Interactive Role of Surrogate Liver Fibrosis Assessment and Insulin Resistance on the Incidence of Major Cardiovascular Events
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Covariables
2.3. Main Variables
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Zhou, B.; Kontis, V.; Bentham, J.; Gunter, M.J.; Ezzati, M. Worldwide burden of cancer attributable to diabetes and high body-mass index: A comparative risk assessment. Lancet Diabetes Endocrinol. 2018, 6, e6–e15. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Otgonsuren, M.; Henry, L.; Venkatesan, C.; Mishra, A.; Erario, M.; Hunt, S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015, 62, 1723–1730. [Google Scholar] [CrossRef]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stål, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015, 149, 389–397. [Google Scholar] [CrossRef]
- Akhtar, D.H.; Iqbal, U.; Vazquez-Montesino, L.M.; Dennis, B.B.; Ahmed, A. Pathogenesis of Insulin Resistance and Atherogenic Dyslipidemia in Nonalcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2019, 7, 362–370. [Google Scholar] [CrossRef]
- Fujii, H.; Kawada, N.; Japan Study Group of Nafld Jsg-Nafld. The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 3863. [Google Scholar] [CrossRef]
- Caussy, C.; Aubin, A.; Loomba, R. The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. Curr. Diab. Rep. 2021, 21, 15. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhou, X.D.; Wu, S.J.; Hu, X.Q.; Tang, B.; Poucke, S.V.; Pan, X.Y.; Wu, W.J.; Gu, X.M.; Fu, S.W.; et al. Synergistic increase in cardiovascular risk in diabetes mellitus with nonalcoholic fatty liver disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Colantonio, L.D.; Cushman, M.; Goff, D.C., Jr.; Howard, G.; Howard, V.J.; Kissela, B.; Levitan, E.B.; Lloyd-Jones, D.M.; Safford, M.M. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA 2014, 311, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188, Erratum in Eur. Heart J. 2020, 41, 4255. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; Clinical Practice Guideline Panel; Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M.; et al. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Reaven, G.M. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: Triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism 2011, 60, 1673–1676. [Google Scholar] [CrossRef]
- Sánchez-Íñigo, L.; Navarro-González, D.; Fernández-Montero, A.; Pastrana-Delgado, J.; Martínez, J.A. The TyG index may predict the development of cardiovascular events. Eur. J. Clin. Investig. 2016, 46, 189–197. [Google Scholar] [CrossRef]
- Zhang, S.; Du, T.; Zhang, J.; Lu, H.; Lin, X.; Xie, J.; Yang, Y.; Yu, X. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017, 16, 15. [Google Scholar] [CrossRef]
- Unger, G.; Benozzi, S.F.; Perruzza, F.; Pennacchiotti, G.L. Triglycerides and glucose index: A useful indicator of insulin resistance. Endocrinol. Nutr. 2014, 61, 533–540. [Google Scholar] [CrossRef]
- Mózes, F.E.; Lee, J.A.; Selvaraj, E.A.; Jayaswal, A.N.A.; Trauner, M.; Boursier, J.; Fournier, C.; Staufer, K.; Stauber, R.E.; Bugianesi, E.; et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: An individual patient data meta-analysis. Gut 2022, 71, 1006–1019. [Google Scholar] [CrossRef]
- Schonmann, Y.; Yeshua, H.; Bentov, I.; Zelber-Sagi, S. Liver fbrosis marker is an independent predictor of cardiovascular morbidity and mortality in the general population. Dig. Liver Dis. 2021, 53, 79–85. [Google Scholar] [CrossRef]
- Martínez-Urbistondo, D.; de la Garza, R.G.; Villares-Fernández, P.; Font, C.; Schellong, S.; López-Núñez, J.J.; Gil-Díaz, A.; Del Carmen Díaz-Pedroche, M.; Hirmerova, J.; Monreal, M.; et al. Liver status and outcomes in patients without previous known liver disease receiving anticoagulant therapy for venous thromboembolism. Intern. Emerg. Med. 2021, 17, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Önnerhag, K.; Hartman, H.; Nilsson, P.M.; Lindgren, S. Non-invasive fibrosis scoring systems can predict future metabolic complications and overall mortality in non-alcoholic fatty liver disease (NAFLD). Scand. J. Gastroenterol. 2019, 54, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Häring, H.U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Navarro-González, D.; Sánchez-Íñigo, L.; Pastrana-Delgado, J.; Fernández-Montero, A.; Martinez, J.A. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The Vascular-Metabolic CUN cohort. Prev. Med. 2016, 86, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.; MacMahon, S.; Mancia, G.; Whitworth, J.; Beilin, L.; Hansson, L.; Neal, B.; Rodgers, A.; Mhurchu, N.; Clark, T. 1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. Clin. Exp. Hypertens. 1999, 21, 1009–1060. [Google Scholar]
- Implementation of the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). Epidemiol. Bull 1997, 18, 1–4.
