Myocarditis Induced by Immunotherapy in Metastatic Melanoma—Review of Literature and Current Guidelines
Abstract
:1. Introduction
2. Clinical Background-Case Report
3. Review and Discussion
3.1. Diagnosis of Myocarditis
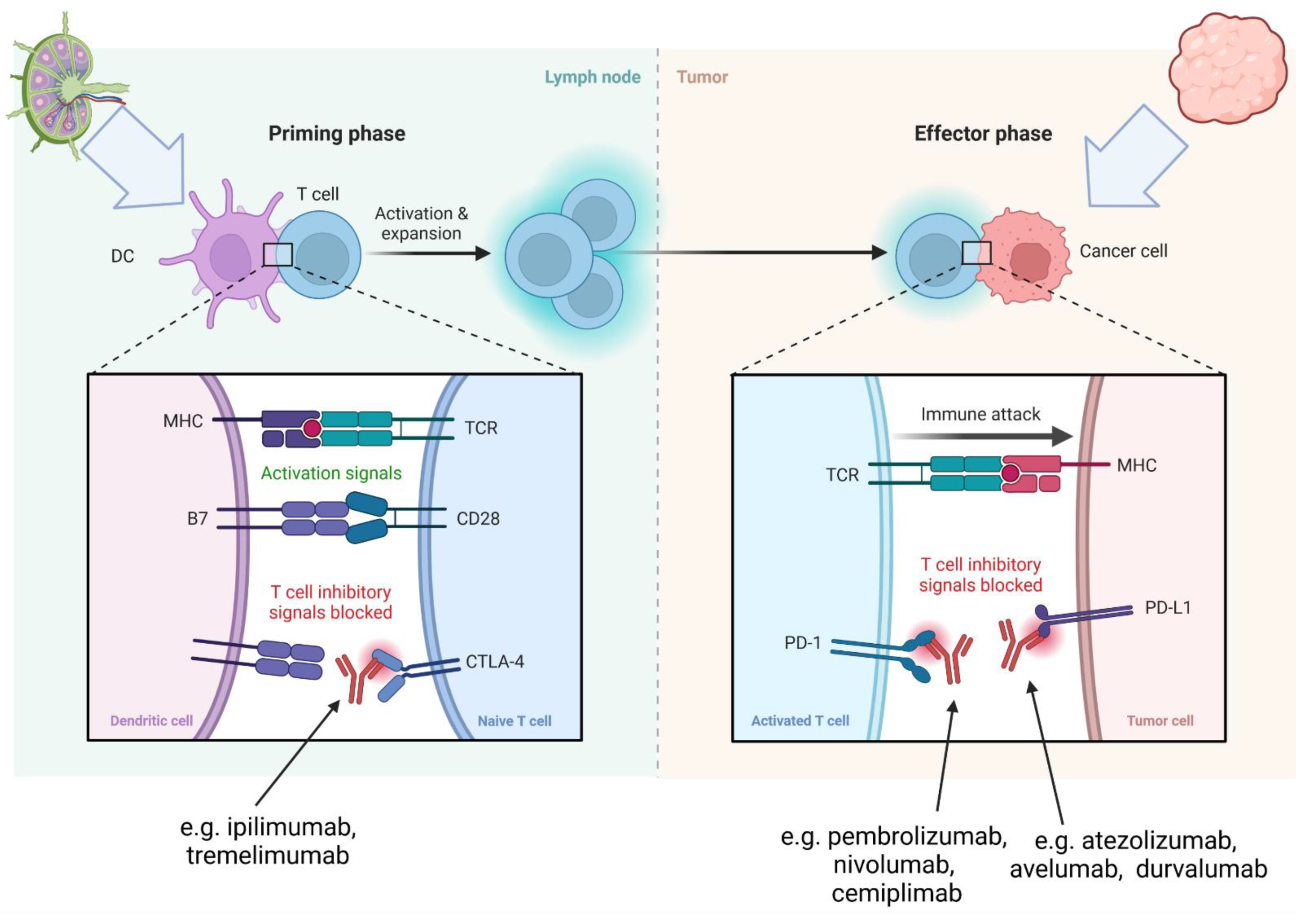
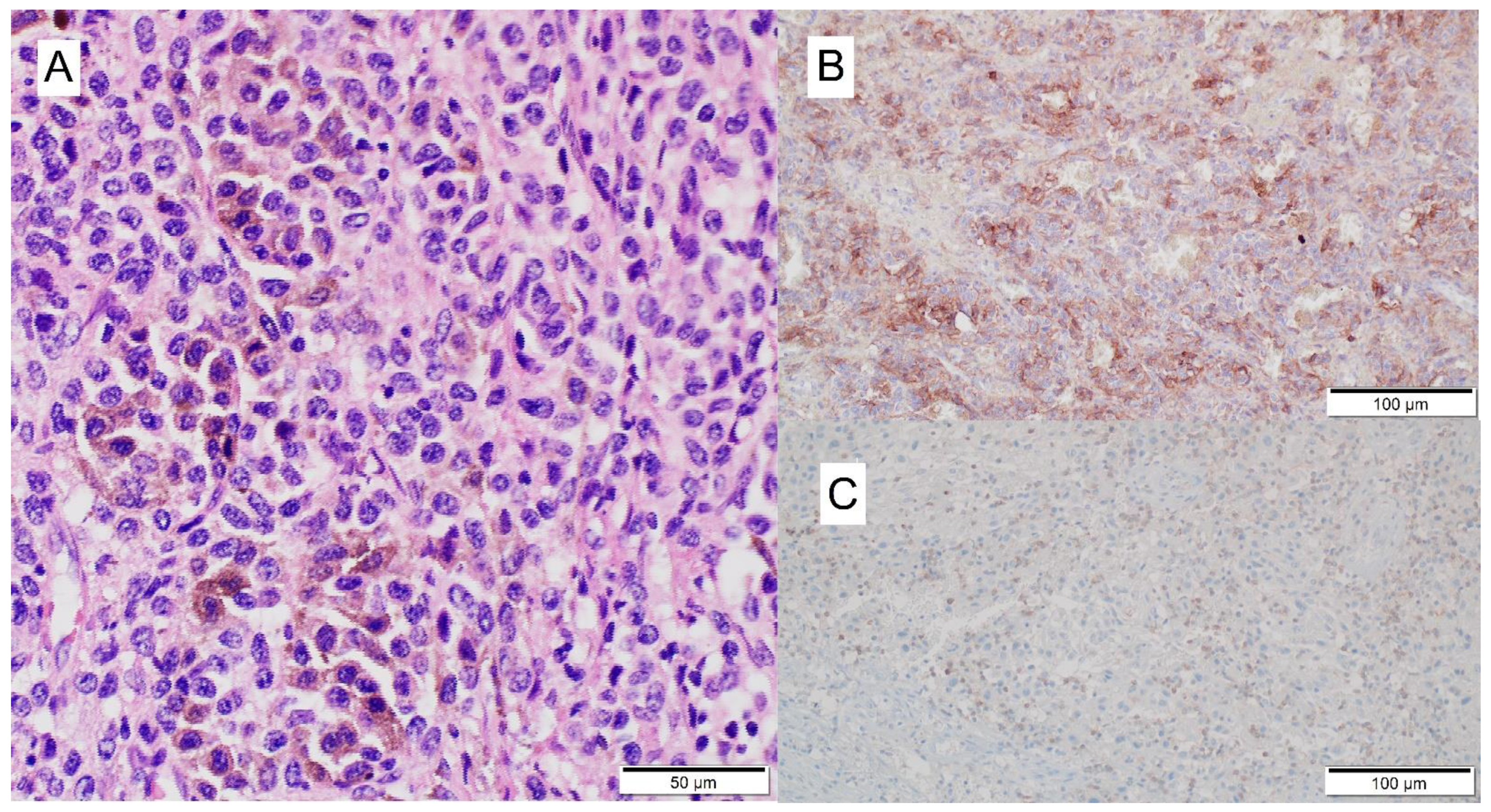
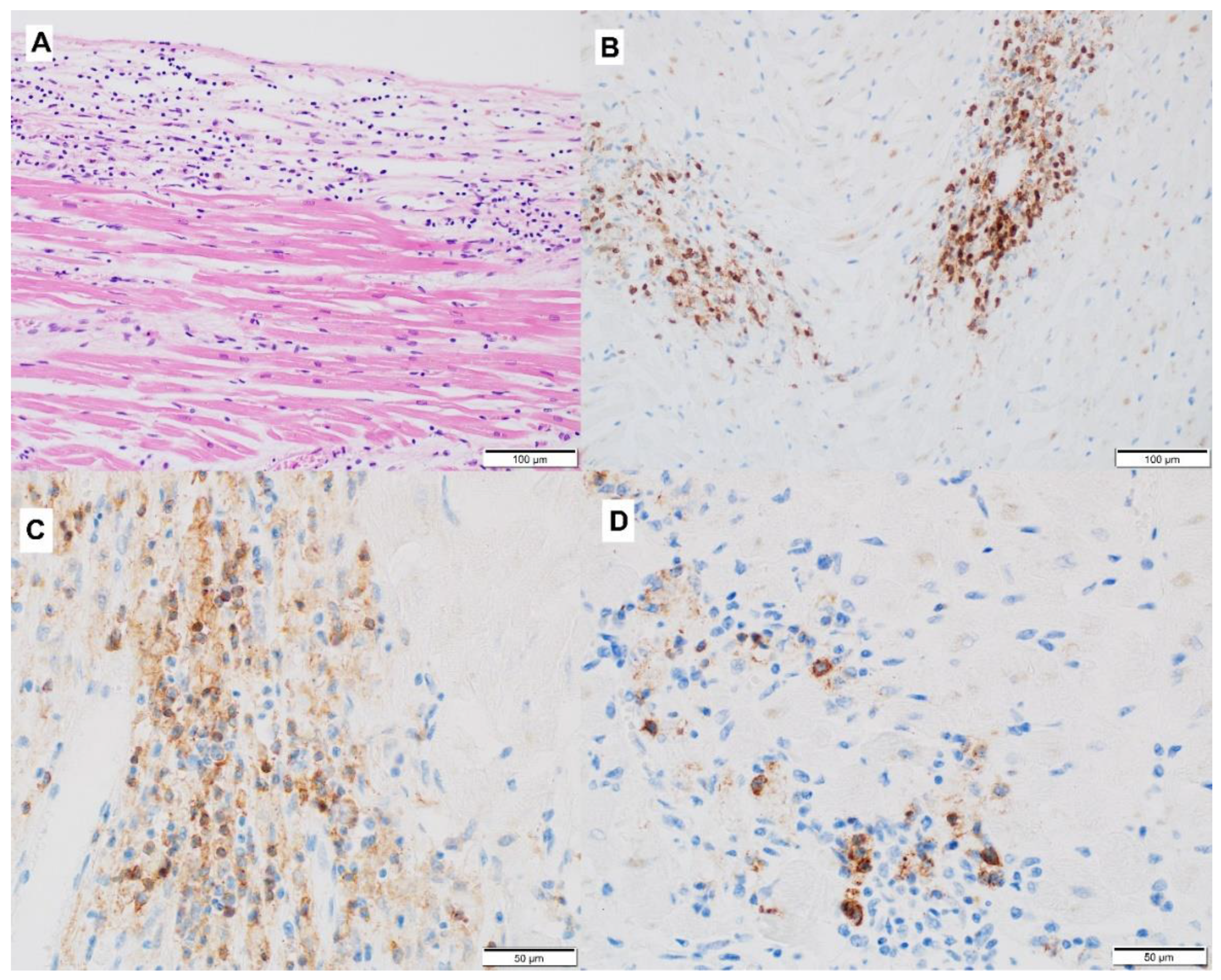
3.2. Mechanism of ICI-Related Myocarditis, Biomarkers and Histopathology
3.3. Clinical Presentation of ICI-Related Myocarditis
3.4. Other Specific Organ Toxicities Associated with ICI
3.5. Risk Factors for ICI-Related Myocarditis
3.6. Treatment of ICI-Related Myocarditis
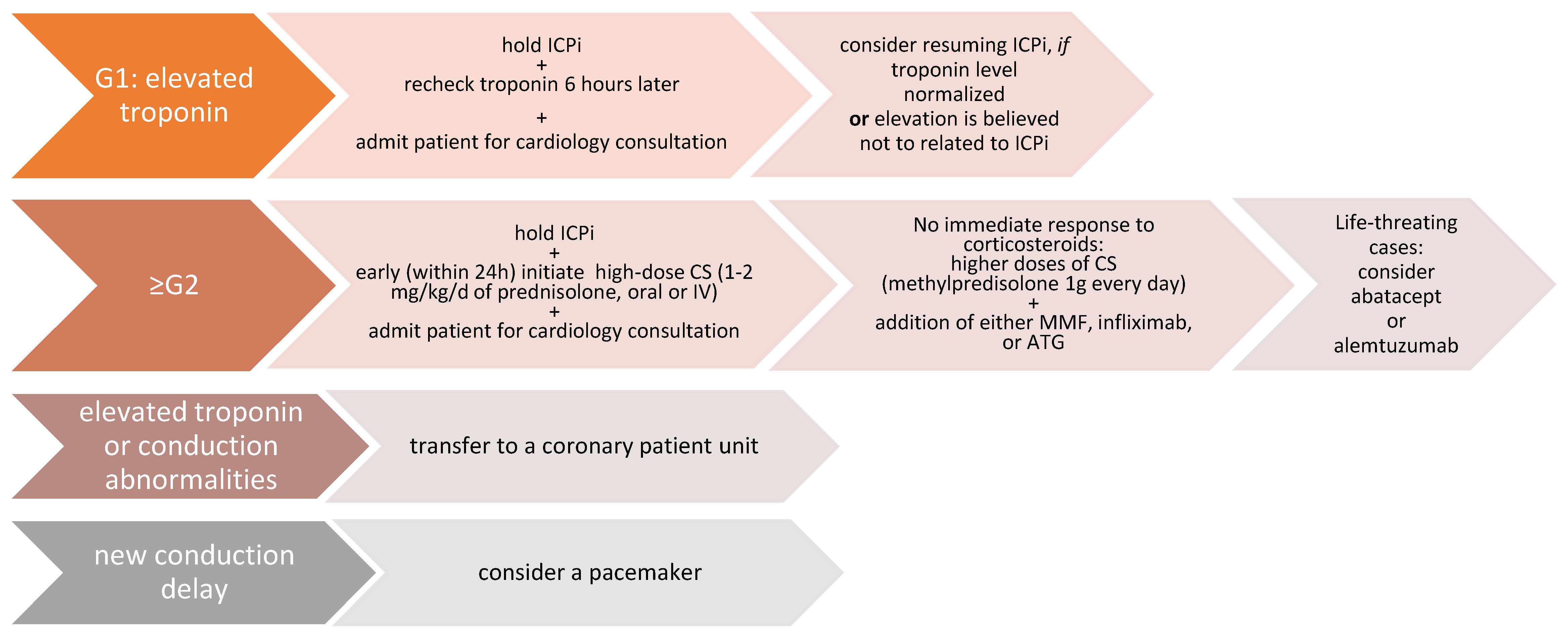
| G1 | Abnormal cardiac biomarker testing without symptoms and with no ECG abnormalities |
| G2 | Abnormal cardiac biomarker testing with mild symptoms or new ECG abnormalities without conduction delay |
| G3 | Abnormal cardiac biomarker testing with either moderate symptoms or new conduction delay |
| G4 | Moderate to severe decompensation, IV medication or intervention required, life-threatening conditions |
3.7. Follow-Up and Surveillance of Patients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, L.B.; Salama, A. A review of cancer immunotherapy toxicity. CA A Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.-H.; Geng, L.-Y.; Jiang, P.-P.; Xu, H.; Nan, K.-J.; Yao, Y.; Jiang, L.-L.; Sun, H.; Qin, T.-J.; Guo, H. The biomarkers related to immune related adverse events caused by immune checkpoint inhibitors. J. Exp. Clin. Cancer Res. 2020, 39, 284. [Google Scholar] [CrossRef] [PubMed]
- Weidhaas, J.; Marco, N.; Scheffler, A.W.; Kalbasi, A.; Wilenius, K.; Rietdorf, E.; Gill, J.; Heilig, M.; Desler, C.; Telesca, D.; et al. Germline biomarkers predict toxicity to anti-PD1/PDL1 checkpoint therapy. J. Immunother. Cancer 2022, 10, e003625. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Johnson, D.B.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, L.; Goldinger, S.M.; Hofmann, L.; Loquai, C.; Ugurel, S.; Thomas, I.; Schmidgen, M.; Gutzmer, R.; Utikal, J.; Heinzerling, L.; et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 210–225. [Google Scholar] [CrossRef]
- Oldfield, K.; Jayasinghe, R.; Niranjan, S.; Chadha, S. Immune checkpoint inhibitor-induced takotsubo syndrome and diabetic ketoacidosis: Rare reactions. BMJ Case Rep. 2021, 14, e237217. [Google Scholar] [CrossRef]
- Voskens, C.J.; Goldinger, S.M.; Loquai, C.; Robert, C.; Kaehler, K.C.; Al, E.; Dummer, R. The price of tumor control: An analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One 2013, 8, e53745. [Google Scholar] [CrossRef]
