Probiotics in the Management of Mental and Gastrointestinal Post-COVID Symptomes
Abstract
:1. Introduction
2. COVID-19 Pandemic and Microbiota
2.1. Environment
2.2. Microbiota and COVID-19
2.3. Post COVID-19 Syndrome
3. Probiotics and COVID-19
3.1. Mechanism of Probiotics Action
3.2. Clinical Efficacy
3.3. Probiotics and Secondary Infections in COVID-19
3.4. Postbiotics
3.5. Safety
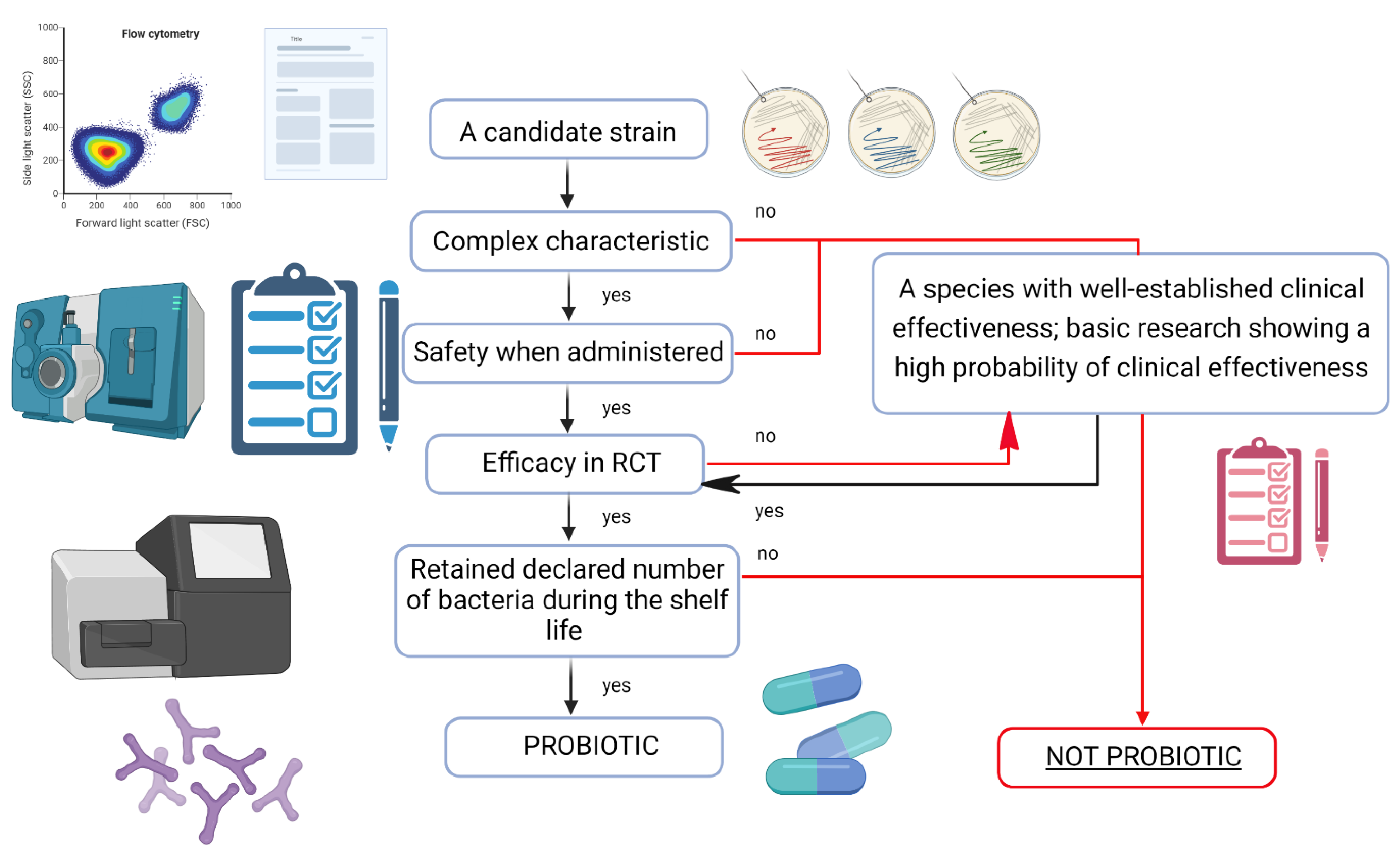
4. Probiotics—Quality Means Effectiveness
5. Special Clinical Situations
5.1. COVID-19 and Central Nervous System
5.2. COVID-19 and Gastrointestinal Tract
5.3. Intensive Care of COVID-19 Patients—Evidence Based Medicine of a Single Center Experience
6. Specific Applications of Probiotics after COVID-19 Infection
6.1. Irritable Bowel Syndrome (IBS)
6.2. Psychobiotics
6.2.1. Depression
6.2.2. Anxiety
6.2.3. Stress
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronavirus COVID-19 (2019-NCoV). Available online: https://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6 (accessed on 13 August 2022).
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kotfis, K.; Skonieczna-Żydecka, K. COVID-19: Gastrointestinal Symptoms and Potential Sources of SARS-CoV-2 Transmission. Anaesthesiol. Intensive Ther. 2020, 52, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Kukla, M.; Skonieczna-Żydecka, K.; Kotfis, K.; Maciejewska, D.; Łoniewski, I.; Lara, L.F.; Pazgan-Simon, M.; Stachowska, E.; Kaczmarczyk, M.; Koulaouzidis, A.; et al. COVID-19, MERS and SARS with Concomitant Liver Injury—Systematic Review of the Existing Literature. J. Clin. Med. 2020, 9, 1420. [Google Scholar] [CrossRef]
- Böhmer, M.M.; Buchholz, U.; Corman, V.M.; Hoch, M.; Katz, K.; Marosevic, D.V.; Böhm, S.; Woudenberg, T.; Ackermann, N.; Konrad, R.; et al. Investigation of a COVID-19 Outbreak in Germany Resulting from a Single Travel-Associated Primary Case: A Case Series. Lancet Infect. Dis. 2020, 20, 920–928. [Google Scholar] [CrossRef]
- Mokhtari, T.; Hassani, F.; Ghaffari, N.; Ebrahimi, B.; Yarahmadi, A.; Hassanzadeh, G. COVID-19 and Multiorgan Failure: A Narrative Review on Potential Mechanisms. J. Mol. Histol. 2020, 51, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, G.; Zhao, L.; Wang, W. Nutritional Modulation of Gut Microbiota Alleviates Severe Gastrointestinal Symptoms in a Patient with Post-Acute COVID-19 Syndrome. mBio 2022, 13, e0380121. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Xiao, H.; Zhang, G. Triclosan, a Common Antimicrobial Ingredient, on Gut Microbiota and Gut Health. Gut Microbes 2018, 10, 434–437. [Google Scholar] [CrossRef]
- Jutkina, J.; Marathe, N.P.; Flach, C.-F.; Larsson, D.G.J. Antibiotics and Common Antibacterial Biocides Stimulate Horizontal Transfer of Resistance at Low Concentrations. Sci. Total Environ. 2018, 616–617, 172–178. [Google Scholar] [CrossRef]
- Ejtahed, H.-S.; Hasani-Ranjbar, S.; Siadat, S.D.; Larijani, B. The Most Important Challenges Ahead of Microbiome Pattern in the Post Era of the COVID-19 Pandemic. J. Diabetes Metab. Disord. 2020, 19, 2031–2033. [Google Scholar] [CrossRef]
- Zang, L.; Ma, Y.; Huang, W.; Ling, Y.; Sun, L.; Wang, X.; Zeng, A.; Dahlgren, R.A.; Wang, C.; Wang, H. Dietary Lactobacillus Plantarum ST-III Alleviates the Toxic Effects of Triclosan on Zebrafish (Danio Rerio) via Gut Microbiota Modulation. Fish Shellfish Immunol. 2019, 84, 1157–1169. [Google Scholar] [CrossRef]
- Woodruff, M.C.; Ramonell, R.P.; Cashman, K.S.; Nguyen, D.C.; Ley, A.M.; Kyu, S.; Saini, A.; Haddad, N.; Chen, W.; Howell, J.C.; et al. Critically Ill SARS-CoV-2 Patients Display Lupus-like Hallmarks of Extrafollicular B Cell Activation. MedRxiv 2020. [Google Scholar] [CrossRef]
- Cai, Q.; Chen, F.; Wang, T.; Luo, F.; Liu, X.; Wu, Q.; He, Q.; Wang, Z.; Liu, Y.; Liu, L.; et al. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care 2020, 43, 1392–1398. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are Patients with Hypertension and Diabetes Mellitus at Increased Risk for COVID-19 Infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Hill, M.A.; Mantzoros, C.; Sowers, J.R. Commentary: COVID-19 in Patients with Diabetes. Metabolism 2020, 107, 154217. [Google Scholar] [CrossRef]
- Moreira-Rosário, A.; Marques, C.; Pinheiro, H.; Araújo, J.R.; Ribeiro, P.; Rocha, R.; Mota, I.; Pestana, D.; Ribeiro, R.; Pereira, A.; et al. Gut Microbiota Diversity and C-Reactive Protein Are Predictors of Disease Severity in COVID-19 Patients. Front. Microbiol. 2021, 12, 1820. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.; Bhattarai, S.; Rojas-Correa, M.; Purkayastha, A.; Holler, D.; Qu, M.D.; Mitchell, W.; Yang, J.; Fountain, S.; Zeamer, A.; et al. The Intestinal and Oral Microbiomes Are Robust Predictors of COVID-19 Severity the Main Predictor of COVID-19-Related Fatality [Preprint]. Univ. Mass. Med. Sch. Fac. Publ. 2021. [Google Scholar] [CrossRef]
- Li, S.; Yang, S.; Zhou, Y.; Disoma, C.; Dong, Z.; Du, A.; Zhang, Y.; Chen, Y.; Huang, W.; Chen, J.; et al. Microbiome Profiling Using Shotgun Metagenomic Sequencing Identified Unique Microorganisms in COVID-19 Patients With Altered Gut Microbiota. Front. Microbiol. 2021, 12, 2930. [Google Scholar] [CrossRef]
- Santacroce, L.; Inchingolo, F.; Topi, S.; Del Prete, R.; Di Cosola, M.; Charitos, I.A.; Montagnani, M. Potential Beneficial Role of Probiotics on the Outcome of COVID-19 Patients: An Evolving Perspective. Diabetes Metab. Syndr. 2021, 15, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Komaroff, A.L. The Tragedy of Long COVID. Available online: https://www.health.harvard.edu/blog/the-tragedy-of-the-post-covid-long-haulers-202010152479 (accessed on 25 April 2022).