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis patients with HIV/HCV co-infection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wong, V.W.-S.; Peleg, N.; et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Csermely, A.; Petracca, G.; Beatrice, G.; Corey, K.E.; Simon, T.G.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: An updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 903–913. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wong, V.W.S.; Castellanos, M.; Aller-de la Fuente, R.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Sanz, M.A.Q.; Conde-Martin, A.F.; et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: A multi-national cohort study. Gastroenterology 2018, 155, 443–457.e17. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Su, X.; Bradley, D.; Fabbrini, E.; Conte, C.; Eagon, J.C.; Varela, J.E.; Brunt, E.M.; Patterson, B.W.; Klein, S. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology 2012, 142, 1444–1446.e2. [Google Scholar] [CrossRef]
- Miura, K.; Yang, L.; van Rooijen, N.; Ohnishi, H.; Seki, E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1310–G1321. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; De Minicis, S.; Gwak, G.Y.; Kluwe, J.; Inokuchi, S.; Bursill, C.A.; Llovet, J.M.; Brenner, D.A.; Schwabe, R.F. CCR1 and CCR5 promote hepatic fibrosis in mice. J. Clin. Investig. 2009, 119, 1858–1870. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Z.; Caviglia, J.M.; Corey, K.E.; Herfel, T.M.; Cai, B.; Masia, R.; Chung, R.T.; Lefkowitch, J.H.; Schwabe, R.F.; et al. Hepatocyte TAZ/WWTR1 Promotes Inflammation and Fibrosis in Nonalcoholic Steatohepatitis. Cell Metab. 2016, 24, 848–862. [Google Scholar] [CrossRef]
- Zhu, C.; Kim, K.; Wang, X.; Bartolome, A.; Salomao, M.; Dongiovanni, P.; Meroni, M.; Graham, M.J.; Yates, K.P.; Diehl, A.M. Hepatocyte Notch activation induces liver fibrosis in nonalcoholic steatohepatitis. Sci. Transl. Med. 2018, 10, eaat0344. [Google Scholar] [CrossRef]