- Tomita, Y.; Sueta, D.; Kakiuchi, Y.; Saeki, S.; Saruwatari, K.; Sakata, S.; Jodai, T.; Migiyama, Y.; Akaike, K.; Hirosako, S.; et al. Acute coronary syndrome as a possible immune-related adverse event in a lung cancer patient achieving a complete response to anti-PD-1 immune checkpoint antibody. Ann. Oncol. 2017, 28, 2893–2895. [Google Scholar] [CrossRef]
- Heery, C.R.; Coyne, G.H.O.; Madan, R.A.; Schlom, J.; Von Heydebreck, A.; Cuillerot, J.-M.; Sabzevari, H.; Gulley, J.L. Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti-PD-L1 monoclonal antibody, in advanced solid malignancies. J. Clin. Oncol. 2014, 32 (Suppl. 15), 3064. [Google Scholar] [CrossRef]
- Tadokoro, T.; Keshino, E.; Makiyama, A.; Sasaguri, T.; Ohshima, K.; Katano, H.; Mohri, M. Acute Lymphocytic Myocarditis with Anti-PD-1 Antibody Nivolumab. Circ. Heart Fail. 2016, 9, e003514. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.; Moslehi, J.J.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.; Damrongwatanasuk, R.; Chen, C.; Neilan, T.G.; et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, P.; Balko, J.; Moslehi, J.J.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Salem, J.E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef]
- Tajiri, K.; Aonuma, K.; Sekine, I. Immune checkpoint inhibitor-related myocarditis. Jpn. J. Clin. Oncol. 2017, 48, 7–12. [Google Scholar] [CrossRef]
- Matzen, E.; Bartels, L.E.; Løgstrup, B.; Horskær, S.; Stilling, C.; Donskov, F. Immune checkpoint inhibitor-induced myocarditis in cancer patients: A case report and review of reported cases. Cardio-Oncol. 2021, 7, 27. [Google Scholar] [CrossRef]
- D’Souza, M.; Nielsen, D.; Svane, I.M.; Iversen, K.; Rasmussen, P.V.; Madelaire, C.; Fosbøl, E.; Køber, L.; Gustafsson, F.; Andersson, C.; et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: A nationwide Danish study. Eur. Heart J. 2020, 42, 1621–1631. [Google Scholar] [CrossRef]
- Scard, C.; Nguyen, J.-M.; Varey, E.; Moustaghfir, I.; Khammari, A.; Dreno, B. Cardiac adverse events associated with anti-PD-1 therapy in patients treated for advanced melanoma: Relevance of dosing troponin T levels. Eur. J. Dermatol. 2021, 31, 205–212. [Google Scholar] [CrossRef]
- Qin, Q.; Patel, V.G.; Wang, B.; Mellgard, G.; Gogerly-Moragoda, M.; Zhong, X.; Parikh, A.B.; Leiter, A.; Gallagher, E.J.; Galsky, M.D.; et al. Type, timing, and patient characteristics associated with immune-related adverse event development in patients with advanced solid tumors treated with immune checkpoint inhibitors. J. Clin. Oncol. 2020, 38 (Suppl. S15), e15160. [Google Scholar] [CrossRef]
- Jain, P.; Bugarin, J.G.; Guha, A.; Jain, C.; Patil, N.; Shen, T.; Stanevich, I.; Nikore, V.; Margolin, K.; Dowlati, A.; et al. Cardiovascular adverse events are associated with usage of immune checkpoint inhibitors in real-world clinical data across the United States. ESMO Open 2021, 6, 100252. [Google Scholar] [CrossRef]