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 Links Amino Acid Malnutrition to Microbial Ecology and Intestinal Inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.-C.; et al. Gut Microbiota Dynamics in a Prospective Cohort of Patients with Post-Acute COVID-19 Syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Li, Y.; Li, J.; Shen, L.; Zhu, L.; Liang, Y.; Lin, X.; Jiao, N.; Cheng, S.; Huang, Y.; et al. Gastrointestinal Sequelae 90 Days after Discharge for COVID-19. Lancet Gastroenterol. Hepatol. 2021, 6, 344–346. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Mohanty, A. Gut Microbiota and Covid-19- Possible Link and Implications. Virus Res. 2020, 285, 198018. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Cavaillon, J.-M. Exotoxins and Endotoxins: Inducers of Inflammatory Cytokines. Toxicon Off. J. Int. Soc. Toxinology 2018, 149, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef]
- Small Intestinal Bacterial Overgrowth (SIBO)|OMICS International. Available online: https://www.omicsonline.org/open-access/small-intestinal-bacterial-overgrowth-sibo-2161-069X.1000225.php?aid=31428 (accessed on 25 April 2022).
- Zaher, S. Nutrition and the Gut Microbiome during Critical Illness: A New Insight of Nutritional Therapy. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2020, 26, 290–298. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial Co-Infection and Secondary Infection in Patients with COVID-19: A Living Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Alberca, R.W.; Oliveira, L.d.M.; Branco, A.C.C.C.; Pereira, N.Z.; Sato, M.N. Obesity as a Risk Factor for COVID-19: An Overview. Crit. Rev. Food Sci. Nutr. 2021, 61, 2262–2276. [Google Scholar] [CrossRef]
- Alberca, G.G.F.; Alberca, R.W. Nutrition and the Microbiota Post-COVID-19. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2021, 27, 111–112. [Google Scholar] [CrossRef]
- Xavier-Santos, D.; Padilha, M.; Fabiano, G.A.; Vinderola, G.; Gomes Cruz, A.; Sivieri, K.; Costa Antunes, A.E. Evidences and Perspectives of the Use of Probiotics, Prebiotics, Synbiotics, and Postbiotics as Adjuvants for Prevention and Treatment of COVID-19: A Bibliometric Analysis and Systematic Review. Trends Food Sci. Technol. 2022, 120, 174–192. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Baindara, P.; Chakraborty, R.; Holliday, Z.M.; Mandal, S.M.; Schrum, A.G. Oral Probiotics in Coronavirus Disease 2019: Connecting the Gut-Lung Axis to Viral Pathogenesis, Inflammation, Secondary Infection and Clinical Trials. New Microbes New Infect. 2021, 40, 100837. [Google Scholar] [CrossRef]
- Kanauchi, O.; Andoh, A.; AbuBakar, S.; Yamamoto, N. Probiotics and Paraprobiotics in Viral Infection: Clinical Application and Effects on the Innate and Acquired Immune Systems. Curr. Pharm. Des. 2018, 24, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Dicks, L.M.T.; Popov, I.V.; Karaseva, A.; Ermakov, A.M.; Suvorov, A.; Tagg, J.R.; Weeks, R.; Chikindas, M.L. Probiotics at War Against Viruses: What Is Missing From the Picture? Front. Microbiol. 2020, 11, 1877. [Google Scholar] [CrossRef]
- Strauss, M.; Mičetić-Turk, D.; Pogačar, M.Š.; Fijan, S. Probiotics for the Prevention of Acute Respiratory-Tract Infections in Older People: Systematic Review. Healthcare 2021, 9, 690. [Google Scholar] [CrossRef] [PubMed]
- Tapiovaara, L.; Lehtoranta, L.; Poussa, T.; Mäkivuokko, H.; Korpela, R.; Pitkäranta, A. Absence of Adverse Events in Healthy Individuals Using Probiotics--Analysis of Six Randomised Studies by One Study Group. Benef. Microbes 2016, 7, 161–169. [Google Scholar] [CrossRef]
- Montazeri-Najafabady, N.; Kazemi, K.; Gholami, A. Recent Advances in Antiviral Effects of Probiotics: Potential Mechanism Study in Prevention and Treatment of SARS-CoV-2. Biologia 2022, 1–18. [Google Scholar] [CrossRef]
- Chiba, Y.; Shida, K.; Nagata, S.; Wada, M.; Bian, L.; Wang, C.; Shimizu, T.; Yamashiro, Y.; Kiyoshima-Shibata, J.; Nanno, M.; et al. Well-Controlled Proinflammatory Cytokine Responses of Peyer’s Patch Cells to Probiotic Lactobacillus Casei. Immunology 2010, 130, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.H.; Cámara, M.; Verma, C.; Eremin, O.; Kulkarni, A.D.; Lobo, D.N. Modulation of T Regulatory and Dendritic Cell Phenotypes Following Ingestion of Bifidobacterium Longum, AHCC® and Azithromycin in Healthy Individuals. Nutrients 2019, 11, 2470. [Google Scholar] [CrossRef]
- Iemoli, E.; Trabattoni, D.; Parisotto, S.; Borgonovo, L.; Toscano, M.; Rizzardini, G.; Clerici, M.; Ricci, E.; Fusi, A.; De Vecchi, E.; et al. Probiotics Reduce Gut Microbial Translocation and Improve Adult Atopic Dermatitis. J. Clin. Gastroenterol. 2012, 46, S33–S40. [Google Scholar] [CrossRef]
- Dwivedi, M.; Kumar, P.; Laddha, N.C.; Kemp, E.H. Induction of Regulatory T Cells: A Role for Probiotics and Prebiotics to Suppress Autoimmunity. Autoimmun. Rev. 2016, 15, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Speer, H.; D’Cunha, N.M.; Botek, M.; McKune, A.J.; Sergi, D.; Georgousopoulou, E.; Mellor, D.D.; Naumovski, N. The Effects of Dietary Polyphenols on Circulating Cardiovascular Disease Biomarkers and Iron Status: A Systematic Review. Nutr. Metab. Insights 2019, 12, 1178638819882739. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Visalli, G.; Cirmi, S.; Lombardo, G.E.; Laganà, P.; Di Pietro, A.; Navarra, M. Natural Iron Chelators: Protective Role in A549 Cells of Flavonoids-Rich Extracts of Citrus Juices in Fe(3+)-Induced Oxidative Stress. Environ. Toxicol. Pharmacol. 2016, 43, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Stiksrud, B.; Nowak, P.; Nwosu, F.C.; Kvale, D.; Thalme, A.; Sonnerborg, A.; Ueland, P.M.; Holm, K.; Birkeland, S.-E.; Dahm, A.E.A.; et al. Reduced Levels of D-Dimer and Changes in Gut Microbiota Composition After Probiotic Intervention in HIV-Infected Individuals on Stable ART. J. Acquir. Immune Defic. Syndr. 1999 2015, 70, 329–337. [Google Scholar] [CrossRef]
- Yeh, T.-L.; Shih, P.-C.; Liu, S.-J.; Lin, C.-H.; Liu, J.-M.; Lei, W.-T.; Lin, C.-Y. The Influence of Prebiotic or Probiotic Supplementation on Antibody Titers after Influenza Vaccination: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Drug Des. Devel. Ther. 2018, 12, 217–230. [Google Scholar] [CrossRef]