- Pajvani, U.B.; Shawber, C.J.; Samuel, V.T.; Birkenfeld, A.L.; Shulman, G.I.; Kitajewski, J.; Accili, D. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat. Med. 2011, 17, 961–967. [Google Scholar] [CrossRef]
- Ratziu, V.; Francque, S.; Sanyal, A. Breakthroughs in therapies for NASH and remaining challenges. Hepatology 2022, 76, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
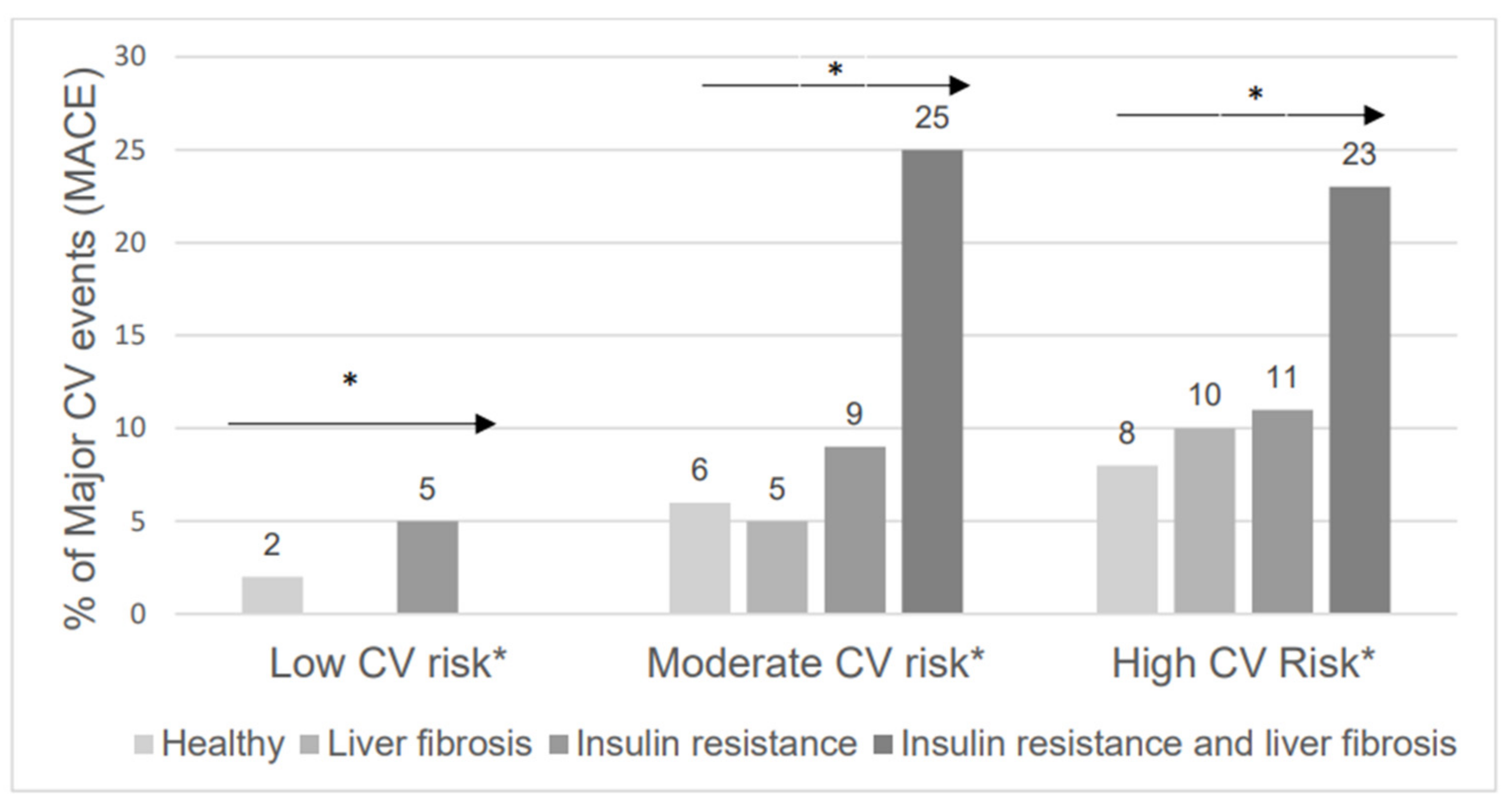
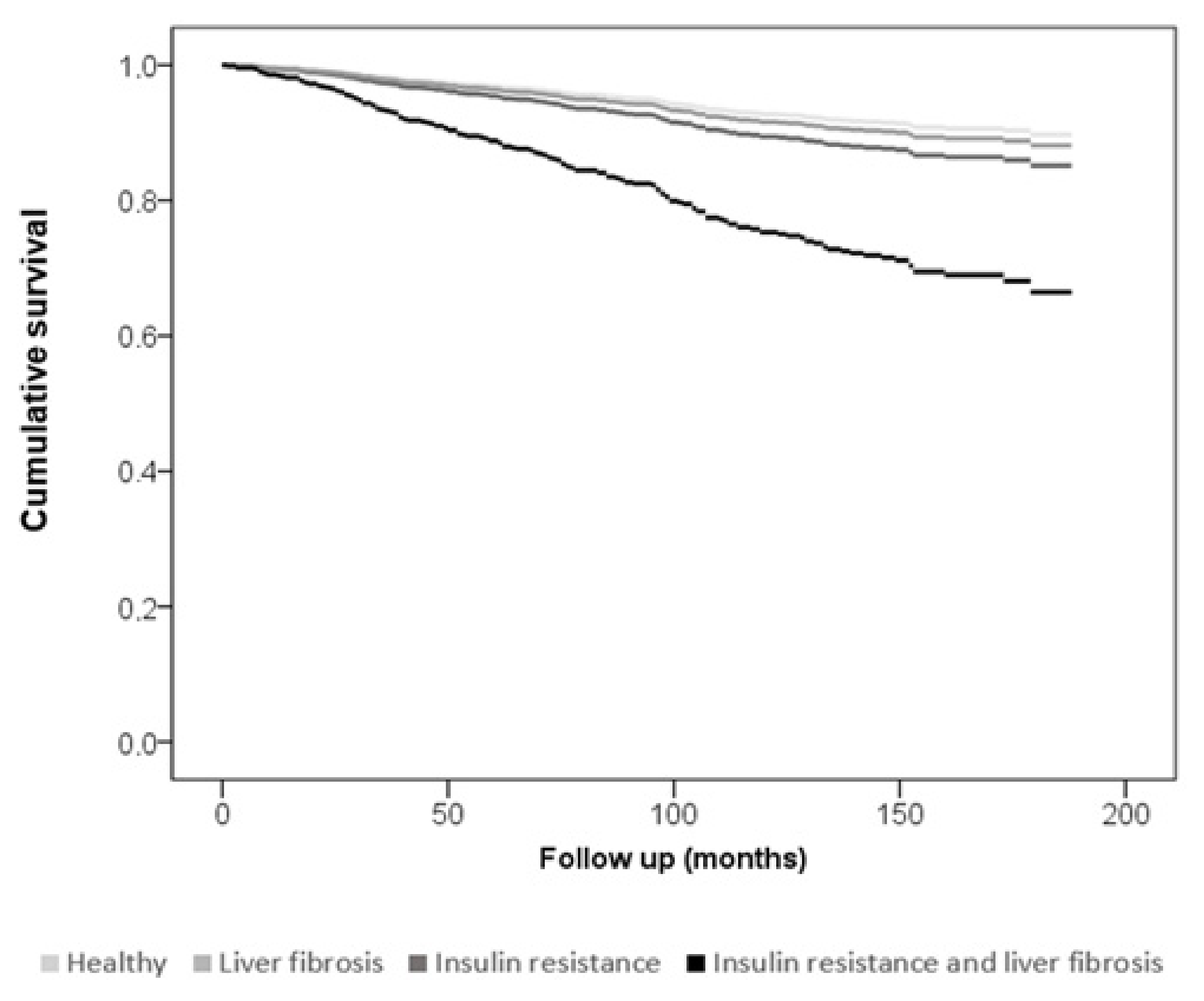
| Variable | Low CV Risk <1% Risk n = 240 | Moderate CV Risk 1–5% Risk n = 770 | High CV Risk >5% Risk n = 1045 | p |
|---|---|---|---|---|
| Age, years, mean ± SD | 54 ± 3 | 55 ± 5 | 63 ± 6 | <0.01 |
| Sex, female % | 212 (88%) | 327 (42%) | 295 (28%) | <0.01 |
| SCORE, % ± SD | 0.29 ± 0.28 | 2.5 ± 1.15 | 8.8 ± 0.15 | <0.01 |
| BMI, mean ± SD | 26.1 ± 4.6 | 27.2 ± 4.9 | 28.0 ± 4.2 | <0.01 |
| BMI > 25 kg/m2, % | 126 (52%) | 553 (71%) | 784 (74%) | <0.01 |
| Co-morbidities at baseline | ||||
| Hypertension, % | 24 (10%) | 192 (24%) | 494 (46%) | <0.01 |
| Controlled LDL (CV group adjusted), % | 45 (19%) | 45 (6%) | 3 (1%) | <0.01 |
| Never smoker, % | 153 (80%) | 342 (55%) | 296 (33%) | <0.01 |
| Daily alcohol consumption, % | 47 (5.8%) | 261 (32%) | 498 (62%) | <0.01 |
| Diabetes mellitus, % | 0 | 0 | 179 (17%) | <0.01 |
| Previous cardiovascular events, % | 0 (0%) | 0 (0%) | 186 (28%) | <0.01 |
| Cardiovascular risk markers | ||||
| Systolic blood pressure | 118 ± 14 | 129 ± 15 | 143 ± 54 | <0.01 |
| Diastolic blood pressure | 75 ± 8 | 81 ± 9 | 84 ± 23 | <0.01 |
| Total cholesterol, mg/dL ± SD | 218 ± 31 | 221 ± 30 | 242 ± 46 | <0.01 |
| c-LDL, mg/dL ± SD | 139 ± 26 | 145 ± 27 | 165 ± 41 | <0.01 |
| HDL, mg/dL ± SD | 62 ± 16 | 55 ± 15 | 53 ± 14 | <0.01 |