- Heinzerling, L.; Ott, P.A.; Hodi, F.S.; Husain, A.N.; Tajmir-Riahi, A.; Tawbi, H.; Pauschinger, M.; Gajewski, T.F.; Lipson, E.J.; Luke, J.J. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J. Immunother. Cancer 2016, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Rahouma, M.; Karim, N.A.; Baudo, M.; Yahia, M.; Kamel, M.; Eldessouki, I.; Abouarab, A.; Saad, I.; Elmously, A.; Gaudino, M.; et al. Cardiotoxicity with immune system targeting drugs: A meta-analysis of anti-PD/PD-L1 immunotherapy randomized clinical trials. Immunotherapy 2019, 11, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, P.; Richards, J.; Testori, A.; et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef]
- Saibil, S.D.; Bonilla, L.; Majeed, H.; Sotov, V.; Hogg, D.; Chappell, M.A.; Cybulsky, M.; Butler, M.O. Fatal Myocarditis and Rhabdomyositis in a Patient with Stage Iv Melanoma Treated with Combined Ipilimumab and Nivolumab. Curr. Oncol. 2019, 26, e418–e421. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Morimoto, R.; Okumura, T.; Yamashita, Y.; Haga, T.; Kuwayama, T.; Yokoi, T.; Hiraiwa, H.; Kondo, T.; Sugiura, Y.; et al. Late-Onset Fulminant Myocarditis with Immune Checkpoint Inhibitor Nivolumab. Can. J. Cardiol. 2018, 34, 812.e1–812.e3. [Google Scholar] [CrossRef]
- Arangalage, D.; Delyon, J.; Lermuzeaux, M.; Ekpe, K.; Ederhy, S.; Pages, C.; Lebbé, C. Survival After Fulminant Myocarditis Induced by Immune-Checkpoint Inhibitors. Ann. Intern. Med. 2017, 167, 683–684. [Google Scholar] [CrossRef]
- Geisler, B.P.; Raad, R.A.; Esaian, D.; Sharon, E.; Schwartz, D.R. Apical ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: A case of takotsubo-like syndrome. J. Immunother. Cancer 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Vincelette, N.D.; Mansour, I.; Hariri, D.; Motamed, S. Late Onset Ipilimumab-Induced Pericarditis and Pericardial Effusion: A Rare but Life Threatening Complication. Case Rep. Oncol. Med. 2015, 2015, 794842. [Google Scholar] [CrossRef]
- Läubli, H.; Balmelli, C.; Bossard, M.; Pfister, O.; Glatz, K.; Zippelius, A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J. Immunother. Cancer 2015, 3, 11. [Google Scholar] [CrossRef]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.; Segal, N.; Callahan, M.; Hellmann, M.D.; et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Göppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 190–209. [Google Scholar] [PubMed]