- Lei, W.-T.; Shih, P.-C.; Liu, S.-J.; Lin, C.-Y.; Yeh, T.-L. Effect of Probiotics and Prebiotics on Immune Response to Influenza Vaccination in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 1175. [Google Scholar] [CrossRef] [PubMed]
- Marlicz, W.; Yung, D.E.; Skonieczna-Żydecka, K.; Loniewski, I.; van Hemert, S.; Loniewska, B.; Koulaouzidis, A. From Clinical Uncertainties to Precision Medicine: The Emerging Role of the Gut Barrier and Microbiome in Small Bowel Functional Diseases. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 961–978. [Google Scholar] [CrossRef]
- d’Ettorre, G.; Ceccarelli, G.; Marazzato, M.; Campagna, G.; Pinacchio, C.; Alessandri, F.; Ruberto, F.; Rossi, G.; Celani, L.; Scagnolari, C.; et al. Challenges in the Management of SARS-CoV-2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front. Med. 2020, 7, 389. [Google Scholar] [CrossRef] [PubMed]
- Ke, E.; Zhang, H. Clinical effects of probiotics in ordinary-type COVID-19 patients with diarrhea. World Chin. J. Dig. 2020, 28, 834–838. [Google Scholar] [CrossRef]
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu, Y.; Abreu, A.T.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic Improves Symptomatic and Viral Clearance in Covid19 Outpatients: A Randomized, Quadruple-Blinded, Placebo-Controlled Trial. Gut Microbes 2022, 14, 2018899. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Bohannon, L.; Lew, M.; Jensen, D.; Jung, S.-H.; Zhao, A.; Sung, A.D.; Wischmeyer, P.E. Randomised, Double-Blind, Placebo-Controlled Trial of Probiotics To Eliminate COVID-19 Transmission in Exposed Household Contacts (PROTECT-EHC): A Clinical Trial Protocol. BMJ Open 2021, 11, e047069. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Aolymat, I.; Al-Holy, M.; Ayyash, M.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Osaili, T.; Apostolopoulos, V.; Liu, S.-Q.; Shah, N.P. The Potential Application of Probiotics and Prebiotics for the Prevention and Treatment of COVID-19. Npj Sci. Food 2020, 4, 17. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Dong, X.; Cao, Y.-Y.; Lu, X.-X.; Zhang, J.-J.; Du, H.; Yan, Y.-Q.; Akdis, C.A.; Gao, Y.-D. Eleven Faces of Coronavirus Disease 2019. Allergy 2020, 75, 1699–1709. [Google Scholar] [CrossRef]
- Manna, S.; Baindara, P.; Mandal, S.M. Molecular Pathogenesis of Secondary Bacterial Infection Associated to Viral Infections Including SARS-CoV-2. J. Infect. Public Health 2020, 13, 1397–1404. [Google Scholar] [CrossRef]
- Tan, F.L.S.; Loo, W.L.; Tan, S.G.; Wong, C.Y.; Tan, Y.-M. Severe Acute Respiratory Syndrome in Surgical Patients: A Diagnostic Dilemma. ANZ J. Surg. 2005, 75, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.-L.; Nobaek, S.; Berggren, A.; Nyman, M.; Björck, I.; Ahrné, S.; Jeppsson, B.; Molin, G. Survival of Lactobacillus Plantarum DSM 9843 (299v), and Effect on the Short-Chain Fatty Acid Content of Faeces after Ingestion of a Rose-Hip Drink with Fermented Oats. Int. J. Food Microbiol. 1998, 42, 29–38. [Google Scholar] [CrossRef]
- Johansson, M.L.; Molin, G.; Jeppsson, B.; Nobaek, S.; Ahrné, S.; Bengmark, S. Administration of Different Lactobacillus Strains in Fermented Oatmeal Soup: In Vivo Colonization of Human Intestinal Mucosa and Effect on the Indigenous Flora. Appl. Environ. Microbiol. 1993, 59, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mangell, P.; Thorlacius, H.; Syk, I.; Ahrné, S.; Molin, G.; Olsson, C.; Jeppsson, B. Lactobacillus Plantarum 299v Does Not Reduce Enteric Bacteria or Bacterial Translocation in Patients Undergoing Colon Resection. Dig. Dis. Sci. 2012, 57, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Nobaek, S.; Johansson, M.-L.; Molin, G.; Ahrné, S.; Jeppsson, B. Alteration of Intestinal Microflora Is Associated With Reduction in Abdominal Bloating and Pain in Patients With Irritable Bowel Syndrome. Off. J. Am. Coll. Gastroenterol. ACG 2000, 95, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Stjernquist-Desatnik, A.; Warfving, H.; Johansson, M.L. Persistence of Lactobacillus Plantarum DSM 9843 on Human Tonsillar Surface after Oral Administration in Fermented Oatmeal Gruel. A Pilot Study. Acta Oto-Laryngol. Suppl. 2000, 543, 215–219. [Google Scholar] [CrossRef]
- Klarin, B.; Johansson, M.-L.; Molin, G.; Larsson, A.; Jeppsson, B. Adhesion of the Probiotic Bacterium Lactobacillus Plantarum 299v onto the Gut Mucosa in Critically Ill Patients: A Randomised Open Trial. Crit. Care 2005, 9, R285. [Google Scholar] [CrossRef]
- Adlerberth, I.; Ahrne, S.; Johansson, M.L.; Molin, G.; Hanson, L.A.; Wold, A.E. A Mannose-Specific Adherence Mechanism in Lactobacillus Plantarum Conferring Binding to the Human Colonic Cell Line HT-29. Appl. Environ. Microbiol. 1996, 62, 2244–2251. [Google Scholar] [CrossRef]
- Mangell, P.; Lennernäs, P.; Wang, M.; Olsson, C.; Ahrné, S.; Molin, G.; Thorlacius, H.; Jeppsson, B. Adhesive Capability of Lactobacillus Plantarum 299v Is Important for Preventing Bacterial Translocation in Endotoxemic Rats. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2006, 114, 611–618. [Google Scholar] [CrossRef]
- Whiting, G.C.; Evans, J.T.; Patel, S.; Gillespie, S.H. Purification of Native Alpha-Enolase from Streptococcus Pneumoniae That Binds Plasminogen and Is Immunogenic. J. Med. Microbiol. 2002, 51, 837–843. [Google Scholar] [CrossRef]