| Triglycerides, mg/dL ± SD | 83 ± 45 | 101 ± 56 | 116 ± 72 | <0.01 |
| Fasting Glucose, mg/dL ± SD | 92 ± 10 | 96 ± 12 | 108 ± 31 | <0.01 |
| Insulin resistance | ||||
| TyG index, mean ± SD | 8.1 ± 0.5 | 8.3 ± 0.5 | 8.6 ± 0.6 | <0.01 |
| TyG index > 8.8, % | 21 (9%) | 141 (18%) | 328 (31%) | <0.01 |
| Chronic inflammation | ||||
| NLR, mean ± SD | 1.94 ± 1.4 | 1.95 ± 0.9 | 2.12 ± 1.1 | <0.01 |
| NLR < 1.5 points, % | 95 (40%) | 253 (33%) | 286 (27%) | <0.01 |
| NLR 1.5–3 points, % | 121 (50%) | 441 (57%) | 616 (59%) | |
| NLR > 3 points, % | 24 (10%) | 78 (10%) | 143 (14%) | |
| MAFLD—HSI index | ||||
| Mean HSI, mean ± SD | 37 ± 6 | 38 ± 6 | 39 ± 6 | <0.01 |
| Low risk, % | 16 (7%) | 49 (6%) | 44 (5%) | <0.01 |
| Indeterminate risk, % | 94 (39%) | 229 (30%) | 301 (29%) | |
| High risk, % | 130 (54%) | 493 (64%) | 700 (67%) | |
| Liver fibrosis—FIB4 index | ||||
| Mean FIB-4 index, mean ± SD | 0.88 ± 0.39 | 1.05 ± 0.78 | 1.18 ± 0.83 | <0.01 |
| Low risk, % | 220 (91%) | 657 (85%) | 769 (73%) | <0.01 |
| Intermediate risk, % | 18 (8%) | 106 (4%) | 254 (25%) | |
| High risk, % | 2 (1%) | 8 (1%) | 22 (2%) | |
| Outcomes | ||||
| Surveillance (months) mean ± SD | 104 ± 60 | 105 ± 60 | 92 ± 60 | <0.01 |
| Acute ischemic cardiopathy, % | 2 (1%) | 30 (4%) | 65 (6%) | <0.01 |
| Cerebral ischemic attack, % | 3 (1%) | 18 (2%) | 43 (4%) | <0.01 |
| Major adverse cardiovascular events (MACE), % | 5 (2%) | 47 (6%) | 108 (10%) | <0.01 |
| Moderate CV Risk HR (95% CI) | p | High CV Risk HR (95% CI) | p | |
|---|---|---|---|---|
| Adiposity | ||||
| BMI kg/m2 | 1.04 (0.97–1.12) | 0.23 | 0.97 (0.92–1.01) | 0.22 |
| Overweight, BMI > 25 kg/m2 | 0.96 (0.52–1.80) | 0.91 | 0.84 (0.55–1.26) | 0.40 |
| Insulin resistance | ||||
| TyG index, mean | 1.43 (0.82–2.50) | 0.19 | 1.76 (1.28–2.41) | <0.01 |