- Dearden, H.; Au, L.; Wang, D.Y.; Zimmer, L.; Eroglu, Z.; Smith, J.L.; Cuvietto, M.; Khoo, C.; Atkinson, V.; Lo, S.; et al. Hyperacute toxicity with combination ipilimumab and anti-PD1 immunotherapy. Eur. J. Cancer 2021, 153, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Shulgin, B.; Kosinsky, Y.; Omelchenko, A.; Chu, L.; Mugundu, G.; Aksenov, S.; Pimentel, R.; DeYulia, G.; Kim, G.; Peskov, K.; et al. Dose dependence of treatment-related adverse events for immune checkpoint inhibitor therapies: A model-based meta-analysis. OncoImmunology 2020, 9, 1748982. [Google Scholar] [CrossRef] [PubMed]
- Arangalage, D.; Degrauwe, N.; Michielin, O.; Monney, P.; Özdemir, B.C. Pathophysiology, diagnosis and management of cardiac toxicity induced by immune checkpoint inhibitors and BRAF and MEK inhibitors. Cancer Treat. Rev. 2021, 100, 102282. [Google Scholar] [PubMed]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Elliott, P.M.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar]
- Cooper, L.T. Myocarditis. New Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef]
- Richardson, P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar]
- Aretz, H.T.; Billingham, M.E.; Edwards, W.D.; Factor, S.M.; Fallon, J.; Fenoglio, J.J.; Olsen, E.G.; Schoen, F.J. Myocarditis. A histopathologic definition and classification. Am. J. Cardiovasc. Pathol. 1987, 1, 3–14. [Google Scholar]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.; Narula, J.; Starling, R.C.; Virmani, R.; et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur. Heart J. 2007, 28, 3076–3093. [Google Scholar]
- Lobenwein, D.; Kocher, F.; Dobner, S.; Gollmann-Tepeköylü, C.; Holfeld, J. Cardiotoxic mechanisms of cancer immunotherapy–A systematic review. Int. J. Cardiol. 2020, 323, 179–187. [Google Scholar]
- Grabie, N.; Gotsman, I.; Dacosta, R.; Pang, H.; Stavrakis, G.; Butte, M.J.; Keir, M.E.; Freeman, G.J.; Sharpe, A.H.; Lichtman, A.H. Endothelial Programmed Death-1 Ligand 1 (PD-L1) Regulates CD8 + T-Cell–Mediated Injury in the Heart. Circulation 2007, 116, 2062–2071. [Google Scholar] [PubMed]
- Seko, Y.; Yagita, H.; Okumura, K.; Azuma, M.; Nagai, R. Roles of programmed death-1 (PD-1)/PD-1 ligands pathway in the development of murine acute myocarditis caused by coxsackievirus B3. Cardiovasc. Res. 2007, 75, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tajiri, K.; Murakoshi, N.; Xu, D.; Yonebayashi, S.; Okabe, Y.; Yuan, Z.; Feng, D.; Inoue, K.; Aonuma, K.; et al. Programmed Death-Ligand 2 Deficiency Exacerbates Experimental Autoimmune Myocarditis in Mice. Int. J. Mol. Sci. 2021, 22, 1426. [Google Scholar] [PubMed]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science 2001, 291, 319–322. [Google Scholar] [PubMed]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar]
- Tay, W.T.; Fang, Y.-H.; Beh, S.T.; Liu, Y.-W.; Hsu, L.-W.; Yen, C.-J.; Liu, P.-Y. Programmed Cell Death-1: Programmed Cell Death-Ligand 1 Interaction Protects Human Cardiomyocytes Against T-Cell Mediated Inflammation and Apoptosis Response In Vitro. Int. J. Mol. Sci. 2020, 21, 2399. [Google Scholar]