- Mölkänen, T.; Tyynelä, J.; Helin, J.; Kalkkinen, N.; Kuusela, P. Enhanced Activation of Bound Plasminogen on Staphylococcus Aureus by Staphylokinase. FEBS Lett. 2002, 517, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Lottenberg, R.; DesJardin, L.E.; Wang, H.; Boyle, M.D. Streptokinase-Producing Streptococci Grown in Human Plasma Acquire Unregulated Cell-Associated Plasmin Activity. J. Infect. Dis. 1992, 166, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Modun, B.; Williams, P. The Staphylococcal Transferrin-Binding Protein Is a Cell Wall Glyceraldehyde-3-Phosphate Dehydrogenase. Infect. Immun. 1999, 67, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Goudot-Crozel, V.; Caillol, D.; Djabali, M.; Dessein, A.J. The Major Parasite Surface Antigen Associated with Human Resistance to Schistosomiasis Is a 37-KD Glyceraldehyde-3P-Dehydrogenase. J. Exp. Med. 1989, 170, 2065–2080. [Google Scholar] [CrossRef]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics Inhibit Enteropathogenic, E. Coli Adherence in Vitro by Inducing Intestinal Mucin Gene Expression. Am. J. Physiol. 1999, 276, G941–G950. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.R.; Ahrne, S.; Hyde, L.; Wei, S.; Hollingsworth, M.A. Extracellular MUC3 Mucin Secretion Follows Adherence of Lactobacillus Strains to Intestinal Epithelial Cells in Vitro. Gut 2003, 52, 827–833. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Altayb, H.N.; Al-Abbasi, F.A.; Al-Malki, A.L.; Kamal, M.A.; Kumar, V. Antiviral Effects of Probiotic Metabolites on COVID-19. J. Biomol. Struct. Dyn. 2020, 39, 4175–4184. [Google Scholar] [CrossRef]
- Balmeh, N.; Mahmoudi, S.; Fard, N.A. Manipulated Bio Antimicrobial Peptides from Probiotic Bacteria as Proposed Drugs for COVID-19 Disease. Inform. Med. Unlocked 2021, 23, 100515. [Google Scholar] [CrossRef]
- Sotoudegan, F.; Daniali, M.; Hassani, S.; Nikfar, S.; Abdollahi, M. Reappraisal of Probiotics’ Safety in Human. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 129, 22–29. [Google Scholar] [CrossRef]
- Costa, R.L.; Moreira, J.; Lorenzo, A.; Lamas, C.C. Infectious Complications Following Probiotic Ingestion: A Potentially Underestimated Problem? A Systematic Review of Reports and Case Series. BMC Complement. Altern. Med. 2018, 18, 329. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.J.; Luijendijk, A.; van Toledo, L.; van Kaam, A.H.; Reiss, I.K.M. Quality of Probiotic Products for Preterm Infants: Contamination and Missing Strains. Acta Paediatr. Oslo Nor. 1992 2020, 109, 276–279. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Update of the List of QPS-Recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 13: Suitability of Taxonomic Units Notified to EFSA until September 2020|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/6377 (accessed on 28 April 2022).
- Center for Veterinary Medicine; Center for Food Safety and Applied Nutrition. Guidance for Industry: Regulatory Framework for Substances Intended for Use in Human Food or Animal Food on the Basis of the Generally Recognized as Safe (GRAS) Provision of the Federal Food, Drug, and Cosmetic Act. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-regulatory-framework-substances-intended-use-human-food-or-animal-food-basis (accessed on 28 April 2022).
- Kumar, H.; Salminen, S.; Verhagen, H.; Rowland, I.; Heimbach, J.; Bañares, S.; Young, T.; Nomoto, K.; Lalonde, M. Novel Probiotics and Prebiotics: Road to the Market. Curr. Opin. Biotechnol. 2015, 32, 99–103. [Google Scholar] [CrossRef] [PubMed]
- EMA European Medicines Agency. Available online: https://www.ema.europa.eu/en (accessed on 14 August 2022).
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L.K. Probiotic Use in Clinical Practice: What Are the Risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264; quiz 1446–1447. [Google Scholar] [CrossRef]
- Verma, D.K.; Niamah, A.K.; Patel, A.R.; Thakur, M.; Singh Sandhu, K.; Chávez-González, M.L.; Shah, N.; Noe Aguilar, C. Chemistry and Microbial Sources of Curdlan with Potential Application and Safety Regulations as Prebiotic in Food and Health. Food Res. Int. 2020, 133, 109136. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Sielatycka, K.; Juzwa, W.; Śliwa-Dominiak, J.; Kaczmarczyk, M.; Łoniewski, I.; Marlicz, W. Multiparameter Flow Cytometric Enumeration of Probiotic-Containing Commercial Powders. Innov. Food Sci. Emerg. Technol. 2021, 68, 102598. [Google Scholar] [CrossRef]
- Kujawa-Szewieczek, A.; Adamczak, M.; Kwiecień, K.; Dudzicz, S.; Gazda, M.; Więcek, A. The Effect of Lactobacillus Plantarum 299v on the Incidence of Clostridium Difficile Infection in High Risk Patients Treated with Antibiotics. Nutrients 2015, 7, 10179–10188. [Google Scholar] [CrossRef]
- Dudzicz, S.; Adamczak, M.; Więcek, A. Clostridium Difficile Infection in the Nephrology Ward. Kidney Blood Press. Res. 2017, 42, 844–852. [Google Scholar] [CrossRef]
- de Simone, C. The Unregulated Probiotic Market. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 809–817. [Google Scholar] [CrossRef]
- Baig, A.M.; Sanders, E.C. Potential Neuroinvasive Pathways of SARS-CoV-2: Deciphering the Spectrum of Neurological Deficit Seen in Coronavirus Disease-2019 (COVID-19). J. Med. Virol. 2020, 92, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Bai, W.-Z.; Hirano, N.; Hayashida, T.; Hashikawa, T. Coronavirus Infection of Rat Dorsal Root Ganglia: Ultrastructural Characterization of Viral Replication, Transfer, and the Early Response of Satellite Cells. Virus Res. 2012, 163, 628–635. [Google Scholar] [CrossRef] [PubMed]
- DosSantos, M.F.; Devalle, S.; Aran, V.; Capra, D.; Roque, N.R.; Coelho-Aguiar, J.d.M.; Spohr, T.C.L.d.S.e.; Subilhaga, J.G.; Pereira, C.M.; D’Andrea Meira, I.; et al. Neuromechanisms of SARS-CoV-2: A Review. Front. Neuroanat. 2020, 14, 37. [Google Scholar] [CrossRef]
- Butowt, R.; Bilinska, K. SARS-CoV-2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection. ACS Chem. Neurosci. 2020, 11, 1200–1203. [Google Scholar] [CrossRef]
- Gudowska-Sawczuk, M.; Mroczko, B. The Role of Neuropilin-1 (NRP-1) in SARS-CoV-2 Infection: Review. J. Clin. Med. 2021, 10, 2772. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Pesce, M.; Seguella, L.; Sanseverino, W.; Lu, J.; Sarnelli, G. Can the Enteric Nervous System Be an Alternative Entrance Door in SARS-CoV2 Neuroinvasion? Brain. Behav. Immun. 2020, 87, 93–94. [Google Scholar] [CrossRef]
