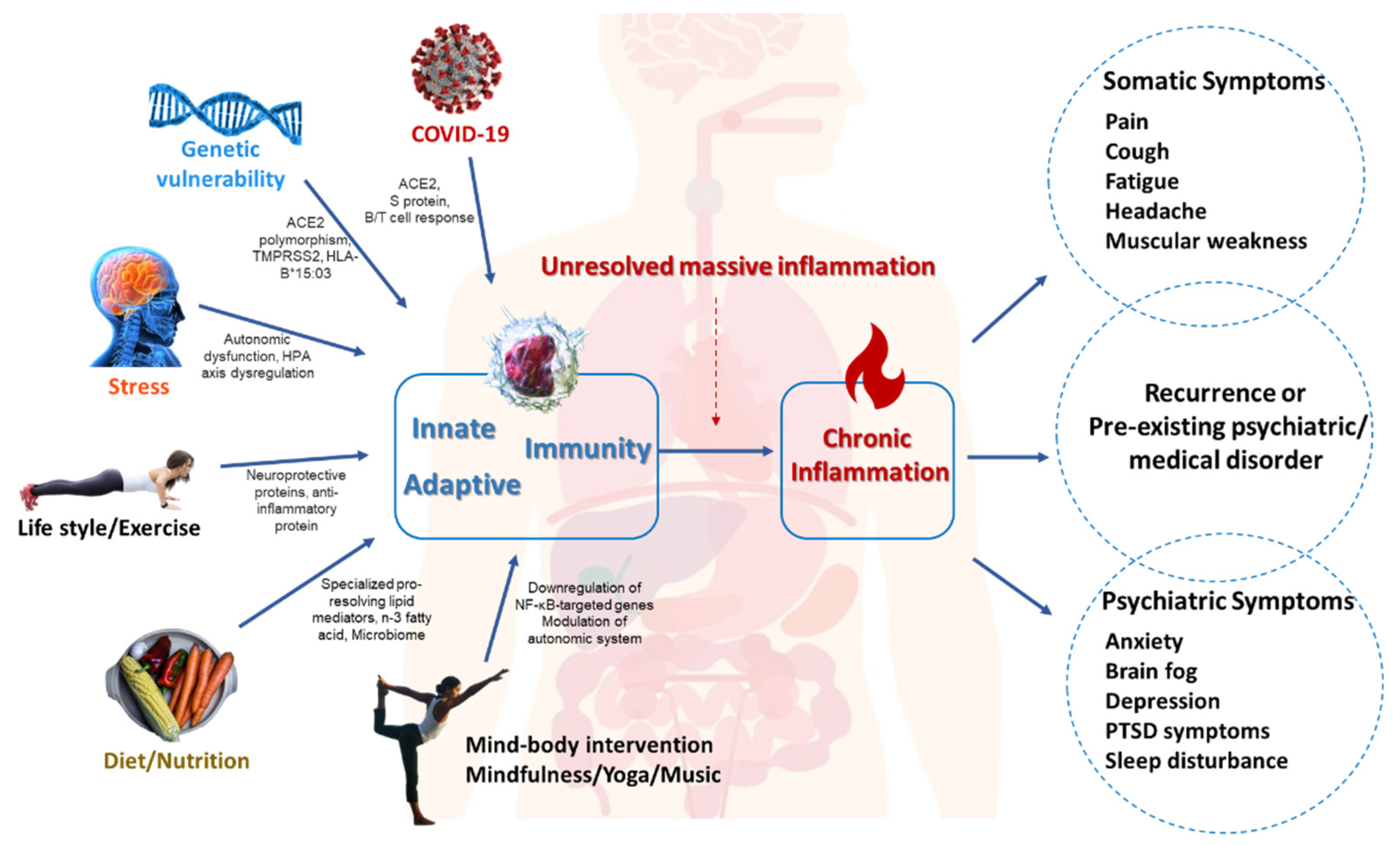
2. Common Pathomechanisms of Somatic and Psychiatric Comorbidity
3. Long COVID, Neuroinflammation, and Somatic and Psychiatric Comorbidity
4. Conclusions
Conflicts of Interest
References
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.; Su, K.P. Nutrition and immunology in mental health: Precision medicine and integrative approaches to address unmet clinical needs in psychiatric treatments. Brain Behav. Immun. 2020, 85, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Santomauro, D.F.; Mantilla Herrera, A.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Simon, N.M. Bridging Knowledge Gaps in the Diagnosis and Management of Neuropsychiatric Sequelae of COVID-19. JAMA Psychiatry 2022, 79, 811–817. [Google Scholar] [CrossRef]
- Wang, S.C.; Su, K.P.; Pariante, C.M. The three frontlines against COVID-19: Brain, Behavior, and Immunity. Brain Behav. Immun. 2021, 93, 409–414. [Google Scholar] [CrossRef]
- Yang, C.P.; Chang, C.M.; Yang, C.C.; Pariante, C.M.; Su, K.P. Long COVID and long chain fatty acids (LCFAs): Psychoneuroimmunity implication of omega-3 LCFAs in delayed consequences of COVID-19. Brain Behav. Immun. 2022, 103, 19–27. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Bonelli, M.; Gadina, M.; O’Shea, J.J. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 2016, 12, 25–36. [Google Scholar] [CrossRef]
- Gałecka, M.; Szemraj, J.; Su, K.-P.; Halaris, A.; Maes, M.; Skiba, A.; Gałecki, P.; Bliźniewska-Kowalska, K. Is the JAK-STAT Signaling Pathway Involved in the Pathogenesis of Depression? J. Clin. Med. 2022, 11, 2056. [Google Scholar] [CrossRef]
- Rawdin, B.J.; Mellon, S.H.; Dhabhar, F.S.; Epel, E.S.; Puterman, E.; Su, Y.; Burke, H.M.; Reus, V.I.; Rosser, R.; Hamilton, S.P.; et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 2013, 31, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015, 144, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Z.; Riwanto, M.; Gao, S.; Levison, B.S.; Gu, X.; Fu, X.; Wagner, M.A.; Besler, C.; Gerstenecker, G.; et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J. Clin. Investig. 2013, 123, 3815–3828. [Google Scholar] [CrossRef]