- Hu, J.R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.; Johnson, D.; Moslehi, J.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar]
- Tarrio, M.L.; Grabie, N.; Bu, D.-X.; Sharpe, A.H.; Lichtman, A.H. PD-1 Protects against Inflammation and Myocyte Damage in T Cell-Mediated Myocarditis. J. Immunol. 2012, 188, 4876–4884. [Google Scholar]
- Koelzer, V.H.; Rothschild, S.I.; Zihler, D.; Wicki, A.; Willi, B.; Willi, N.; Voegeli, M.; Cathomas, G.; Zippelius, A.; Mertz, K.D. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors—an autopsy study. J. Immunother. Cancer 2016, 4, 13. [Google Scholar]
- Saade, A.; Mansuet-Lupo, A.; Arrondeau, J.; Thibault, C.; Mirabel, M.; Goldwasser, F.; Oudard, S.; Weiss, L. Pericardial effusion under nivolumab: Case-reports and review of the literature. J. Immunother Cancer 2019, 7, 266. [Google Scholar] [CrossRef] [Green Version]
- Ang, E.; Mweempwa, A.; Heron, C.; Ahn, Y.; Rivalland, G.; Ha, L.Y.; Deva, S. Cardiac Troponin I and T in Checkpoint Inhibitor–associated Myositis and Myocarditis. J. Immunother. 2021, 44, 162–163. [Google Scholar] [PubMed]
- Arponen, O.; Skyttä, T. Immune checkpoint inhibitor-induced myocarditis not visible with cardiac magnetic resonance imaging but detected with PET-CT: A case report. Acta Oncol. 2020, 59, 490–492. [Google Scholar] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.; Crea, F.; Goudevenos, F.; Widimský, P.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Pohl, J.; Mincu, R.-I.; Mrotzek, S.M.; Hinrichs, L.; Michel, L.; Livingstone, E.; Zimmer, L.; Wakili, R.; Schadendorf, D.; Rassaf, T.; et al. ECG Changes in Melanoma Patients Undergoing Cancer Therapy–Data from the ECoR Registry. J. Clin. Med. 2020, 9, 2060. [Google Scholar]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; delle Miocarditi, R.L.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients With Acute Myocarditis: Multicenter Lombardy Registry. Circulation 2018, 138, 1088–1099. [Google Scholar]
- Awadalla, M.; Mahmood, S.S.; Groarke, J.D.; Hassan, M.Z.; Nohria, A.; Rokicki, A.; Murphy, S.; Mercaldo, N.; Zhang, L.; Neilan, T.G.; et al. Global Longitudinal Strain and Cardiac Events in Patients With Immune Checkpoint Inhibitor-Related Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 467–478. [Google Scholar]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar]
- Olimulder, M.A.; van Es, J.; Galjee, M.A. The importance of cardiac MRI as a diagnostic tool in viral myocarditis-induced cardiomyopathy. Neth. Heart J. 2009, 17, 481–486. [Google Scholar]
- Nensa, F.; Kloth, J.; Tezgah, E.; Poeppel, T.D.; Heusch, P.; Goebel, J.; Nassenstein, K.; Schlosser, T. Feasibility of FDG-PET in myocarditis: Comparison to CMR using integrated PET/MRI. J. Nucl. Cardiol. 2018, 25, 785–794. [Google Scholar]