- Skonieczna-Żydecka, K.; Marlicz, W.; Misera, A.; Koulaouzidis, A.; Łoniewski, I. Microbiome-The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. J. Clin. Med. 2018, 7, 521. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Deng, Q.; Zhang, G.; Wu, K.; Ni, L.; Yang, Y.; Liu, B.; Wang, W.; Wei, C.; et al. The Presence of SARS-CoV-2 RNA in the Feces of COVID-19 Patients. J. Med. Virol. 2020, 92, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Ogier, M.; Andéol, G.; Sagui, E.; Dal Bo, G. How to Detect and Track Chronic Neurologic Sequelae of COVID-19? Use of Auditory Brainstem Responses and Neuroimaging for Long-Term Patient Follow-Up. Brain Behav. Immun.-Health 2020, 5, 100081. [Google Scholar] [CrossRef] [PubMed]
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central Nervous System Involvement by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). J. Med. Virol. 2020, 92, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Reza-Zaldívar, E.E.; Hernández-Sapiéns, M.A.; Minjarez, B.; Gómez-Pinedo, U.; Márquez-Aguirre, A.L.; Mateos-Díaz, J.C.; Matias-Guiu, J.; Canales-Aguirre, A.A. Infection Mechanism of SARS-CoV-2 and Its Implication on the Nervous System. Front. Immunol. 2021, 11, 3738. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, T.; Yang, N.; Han, D.; Mi, X.; Li, Y.; Liu, K.; Vuylsteke, A.; Xiang, H.; Guo, X. Neurological Manifestations of Patients with COVID-19: Potential Routes of SARS-CoV-2 Neuroinvasion from the Periphery to the Brain. Front. Med. 2020, 14, 533–541. [Google Scholar] [CrossRef]
- Miner, J.J.; Diamond, M.S. Mechanisms of Restriction of Viral Neuroinvasion at the Blood-Brain Barrier. Curr. Opin. Immunol. 2016, 38, 18–23. [Google Scholar] [CrossRef]
- Cheng, Q.; Yang, Y.; Gao, J. Infectivity of Human Coronavirus in the Brain. EBioMedicine 2020, 56, 102799. [Google Scholar] [CrossRef]
- Troyer, E.A.; Kohn, J.N.; Hong, S. Are We Facing a Crashing Wave of Neuropsychiatric Sequelae of COVID-19? Neuropsychiatric Symptoms and Potential Immunologic Mechanisms. Brain. Behav. Immun. 2020, 87, 34–39. [Google Scholar] [CrossRef]
- Mak, I.W.C.; Chu, C.M.; Pan, P.C.; Yiu, M.G.C.; Chan, V.L. Long-Term Psychiatric Morbidities among SARS Survivors. Gen. Hosp. Psychiatry 2009, 31, 318–326. [Google Scholar] [CrossRef]
- Moreno, C.; Wykes, T.; Galderisi, S.; Nordentoft, M.; Crossley, N.; Jones, N.; Cannon, M.; Correll, C.U.; Byrne, L.; Carr, S.; et al. How Mental Health Care Should Change as a Consequence of the COVID-19 Pandemic. Lancet Psychiatry 2020, 7, 813–824. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and Disorders of the Stress System. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Cabello, M.; Miret, M.; Caballero, F.F.; Chatterji, S.; Naidoo, N.; Kowal, P.; D’Este, C.; Ayuso-Mateos, J.L. The Role of Unhealthy Lifestyles in the Incidence and Persistence of Depression: A Longitudinal General Population Study in Four Emerging Countries. Glob. Health 2017, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Estradé, A.; Thompson, T.; Agorastos, A.; Radua, J.; Cortese, S.; Dragioti, E.; Leisch, F.; Vancampfort, D.; Thygesen, L.C.; et al. Physical and Mental Health Impact of COVID-19 on Children, Adolescents, and Their Families: The Collaborative Outcomes Study on Health and Functioning during Infection Times—Children and Adolescents (COH-FIT-C&A). J. Affect. Disord. 2021, 299, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Estradé, A.; Thompson, T.; Agorastos, A.; Radua, J.; Cortese, S.; Dragioti, E.; Leisch, F.; Vancampfort, D.; Thygesen, L.C.; et al. The Collaborative Outcomes Study on Health and Functioning during Infection Times in Adults (COH-FIT-Adults): Design and Methods of an International Online Survey Targeting Physical and Mental Health Effects of the COVID-19 Pandemic. J. Affect. Disord. 2022, 299, 393–407. [Google Scholar] [CrossRef]
- Dragioti, E.; Li, H.; Tsitsas, G.; Lee, K.H.; Choi, J.; Kim, J.; Choi, Y.J.; Tsamakis, K.; Estradé, A.; Agorastos, A.; et al. A Large Scale Meta-Analytic Atlas of Mental Health Problems Prevalence during the COVID-19 Early Pandemic. J. Med. Virol. 2022, 94, 1935–1949. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Lian, J.-S.; Hu, J.-H.; Gao, J.; Zheng, L.; Zhang, Y.-M.; Hao, S.-R.; Jia, H.-Y.; Cai, H.; Zhang, X.-L.; et al. Epidemiological, Clinical and Virological Characteristics of 74 Cases of Coronavirus-Infected Disease 2019 (COVID-19) with Gastrointestinal Symptoms. Gut 2020, 69, 1002–1009. [Google Scholar] [CrossRef]
- Wan, Y.; Li, J.; Shen, L.; Zou, Y.; Hou, L.; Zhu, L.; Faden, H.S.; Tang, Z.; Shi, M.; Jiao, N.; et al. Enteric Involvement in Hospitalised Patients with COVID-19 Outside Wuhan. Lancet Gastroenterol. Hepatol. 2020, 5, 534–535. [Google Scholar] [CrossRef]
- Redd, W.D.; Zhou, J.C.; Hathorn, K.E.; McCarty, T.R.; Bazarbashi, A.N.; Thompson, C.C.; Shen, L.; Chan, W.W. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology 2020, 159, 765–767.e2. [Google Scholar] [CrossRef]
- Cholankeril, G.; Podboy, A.; Aivaliotis, V.I.; Tarlow, B.; Pham, E.A.; Spencer, S.P.; Kim, D.; Hsing, A.; Ahmed, A. High Prevalence of Concurrent Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2: Early Experience From California. Gastroenterology 2020, 159, 775–777. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Dong, X.; Cao, Y.-Y.; Yuan, Y.-D.; Yang, Y.-B.; Yan, Y.-Q.; Akdis, C.A.; Gao, Y.-D. Clinical Characteristics of 140 Patients Infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-Analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef]