- Bliźniewska-Kowalska, K.; Gałecki, P.; Su, K.-P.; Halaris, A.; Szemraj, J.; Gałecka, M. Expression of PON1, PON2, PON3 and MPO Genes in Patients with Depressive Disorders. J. Clin. Med. 2022, 11, 3321. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Wachowska, K.; Gałecki, P. Inflammation and Cognition in Depression: A Narrative Review. J. Clin. Med. 2021, 10, 5859. [Google Scholar] [CrossRef]
- Herrman, H.; Patel, V.; Kieling, C.; Berk, M.; Buchweitz, C.; Cuijpers, P.; Furukawa, T.A.; Kessler, R.C.; Kohrt, B.A.; Maj, M.; et al. Time for united action on depression: A Lancet-World Psychiatric Association Commission. Lancet 2022, 399, 957–1022. [Google Scholar] [CrossRef]
- Gałecki, P.; Samochowiec, J.; Mikułowska, M.; Szulc, A. Treatment-Resistant Depression in Poland—Epidemiology and Treatment. J. Clin. Med. 2022, 11, 480. [Google Scholar] [CrossRef]
- Yu, C.-L.; Liang, C.-S.; Yang, F.-C.; Tu, Y.-K.; Hsu, C.-W.; Carvalho, A.F.; Stubbs, B.; Thompson, T.; Tsai, C.-K.; Yeh, T.-C.; et al. Trajectory of Antidepressant Effects after Single- or Two-Dose Administration of Psilocybin: A Systematic Review and Multivariate Meta-Analysis. J. Clin. Med. 2022, 11, 938. [Google Scholar] [CrossRef]
- Orzechowska, A.; Maruszewska, P.; Gałecki, P. Cognitive Behavioral Therapy of Patients with Somatic Symptoms—Diagnostic and Therapeutic Difficulties. J. Clin. Med. 2021, 10, 3159. [Google Scholar] [CrossRef] [PubMed]
- Ting, B.; Tsai, C.-L.; Hsu, W.-T.; Shen, M.-L.; Tseng, P.-T.; Chen, D.T.-L.; Su, K.-P.; Jingling, L. Music Intervention for Pain Control in the Pediatric Population: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Perl, D.P.; Nair, G.; Li, W.; Maric, D.; Murray, H.; Dodd, S.J.; Koretsky, A.P.; Watts, J.A.; Cheung, V.; et al. Microvascular Injury in the Brains of Patients with COVID-19. N. Engl. J. Med. 2020, 384, 481–483. [Google Scholar] [CrossRef]
- Wachowska, K.; Szemraj, J.; Śmigielski, J.; Gałecki, P. Inflammatory Markers and Episodic Memory Functioning in Depressive Disorders. J. Clin. Med. 2022, 11, 693. [Google Scholar] [CrossRef]
- Wachowska, K.; Gałecki, P.; Szemraj, J.; Śmigielski, J.; Orzechowska, A. Personality Traits and Inflammation in Depressive Disorders. J. Clin. Med. 2022, 11, 1974. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Marshall, M. How COVID-19 can damage the brain. Nature 2020, 585, 342–343. [Google Scholar] [CrossRef]