- Hazebroek, M.R.; Everaerts, K.; Heymans, S. Diagnostic approach of myocarditis: Strike the golden mean. Neth. Heart J. 2014, 22, 80–84. [Google Scholar]
- Ansari-Gilani, K.; Tirumani, S.H.; Smith, D.A.; Nelson, A.; Alahmadi, A.; Hoimes, C.J.; Ramaiya, N.H. Myocarditis associated with immune checkpoint inhibitor therapy: A case report of three patients. Emerg. Radiol. 2020, 27, 455–460. [Google Scholar] [PubMed]
- Samara, Y.; Yu, C.L.; Dasanu, C.A. Acute autoimmune myocarditis and hepatitis due to ipilimumab monotherapy for malignant melanoma. J. Oncol. Pharm. Pract. 2018, 25, 966–968. [Google Scholar]
- Fukasawa, Y.; Sasaki, K.; Natsume, M.; Nakashima, M.; Ota, S.; Watanabe, K.; Takahashi, Y.; Kondo, F.; Kozuma, K.; Seki, N. Nivolumab-Induced Myocarditis Concomitant with Myasthenia Gravis. Case Rep. Oncol. 2017, 10, 809–812. [Google Scholar] [PubMed]
- Pathak, R.; Katel, A.; Massarelli, E.; Villaflor, V.M.; Sun, V.; Salgia, R. Immune Checkpoint Inhibitor–Induced Myocarditis with Myositis/Myasthenia Gravis Overlap Syndrome: A Systematic Review of Cases. Oncologist 2021, 26, 1052–1061. [Google Scholar] [PubMed]
- Suzuki, S.; Ishikawa, N.; Konoeda, F.; Seki, N.; Fukushima, S.; Takahashi, K.; Uhara, H.; Hasegawa, Y.; Inomata, S.; Matsui, M.; et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017, 89, 1127–1134. [Google Scholar]
- Bawek, S.J.; Ton, R.; McGovern-Poore, M.; Khoncarly, B.; Narvel, R. Nivolumab-Induced Myasthenia Gravis Concomitant with Myocarditis, Myositis, and Hepatitis. Cureus 2021, 13, e18040. [Google Scholar]
- Diamantopoulos, P.T.; Tsatsou, K.; Benopoulou, O.; Bonou, M.; Anastasopoulou, A.; Mastrogianni, E.; Gogas, H. Concomitant development of neurologic and cardiac immune-related adverse effects in patients treated with immune checkpoint inhibitors for melanoma. Melanoma Res. 2020, 30, 484–491. [Google Scholar]
- Shalata, W.; Peled, N.; Gabizon, I.; Saleh, O.A.; Kian, W.; & Yakobson, A. Associated Myocarditis: A Predictive Factor for Response? Case Rep. Oncol. 2020, 13, 550–557. [Google Scholar]
- Barham, W.; Guo, R.; Park, S.S.; Herrmann, J.; Dong, H.; Yan, Y. Case Report: Simultaneous Hyperprogression and Fulminant Myocarditis in a Patient with Advanced Melanoma Following Treatment With Immune Checkpoint Inhibitor Therapy. Front. Immunol. 2021, 11, 561083. [Google Scholar]
- Arangalage, D.; Pavon, A.G.; Özdemir, B.C.; Michielin, O.; Schwitter, J.; Monney, P. Acute cardiac manifestations under immune checkpoint inhibitors—beware of the obvious: A case report. Eur. Heart J.-Case Rep. 2021, 5, ytab262. [Google Scholar]