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A Systematic Review and Meta-Analysis of Clinical Characteristics, Risk Factors, and Outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, H.; Yang, H. Lack of Marked Association Between Gastrointestinal Symptoms and COVID-19 Mortality: An Updated Meta-Analysis Based on Adjusted Effect Estimates. Mayo Clin. Proc. 2021, 96, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, X.; Xiong, L.; Cai, K. Imaging and Clinical Features of Patients with 2019 Novel Coronavirus SARS-CoV-2: A Systematic Review and Meta-Analysis. J. Med. Virol. 2020, 92, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Altayar, O.; Siddique, S.M.; Davitkov, P.; Feuerstein, J.D.; Lim, J.K.; Falck-Ytter, Y.; El-Serag, H.B.; AGA Institute. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology 2020, 159, 320–334.e27. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple Early Factors Anticipate Post-Acute COVID-19 Sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Bujko, K.; Ciechanowicz, A.; Sielatycka, K.; Cymer, M.; Marlicz, W.; Kucia, M. SARS-CoV-2 Entry Receptor ACE2 Is Expressed on Very Small CD45- Precursors of Hematopoietic and Endothelial Cells and in Response to Virus Spike Protein Activates the Nlrp3 Inflammasome. Stem Cell Rev. Rep. 2021, 17, 266–277. [Google Scholar] [CrossRef]
- Penninger, J.M.; Grant, M.B.; Sung, J.J.Y. The Role of Angiotensin Converting Enzyme 2 in Modulating Gut Microbiota, Intestinal Inflammation, and Coronavirus Infection. Gastroenterology 2021, 160, 39–46. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 20. [Google Scholar] [CrossRef]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA Axis Dysregulation in Severe Mental Illness: Can We Shift the Blame to Gut Microbiota? Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.; Szulińska, M.; Łoniewski, I.; Kręgielska-Narożna, M.; Skonieczna-Żydecka, K.; Kosciolek, T.; Bezshapkin, V.; Bogdański, P. Treatment With Multi-Species Probiotics Changes the Functions, Not the Composition of Gut Microbiota in Postmenopausal Women With Obesity: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Cell. Infect. Microbiol. 2022, 12, 815798. [Google Scholar] [CrossRef]
- Cooney, J.; Appiahene, P.; Findlay, R.; Al-Hillawi, L.; Rafique, K.; Laband, W.; Shandro, B.; Poullis, A. COVID-19 Infection Causing Residual Gastrointestinal Symptoms—A Single UK Centre Case Series. Clin. Med. 2022, 22, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Abate, S.M.; Ahmed Ali, S.; Mantfardo, B.; Basu, B. Rate of Intensive Care Unit Admission and Outcomes among Patients with Coronavirus: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0235653. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Kane, A.D.; Cook, T.M. Outcomes from Intensive Care in Patients with COVID-19: A Systematic Review and Meta-Analysis of Observational Studies. Anaesthesia 2020, 75, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, L.A.; Costa, I.B.S.d.S.; Rizk, S.I.; Biselli, B.; Gomes, B.R.; Bittar, C.S.; de Oliveira, G.Q.; de Almeida, J.P.; de Oliveira Bello, M.V.; Garzillo, C.; et al. Intensive Care Management of Patients with COVID-19: A Practical Approach. Ann. Intensive Care 2021, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Sigfrid, L.; Drake, T.M.; Pauley, E.; Jesudason, E.C.; Olliaro, P.; Lim, W.S.; Gillesen, A.; Berry, C.; Lowe, D.J.; McPeake, J.; et al. Long Covid in Adults Discharged from UK Hospitals after Covid-19: A Prospective, Multicentre Cohort Study Using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg. Health-Eur. 2021, 8, 100186. [Google Scholar] [CrossRef]
- Niedzielin, K.; Kordecki, H.; Birkenfeld, B. A Controlled, Double-Blind, Randomized Study on the Efficacy of Lactobacillus Plantarum 299V in Patients with Irritable Bowel Syndrome. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1143–1147. [Google Scholar] [CrossRef]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology Guidelines on the Management of Irritable Bowel Syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef]
- Marlicz, W.; Skonieczna-Żydecka, K.; Krynicka, P.; Łoniewski, I.; Rydzewska, G. Probiotics in Irritable Bowel Syndrome—Is the Quest for the Right Strain over? Rapid Review of Existing Guidelines and Recommendations. Gastroenterol. Rev. Gastroenterol. 2021, 16, 369–382. [Google Scholar] [CrossRef]
- McFarland, L.V.; Karakan, T.; Karatas, A. Strain-Specific and Outcome-Specific Efficacy of Probiotics for the Treatment of Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. EClinicalMedicine 2021, 41, 101154. [Google Scholar] [CrossRef]
- Krammer, H.; Storr, M.; Madisch, A.; Riffel, J. [Treatment of IBS with Lactobacillus plantarum 299v: Therapeutic success increases with length of treatment—Real-life data of a non-interventional study in Germany]. Z. Gastroenterol. 2021, 59, 125–134. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, Y.A.; Bowyer, R.K.; Leach, H.; Gulia, P.; Horobin, J.; O’Sullivan, N.A.; Pettitt, C.; Reeves, L.B.; Seamark, L.; Williams, M.; et al. British Dietetic Association Systematic Review and Evidence-Based Practice Guidelines for the Dietary Management of Irritable Bowel Syndrome in Adults (2016 Update). J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2016, 29, 549–575. [Google Scholar] [CrossRef]
- McKenzie, Y.A.; Alder, A.; Anderson, W.; Wills, A.; Goddard, L.; Gulia, P.; Jankovich, E.; Mutch, P.; Reeves, L.B.; Singer, A.; et al. British Dietetic Association Evidence-Based Guidelines for the Dietary Management of Irritable Bowel Syndrome in Adults. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2012, 25, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Floch, M.H.; Walker, W.A.; Sanders, M.E.; Nieuwdorp, M.; Kim, A.S.; Brenner, D.A.; Qamar, A.A.; Miloh, T.A.; Guarino, A.; Guslandi, M.; et al. Recommendations for Probiotic Use—2015 Update: Proceedings and Consensus Opinion. J. Clin. Gastroenterol. 2015, 49, S69–S73. [Google Scholar] [CrossRef] [PubMed]
- Floch, M.H.; Walker, W.A.; Madsen, K.; Sanders, M.E.; Macfarlane, G.T.; Flint, H.J.; Dieleman, L.A.; Ringel, Y.; Guandalini, S.; Kelly, C.P.; et al. Recommendations for Probiotic Use-2011 Update. J. Clin. Gastroenterol. 2011, 45, S168–S171. [Google Scholar] [CrossRef]
- World Gastroenterology Organisation (WGO). Available online: https://www.worldgastroenterology.org (accessed on 14 August 2022).