- Allison, D.J.; Ditor, D.S. The common inflammatory etiology of depression and cognitive impairment: A therapeutic target. J. Neuroinflamm. 2014, 11, 151. [Google Scholar] [CrossRef]
- Kato, T. Coping with Stress, Executive Functions, and Depressive Symptoms: Focusing on Flexible Responses to Stress. J. Clin. Med. 2021, 10, 3122. [Google Scholar] [CrossRef]
- Chen, L.; Qu, L.; Hong, R.Y. Pathways Linking the Big Five to Psychological Distress: Exploring the Mediating Roles of Stress Mindset and Coping Flexibility. J. Clin. Med. 2022, 11, 2272. [Google Scholar] [CrossRef]
- Gold, S.M.; Kohler-Forsberg, O.; Moss-Morris, R.; Mehnert, A.; Miranda, J.J.; Bullinger, M.; Steptoe, A.; Whooley, M.A.; Otte, C. Comorbid depression in medical diseases. Nat. Rev. Dis. Primers 2020, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Schöler, D.; Kostev, K.; Demir, M.; Luedde, M.; Konrad, M.; Luedde, T.; Roderburg, C.; Loosen, S.H. An Elevated FIB-4 Score Is Associated with an Increased Incidence of Depression among Outpatients in Germany. J. Clin. Med. 2022, 11, 2214. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, S.; Jo, J.K. The Relationships between Abnormal Serum Lipid Levels, Depression, and Suicidal Ideation According to Sex. J. Clin. Med. 2022, 11, 2119. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.-S.; Gałecki, P.; Su, K.-P. Unresolved Systemic Inflammation, Long COVID, and the Common Pathomechanisms of Somatic and Psychiatric Comorbidity. J. Clin. Med. 2022, 11, 5114. https://doi.org/10.3390/jcm11175114
Liang C-S, Gałecki P, Su K-P. Unresolved Systemic Inflammation, Long COVID, and the Common Pathomechanisms of Somatic and Psychiatric Comorbidity. Journal of Clinical Medicine. 2022; 11(17):5114. https://doi.org/10.3390/jcm11175114
Chicago/Turabian StyleLiang, Chih-Sung, Piotr Gałecki, and Kuan-Pin Su. 2022. "Unresolved Systemic Inflammation, Long COVID, and the Common Pathomechanisms of Somatic and Psychiatric Comorbidity" Journal of Clinical Medicine 11, no. 17: 5114. https://doi.org/10.3390/jcm11175114