- Fazel, M.; Jedlowski, P.M. Severe Myositis, Myocarditis, and Myasthenia Gravis with Elevated Anti-Striated Muscle Antibody following Single Dose of Ipilimumab-Nivolumab Therapy in a Patient with Metastatic Melanoma. Case Rep. Immunol. 2019, 2019, 2539493. [Google Scholar]
- Wakefield, C.; Shultz, C.; Patel, B.; Malla, M. Life-threatening immune checkpoint inhibitor-induced myocarditis and myasthenia gravis overlap syndrome treated with abatacept: A case report. BMJ Case Rep. 2021, 14, e244334. [Google Scholar] [PubMed]
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association Between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311. [Google Scholar]
- Kanz, B.A.; Pollack, M.H.; Johnpulle, R.; Puzanov, I.; Horn, L.; Morgans, A.; Sosman, J.A.; Rapisuwon, S.; Conry, R.M.; Eroglu, Z.; et al. Safety and efficacy of anti-PD-1 in patients with baseline cardiac, renal, or hepatic dysfunction. J. Immunother. Cancer 2016, 4, 60. [Google Scholar] [PubMed]
- Shah, K.P.; Song, H.; Ye, F.; Moslehi, J.J.; Balko, J.M.; Salem, J.-E.; Johnson, D.B. Demographic Factors Associated with Toxicity in Patients Treated with Anti–Programmed Cell Death-1 Therapy. Cancer Immunol. Res. 2020, 8, 851–855. [Google Scholar] [PubMed]
- Johnson, D.B.; Sullivan, R.J.; Ott, P.A.; Carlino, M.S.; Khushalani, N.I.; Ye, F.; Buchbinder, E.; Mudigonda, T.; Spencer, K.; Clark, J.I.; et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2016, 2, 234–240. [Google Scholar]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.; Mammen, J.; Naing, A.; Bollin, K.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar]
- Guo, C.W.; Alexander, M.; Dib, Y.; Lau, P.K.; Weppler, A.M.; Au-Yeung, G.; Lee, B.; Khoo, C.; Mooney, D.; Joshi, S.B.; et al. A closer look at immune-mediated myocarditis in the era of combined checkpoint blockade and targeted therapies. Eur. J. Cancer 2019, 124, 15–24. [Google Scholar]
- Norwood, T.G.; Lenneman, C.A.; Westbrook, B.C.; Litovsky, S.H.; McKee, S.B.; Conry, R.M. Evolution of Immune Checkpoint Blockade–Induced Myocarditis Over 2 Years. JACC Case Rep. 2020, 2, 203–209. [Google Scholar]
- Michel, L.; Helfrich, I.; Hendgen-Cotta, U.B.; Mincu, R.-I.; Korste, S.; Mrotzek, S.M.; Spomer, A.; Odersky, A.; Rischpler, C.; Herrmann, K.; et al. Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur. Heart J. 2021, 43, 316–329. [Google Scholar]
- Balanescu, D.V.; Donisan, T.; Palaskas, N.; Lopez-Mattei, J.; Kim, P.Y.; Buja, L.M.; McNamara, D.M.; Kobashigawa, J.A.; Durand, J.-B.; Iliescu, C.A. Immunomodulatory treatment of immune checkpoint inhibitor-induced myocarditis: Pathway toward precision-based therapy. Cardiovasc. Pathol. 2020, 47, 107211. [Google Scholar] [PubMed]
- Benassaia, E.; Vallet, A.; Rouleau, E.; Ederhy, S.; Robert, C. Troponin increase during immunotherapy: Not always myocarditis. Eur. J. Cancer 2021, 157, 424–427. [Google Scholar]