- Dhar, D. Impending Mental Health Issues During Coronavirus Disease 2019—Time for Personalized Nutrition Based on the Gut Microbiota to Tide Over the Crisis? Front. Neurosci. 2022, 15, 831193. [Google Scholar] [CrossRef]
- Pappa, S.; Ntella, V.; Giannakas, T.; Giannakoulis, V.G.; Papoutsi, E.; Katsaounou, P. Prevalence of Depression, Anxiety, and Insomnia among Healthcare Workers during the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Brain. Behav. Immun. 2020, 88, 901–907. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and Neuropsychiatric Presentations Associated with Severe Coronavirus Infections: A Systematic Review and Meta-Analysis with Comparison to the COVID-19 Pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Łoniewski, I.; Misera, A.; Skonieczna-Żydecka, K.; Kaczmarczyk, M.; Kaźmierczak-Siedlecka, K.; Misiak, B.; Marlicz, W.; Samochowiec, J. Major Depressive Disorder and Gut Microbiota—Association Not Causation. A Scoping Review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110111. [Google Scholar] [CrossRef]
- Mörkl, S.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182. [Google Scholar] [CrossRef]
- Ettman, C.K.; Abdalla, S.M.; Cohen, G.H.; Sampson, L.; Vivier, P.M.; Galea, S. Prevalence of Depression Symptoms in US Adults Before and During the COVID-19 Pandemic. JAMA Netw. Open 2020, 3, e2019686. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Arseneault-Bréard, J.; Rondeau, I.; Gilbert, K.; Girard, S.-A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Combination of Lactobacillus Helveticus R0052 and Bifidobacterium Longum R0175 Reduces Post-Myocardial Infarction Depression Symptoms and Restores Intestinal Permeability in a Rat Model. Br. J. Nutr. 2012, 107, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Malick, M.; Gilbert, K.; Daniel, J.; Arseneault-Breard, J.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Vagotomy Prevents the Effect of Probiotics on Caspase Activity in a Model of Postmyocardial Infarction Depression. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2015, 27, 663–671. [Google Scholar] [CrossRef]
- Cowan, C.S.M.; Callaghan, B.L.; Richardson, R. The Effects of a Probiotic Formulation (Lactobacillus Rhamnosus and L. Helveticus) on Developmental Trajectories of Emotional Learning in Stressed Infant Rats. Transl. Psychiatry 2016, 6, e823. [Google Scholar] [CrossRef]
- Wang, H.; Lee, I.-S.; Braun, C.; Enck, P. Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review. J. Neurogastroenterol. Motil. 2016, 22, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of Psychotropic-like Properties of a Probiotic Formulation (Lactobacillus Helveticus R0052 and Bifidobacterium Longum R0175) in Rats and Human Subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Gareau, M.G.; Jury, J.; MacQueen, G.; Sherman, P.M.; Perdue, M.H. Probiotic Treatment of Rat Pups Normalises Corticosterone Release and Ameliorates Colonic Dysfunction Induced by Maternal Separation. Gut 2007, 56, 1522–1528. [Google Scholar] [CrossRef]
- McEwen, B.S.; Stellar, E. Stress and the Individual. Mechanisms Leading to Disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Frydecka, D.; Zawadzki, M.; Krefft, M.; Kiejna, A. Refining and Integrating Schizophrenia Pathophysiology—Relevance of the Allostatic Load Concept. Neurosci. Biobehav. Rev. 2014, 45, 183–201. [Google Scholar] [CrossRef]
- Shiels, P.G.; Buchanan, S.; Selman, C.; Stenvinkel, P. Allostatic Load and Ageing: Linking the Microbiome and Nutrition with Age-Related Health. Biochem. Soc. Trans. 2019, 47, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.-A.; Bah, T.M.; Kaloustian, S.; Lada-Moldovan, L.; Rondeau, I.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Lactobacillus Helveticus and Bifidobacterium Longum Taken in Combination Reduce the Apoptosis Propensity in the Limbic System after Myocardial Infarction in a Rat Model. Br. J. Nutr. 2009, 102, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.L.; Cowan, C.S.M.; Richardson, R. Treating Generational Stress: Effect of Paternal Stress on Development of Memory and Extinction in Offspring Is Reversed by Probiotic Treatment. Psychol. Sci. 2016, 27, 1171–1180. [Google Scholar] [CrossRef]
- Diop, L.; Guillou, S.; Durand, H. Probiotic Food Supplement Reduces Stress-Induced Gastrointestinal Symptoms in Volunteers: A Double-Blind, Placebo-Controlled, Randomized Trial. Nutr. Res. 2008, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liśkiewicz, P.; Kaczmarczyk, M.; Misiak, B.; Wroński, M.; Bąba-Kubiś, A.; Skonieczna-Żydecka, K.; Marlicz, W.; Bieńkowski, P.; Misera, A.; Pełka-Wysiecka, J.; et al. Analysis of Gut Microbiota and Intestinal Integrity Markers of Inpatients with Major Depressive Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110076. [Google Scholar] [CrossRef]
- Liśkiewicz, P.; Pełka-Wysiecka, J.; Kaczmarczyk, M.; Łoniewski, I.; Wroński, M.; Bąba-Kubiś, A.; Skonieczna-Żydecka, K.; Marlicz, W.; Misiak, B.; Samochowiec, J. Fecal Microbiota Analysis in Patients Going through a Depressive Episode during Treatment in a Psychiatric Hospital Setting. J. Clin. Med. 2019, 8, 164. [Google Scholar] [CrossRef]
- Misera, A.; Liśkiewicz, P.; Łoniewski, I.; Skonieczna-Żydecka, K.; Samochowiec, J. Effect of Psychobiotics on Psychometric Tests and Inflammatory Markers in Major Depressive Disorder: Meta-Analysis of Randomized Controlled Trials with Meta-Regression. Pharmaceuticals 2021, 14, 952. [Google Scholar] [CrossRef]
- Heidarzadeh-Rad, N.; Gökmen-Özel, H.; Kazemi, A.; Almasi, N.; Djafarian, K. Effects of a Psychobiotic Supplement on Serum Brain-Derived Neurotrophic Factor Levels in Depressive Patients: A Post Hoc Analysis of a Randomized Clinical Trial. J. Neurogastroenterol. Motil. 2020, 26, 486–495. [Google Scholar] [CrossRef]
- Eskandarzadeh, S.; Effatpanah, M.; Khosravi-Darani, K.; Askari, R.; Hosseini, A.F.; Reisian, M.; Jazayeri, S. Efficacy of a Multispecies Probiotic as Adjunctive Therapy in Generalized Anxiety Disorder: A Double Blind, Randomized, Placebo-Controlled Trial. Nutr. Neurosci. 2021, 24, 102–108. [Google Scholar] [CrossRef]
- Gao, F.; Guo, R.; Ma, Q.; Li, Y.; Wang, W.; Fan, Y.; Ju, Y.; Zhao, B.; Gao, Y.; Qian, L.; et al. Stressful Events Induce Long-Term Gut Microbiota Dysbiosis and Associated Post-Traumatic Stress Symptoms in Healthcare Workers Fighting against COVID-19. J. Affect. Disord. 2022, 303, 187–195. [Google Scholar] [CrossRef]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Probiotic Lactobacillus Casei Strain Shirota Relieves Stress-Associated Symptoms by Modulating the Gut-Brain Interaction in Human and Animal Models. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2016, 28, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.X.; Yusoff, N.a.A.; Hor, Y.-Y.; Lew, L.-C.; Jaafar, M.H.; Choi, S.-B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus Plantarum DR7 Alleviates Stress and Anxiety in Adults: A Randomised, Double-Blind, Placebo-Controlled Study. Benef. Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Bagga, D.; Reichert, J.L.; Koschutnig, K.; Aigner, C.S.; Holzer, P.; Koskinen, K.; Moissl-Eichinger, C.; Schöpf, V. Probiotics Drive Gut Microbiome Triggering Emotional Brain Signatures. Gut Microbes 2018, 9, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Williams, C.; Brown, A. Impact of Consuming a Milk Drink Containing a Probiotic on Mood and Cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A Randomized, Double-Blind, Placebo-Controlled Pilot Study of a Probiotic in Emotional Symptoms of Chronic Fatigue Syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef]
