Invasiveness of Ventilation Therapy Is Associated to Prevalence of Secondary Bacterial and Fungal Infections in Critically Ill COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
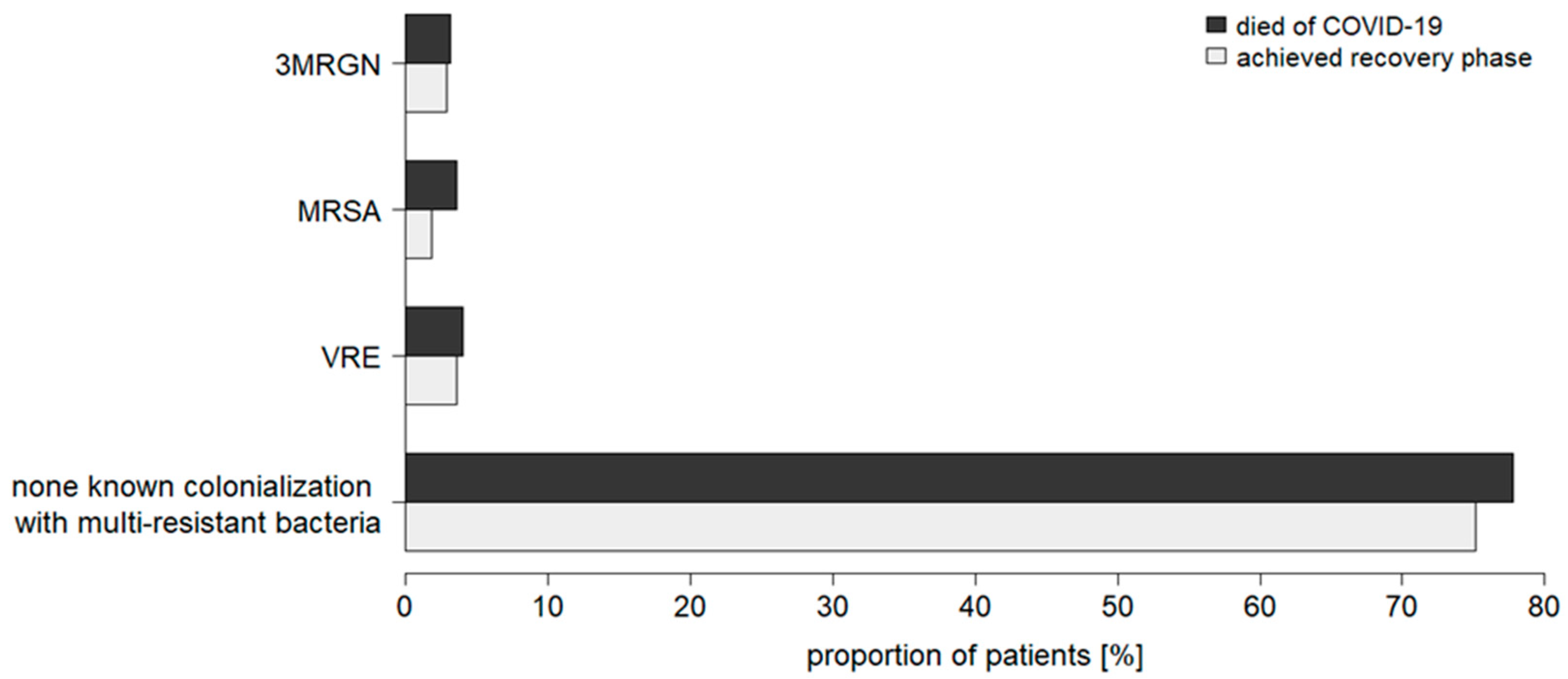
3.2. Community-Acquired Colonializations with Multidrug-Resistant Bacteria
3.3. Healthcare-Associated Bacterial and Fungal Infections
3.4. Antimicrobial Therapy: Frequency of Use and Clinical Benefit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorbello, M.; El-Boghdadly, K.; Di Giacinto, I.; Cataldo, R.; Esposito, C.; Falcetta, S.; Merli, G.; Cortese, G.; Corso, R.M.; Bressan, F.; et al. The Italian coronavirus disease 2019 outbreak: Recommendations from clinical practice. Anaesthesia 2020, 75, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Der Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Pfeifer, M.; Ewig, S.; Voshaar, T.; Randerath, W.J.; Bauer, T.; Geiseler, J.; Dellweg, D.; Westhoff, M.; Windisch, W.; Schoenhofer, B.; et al. Position Paper for the State-of-the-Art Application of Respiratory Support in Patients with COVID-19. Respiration 2020, 99, 521–541. [Google Scholar] [CrossRef]
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Marx, G.; Karagiannidis, C. German recommendations for critically ill patients with COVID-19. Med. Klin. Intensivmed. 2020, 115, 111–114. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Resp. Med. 2020, 8, 853–862. [Google Scholar] [CrossRef]
- Thomas-Rueddel, D.; Winning, J.; Dickmann, P.; Ouart, D.; Kortgen, A.; Janssens, U.; Bauer, M. Coronavirus disease 2019 (COVID-19): Update for anesthesiologists and intensivists March 2020. Anaesthesist 2021, 70, 1–10. [Google Scholar] [CrossRef]
- Alhazzani, W.; Moller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Int. Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef]
- Fachgruppe COVRIIN beim Robert-Koch-Institut. Antivirale Therapie in der Frühphase einer SARS-CoV-2-Infektion: Bei Patienten mit Risikofaktoren für einen Schweren Verlauf von COVID-19 (bei Asymptomatischen Patienten oder Patienten mit milder COVID-19); Bewertung durch die Fachgruppe COVRIIN beim Robert Koch-Institut. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/COVRIIN_Dok/Antivirale_Therapie_Fruehphase.pdf?__blob=publicationFile (accessed on 29 June 2022).