| Insulin resistance (TyG > 8.8) | 2.00 (1.07–3.73) | 0.03 | 1.73 (1.18–2.55) | <0.01 |
| Cholesterol levels | ||||
| LDL levels, mg/dL | 1.00 (0.99–1.01) | 0.92 | 1.00 (0.99–1.01) | 0.89 |
| Low LDL | 1.48 (0.21–1.38) | 0.45 | - | - |
| Chronic inflammation | ||||
| NLR, mean ± SD | 1.05 (0.76–1.45) | 0.74 | 0.97 (0.80–1.18) | 0.77 |
| NLR < 1.5 points | ||||
| NLR 1.5–3 points | 0.67 (0.36–1.23) | 0.28 | 0.68 (0.45–1.04) | 0.08 |
| NLR > 3 points | 1.48 (0.62–3.53) | 0.29 | 0.81 (0.44–1.49) | 0.51 |
| Alcohol | ||||
| Alcohol consumption (yes/no) | 1.39 (0.72–2.71) | 0.38 | 1.05 (0.70–1.60) | 0.77 |
| MAFLD (HSI index) | ||||
| HSI, mean ± SD | 1.03 (0.98–1.08) | 0.18 | 0.99 (0.92–1.02) | 0.56 |
| High risk (HSI > 36), % | 1.26 (0.69–2.33) | 0.44 | 0.96 (0.65–1.43) | 0.86 |
| Liver fibrosis (FIB4 index) | ||||
| FIB-4 index, mean ± SD | 1.11 (0.47–2.64) | 0.86 | 1.49 (1.05–2.13) | 0.03 |
| Liver fibrosis risk (FIB-4 > 1.3), % | 1.69 (0.81–3.50) | 0.15 | 1.40 (0.93–2.09) | 0.09 |
| Variable | HR (CI 95%) | p |
|---|---|---|
| Model 1 linear Adjusted by SCORE subgroup | ||
| Non-invasive assessment of liver fibrosis (FIB-4) | 1.48 (1.07–2.04) | 0.01 |
| Insulin resistance assessment (TyG index) | 1.71 (1.30–2.24) | <0.01 |
| Model 2 interaction Adjusted by SCORE subgroup | ||
| Insulin resistance assessment (TyG index) | 1.61 (1.23–2.12) | 0.01 |
| FIB-4 index x TyG index (interaction) | 1.05 (1.01–1.08) | 0.01 |
| Model 3 Adjusted by SCORE categorical interaction | ||
| Insulin resistance assessment (TyG index) | 1.42 (1.06–1.92) | 0.02 |
| FIB-4 index > 1.3 points x TyG index > 8.8 points (interaction) | 2.57 (1.52–4.34) | <0.01 |
| Model 4 Adjusted by SCORE subgroup, age and sex | ||
| Insulin resistance assessment (TyG index) | 1.54 (1.14–2.09) | 0.01 |
| FIB-4 index > 1.3 points x TyG index > 8.8 points (interaction) | 2.12 (1.24–3.64) | <0.01 |