- Messaoudi, M.; Violle, N.; Bisson, J.-F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial Psychological Effects of a Probiotic Formulation (Lactobacillus Helveticus R0052 and Bifidobacterium Longum R0175) in Healthy Human Volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A Randomized Controlled Trial to Test the Effect of Multispecies Probiotics on Cognitive Reactivity to Sad Mood. Brain. Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v Decreases Kynurenine Concentration and Improves Cognitive Functions in Patients with Major Depression: A Double-Blind, Randomized, Placebo Controlled Study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Djafarian, K. Effect of Probiotic and Prebiotic versus Placebo on Appetite in Patients with Major Depressive Disorder: Post Hoc Analysis of a Randomised Clinical Trial. J. Hum. Nutr. Diet. 2020, 33, 56–65. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Djafarian, K. Effect of Prebiotic and Probiotic Supplementation on Circulating Pro-Inflammatory Cytokines and Urinary Cortisol Levels in Patients with Major Depressive Disorder: A Double-Blind, Placebo-Controlled Randomized Clinical Trial. J. Funct. Foods 2019, 52, 596–602. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Nazari, S.; Etesam, F.; Nourimajd, S.; Ahmadpanah, M.; Jahromi, S.R. The Effect of Synbiotic as an Adjuvant Therapy to Fluoxetine in Moderate Depression: A Randomized Multicenter Trial. Arch. Neurosci. 2018, 5, e60507. [Google Scholar] [CrossRef]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut Feelings: A Randomised, Triple-Blind, Placebo-Controlled Trial of Probiotics for Depressive Symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and Metabolic Response to Probiotic Administration in Patients with Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Saccarello, A.; Montarsolo, P.; Massardo, I.; Picciotto, R.; Pedemonte, A.; Castagnaro, R.; Brasesco, P.C.; Guida, V.; Picco, P.; Fioravanti, P. Oral Administration of S-Adenosylmethionine (SAMe) and Lactobacillus Plantarum HEAL9 Improves the Mild-To-Moderate Symptoms of Depression: A Randomized, Double-Blind, Placebo-Controlled Study. Prim. Care Companion CNS Disord. 2020, 22, 19m02578. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H.; et al. PROVIT: Supplementary Probiotic Treatment and Vitamin B7 in Depression—A Randomized Controlled Trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef]
- Reiter, A.; Bengesser, S.A.; Hauschild, A.-C.; Birkl-Töglhofer, A.-M.; Fellendorf, F.T.; Platzer, M.; Färber, T.; Seidl, M.; Mendel, L.-M.; Unterweger, R.; et al. Interleukin-6 Gene Expression Changes after a 4-Week Intake of a Multispecies Probiotic in Major Depressive Disorder-Preliminary Results of the PROVIT Study. Nutrients 2020, 12, 2575. [Google Scholar] [CrossRef]

| Strains | Daily Dose (CFU) |
|---|---|
| L. plantarum 299v (DSM 9843) | 1 × 109–2 × 1010 |
| B. infantis 35624 | 1 × 108 |
| S. boulardii CNCM I-745 | 2 × (5 × 109)/or 250 mg–4 × 1011 |
| E. coli DSM17252 | 3.375 × 107–2.475 × 108 |
| Bacillus coagulans GB-1-30, 6068 | 2 × 109 |
| S. cerevisiae | 4 × 109 |
| B. lactis DN 173010 | 2 × 125 g |
| L. rhamnosus NCIMB 30174, L. plantarum NCIMB 30173, L. acidophilus NCIMB 30175, and Enterococcus faecium NCIMB 30176 | 2 × 107/kg body weight |
| S. thermophilus DSM24731, B. longum DSM24736, B. breve DSM24732, B. infantis DSM24737, L. acidophilus DSM24735, L. plantarum DSM24730, L. paracasei DSM24733 and L. delbrueckii spp. bulgaricus DSM24734 | 4.5–9.0 × 1011 |
| L. aciophilus CUL60 [NCIMB 30157], L. acidophilus CUL21 [NCIMB 30156], B. animalis subsp. lactis CUL34 [NCIMB 30172] and B. bifidum CUL20 | 2.5 × 1010 |
| L. plantarum CECT7484 and CECT7485 and Pediococcus acidilactici CECT7483 | 3.6 × 109 |
| L. sp. HY7801, B. longum HY8004 and L. brevis HY7401 | 4 × 1010 |
| B. bifidum BGN4, B. lactis AD011, L. acidophilus AD031 and L. casei IBS041 | 4 × 1010 |
| B. lactis CNCM I-2494, with S. thermophilus and L. bulgaricus | 1.25 × 1010 + 1.2 × 109 |
| Strains | Daily Dose (CFU) | Ref. |
|---|---|---|
| Stress | ||
| L. acidophilus Rosell-52; B. longum Rosell-175 | 3 × 109 | [172] |
| L. casei Shirota YIT 9029 | 1 × 1010 | [179] |
| L. plantarum DR7 | 1 × 109 | [180] |
| Anxiety and/or depressive symptoms | ||
| L. casei W56, L. acidophilus W22, L. paracasei W20, B. lactis W51, L. salivarius W24, Lactococcus lactis W19, B. lactis W52, L. plantarum W62 and B. bifidum W23 | 7.5 × 106 | [181] |
| L. casei Shirota | 2.4 × 1010–4.2 × 1011 | [182,183] |
| L. helveticus R0052, B. longum R0175 | 3 × 109 | [165,184] |
| B. lactis W52, L. brevis W63, L. casei W56, Lactococcus lactis W19 and W58, L. acidophilus W37, B. bifidum W23, B. lactis W51, L. salivarius W24 | 5 × 109 | [185] |
| Major Depressive Disorder (MDD) | ||
| L. plantarum 299v | 2 × 1010 | [186] |
| L. helveticus R0052 (CNCM strain I-1722) and B. longum R0175 (CNCM strain I-3470) | 1 × 1010 | [187,188] |
| L. casei, L. acidofilus, L. bulgarigus, L. rhamnosus, B. breve, B. longum, S. thermophilus and FOS | L. casei 3 × 108; L. acidofilus 2 × 108, L. bulgarigus 2 × 109, L. rhamnosus 3 × 108, B. breve 2 × 108, B. longum 1 × 109, S. thermophilus 3 × 108 and 200 mg fructooligosaccharide | [189] |
| B. bifidum W23, B. lactis W51 and W52, L. acidophilus W37, L. brevis W63, L.casei W56, L. salivarius W24, L. lactis W19 W58 | 1 × 1010 | [190] |
| L. acidophilus, L. casei and B. bifidum | 1 capsule daily | [191] |
| L. plantarum Heal 9 + SAMe | 1 × 109 + 200 mg SAMe | [192] |
| B. bifidum W23, B. lactis W51, and W52, L. acidophilus W22, L. casei W56, L. paracasei W20, L. plantarum W62, L. salivarius W24, L. lactis W19 and FOS | 7.5 × 109 | [193,194] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łoniewski, I.; Skonieczna-Żydecka, K.; Sołek-Pastuszka, J.; Marlicz, W. Probiotics in the Management of Mental and Gastrointestinal Post-COVID Symptomes. J. Clin. Med. 2022, 11, 5155. https://doi.org/10.3390/jcm11175155
Łoniewski I, Skonieczna-Żydecka K, Sołek-Pastuszka J, Marlicz W. Probiotics in the Management of Mental and Gastrointestinal Post-COVID Symptomes. Journal of Clinical Medicine. 2022; 11(17):5155. https://doi.org/10.3390/jcm11175155
Chicago/Turabian StyleŁoniewski, Igor, Karolina Skonieczna-Żydecka, Joanna Sołek-Pastuszka, and Wojciech Marlicz. 2022. "Probiotics in the Management of Mental and Gastrointestinal Post-COVID Symptomes" Journal of Clinical Medicine 11, no. 17: 5155. https://doi.org/10.3390/jcm11175155
APA StyleŁoniewski, I., Skonieczna-Żydecka, K., Sołek-Pastuszka, J., & Marlicz, W. (2022). Probiotics in the Management of Mental and Gastrointestinal Post-COVID Symptomes. Journal of Clinical Medicine, 11(17), 5155. https://doi.org/10.3390/jcm11175155