- Bussani, R.; De-Giorgio, F.; Abbate, A.; Silvestri, F. Cardiac metastases. J. Clin. Pathol. 2007, 60, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kurzhals, J.K.; Graf, T.; Boch, K.; Grzyska, U.; Frydrychowicz, A.; Zillikens, D.; Terheyden, P.; Langan, E.A. Serum Troponin T Concentrations Are Frequently Elevated in Advanced Skin Cancer Patients Prior to Immune Checkpoint Inhibitor Therapy: Experience from a Single Tertiary Referral Center. Front. Med. 2021, 8, 691618. [Google Scholar]
| Parameter | Reference Range | Before Initiation of Immunotherapy | After 3rd Course of Nivolumab | On Admission to the 4th Course |
|---|---|---|---|---|
| ASPAT | <50 (IU/L) | 16 | 71 | 178 |
| ALT | <50 (IU/L) | 10 | 49 | 207 |
| CK | <171 (IU/L) | 60 | - | 2194 |
| CRP | <5 (mg/L) | 2.8 | - | 6.1 |
| LDH | <247 (IU/L) | 222 | 377 | 943 |
| Definitive diagnosis | Histology—EMB (according to Dallas criteria [38]) | Active myocarditis: an inflammatory infiltrate of the myocardium with necrosis and/or degeneration of adjacent myocytes not typical of the ischemic damage associated with coronary artery disease. Borderline myocarditis: sparse inflammatory infiltrate or myocytes without evident injury. |
| Diagnosis of clinically suspected myocarditis [35]: ≥1 of the clinical presentations of myocarditis and ≥1 diagnostic criteria (if the patient is asymptomatic, ≥2 diagnostic criteria are required) | Clinical presentations |
|
| Diagnostic criteria |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnecka, A.M.; Kleibert, M.; Płachta, I.; Rogala, P.; Wągrodzki, M.; Leszek, P.; Rutkowski, P. Myocarditis Induced by Immunotherapy in Metastatic Melanoma—Review of Literature and Current Guidelines. J. Clin. Med. 2022, 11, 5182. https://doi.org/10.3390/jcm11175182
Czarnecka AM, Kleibert M, Płachta I, Rogala P, Wągrodzki M, Leszek P, Rutkowski P. Myocarditis Induced by Immunotherapy in Metastatic Melanoma—Review of Literature and Current Guidelines. Journal of Clinical Medicine. 2022; 11(17):5182. https://doi.org/10.3390/jcm11175182
Chicago/Turabian StyleCzarnecka, Anna M., Marcin Kleibert, Iga Płachta, Paweł Rogala, Michał Wągrodzki, Przemysław Leszek, and Piotr Rutkowski. 2022. "Myocarditis Induced by Immunotherapy in Metastatic Melanoma—Review of Literature and Current Guidelines" Journal of Clinical Medicine 11, no. 17: 5182. https://doi.org/10.3390/jcm11175182
APA StyleCzarnecka, A. M., Kleibert, M., Płachta, I., Rogala, P., Wągrodzki, M., Leszek, P., & Rutkowski, P. (2022). Myocarditis Induced by Immunotherapy in Metastatic Melanoma—Review of Literature and Current Guidelines. Journal of Clinical Medicine, 11(17), 5182. https://doi.org/10.3390/jcm11175182