| Model 5 Adjusted by age, sex, SCORE, DM and previous CVE | ||
| Insulin resistance assessment (TyG index) | 1.51 (1.10–2.10) | 0.02 |
| FIB-4 index > 1.3 points x TyG index > 8.8 points (interaction) | 2.29 (1.33–3.94) | 0.01 |
| Model 6 Adjusted by age, sex, SCORE, DM and previous CVE | ||
| Healthy individuals FIB-4 < 1.3 points/TyG < 8.8 points | 1 | |
| Liver fibrosis FIB-4 > 1.3 points/TyG < 8.8 points | 1.00 (0.63–1.58) | 0.98 |
| Insulin resistance FIB-4 < 1.3 points/TyG > 8.8 points | 1.51 (1.01–2.28) | 0.04 |
| Insulin resistance and liver fibrosis FIB-4 > 1.3 points/TyG > 8.8 points | 3.34 (1.94–5.77) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Urbistondo, D.; D’Avola, D.; Navarro-González, D.; Sanchez-Iñigo, L.; Fernandez-Montero, A.; Perez-Diaz-del-Campo, N.; Bugianesi, E.; Martinez, J.A.; Pastrana, J.C. Interactive Role of Surrogate Liver Fibrosis Assessment and Insulin Resistance on the Incidence of Major Cardiovascular Events. J. Clin. Med. 2022, 11, 5190. https://doi.org/10.3390/jcm11175190
Martinez-Urbistondo D, D’Avola D, Navarro-González D, Sanchez-Iñigo L, Fernandez-Montero A, Perez-Diaz-del-Campo N, Bugianesi E, Martinez JA, Pastrana JC. Interactive Role of Surrogate Liver Fibrosis Assessment and Insulin Resistance on the Incidence of Major Cardiovascular Events. Journal of Clinical Medicine. 2022; 11(17):5190. https://doi.org/10.3390/jcm11175190
Chicago/Turabian StyleMartinez-Urbistondo, Diego, Delia D’Avola, David Navarro-González, Laura Sanchez-Iñigo, Alejandro Fernandez-Montero, Nuria Perez-Diaz-del-Campo, Elisabetta Bugianesi, Jose Alfredo Martinez, and Juan Carlos Pastrana. 2022. "Interactive Role of Surrogate Liver Fibrosis Assessment and Insulin Resistance on the Incidence of Major Cardiovascular Events" Journal of Clinical Medicine 11, no. 17: 5190. https://doi.org/10.3390/jcm11175190