- Fichtner, F.; Moerer, O.; Weber-Carstens, S.; Nothacker, M.; Kaisers, U.; Laudi, S.; Grp, G. Clinical Guideline for Treating Acute Respiratory Insufficiency with Invasive Ventilation and Extracorporeal Membrane Oxygenation: Evidence-Based Recommendations for Choosing Modes and Setting Parameters of Mechanical Ventilation. Respiration 2019, 98, 357–372. [Google Scholar] [CrossRef]
- Vincent, J.L.; Bihari, D.J.; Suter, P.M.; Bruining, H.A.; White, J.; Nicolaschanoin, M.H.; Wolff, M.; Spencer, R.C.; Hemmer, M. The Prevalence of Nosocomial Infection in Intensive-Care Units in Europe—Results of the European Prevalence of Infection in Intensive-Care (EPIC) Study. JAMA-J. Am. Med. Assoc. 1995, 274, 639–644. [Google Scholar] [CrossRef]
- Tobin, M.J. Basing Respiratory Management of COVID-19 on Physiological Principles. Am. J. Respir. Crit. Care Med. 2020, 201, 1319–1320. [Google Scholar] [CrossRef] [Green Version]
- Chiurlo, M.; Mastrangelo, A.; Ripa, M.; Scarpellini, P. Invasive fungal infections in patients with COVID-19: A review on pathogenesis, epidemiology, clinical features, treatment, and outcomes. New Microbiol. 2021, 44, 71–83. [Google Scholar] [PubMed]
- Chong, W.H.; Saha, B.K.; Ramani, A.; Chopra, A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 2021, 49, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, E354–E365. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Baindara, P.; Mandal, S.M. Molecular pathogenesis of secondary bacterial infection associated to viral infections including SARS-CoV-2. J. Infect. Public Health 2020, 13, 1397–1404. [Google Scholar] [CrossRef]
- Pasero, D.; Cossu, A.P.; Terragni, P. Multi-Drug Resistance Bacterial Infections in Critically Ill Patients Admitted with COVID-19. Microorganisms 2021, 9, 1773. [Google Scholar] [CrossRef]
- Fehér, Á.; Szarvas, Z.; Lehoczki, A.; Fekete, M.; Fazekas-Pongor, V. Co-infections in COVID-19 patients and correlation with mortality rate. Minireview. Physiol. Int. 2022, 109, 1–8. [Google Scholar] [CrossRef]
- Roudbary, M.; Kumar, S.; Kumar, A.; Cernakova, L.; Nikoomanesh, F.; Rodrigues, C.F. Overview on the Prevalence of Fungal Infections, Immune Response, and Microbiome Role in COVID-19 Patients. J. Fungi 2021, 7, 720. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients With COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F.; et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 2021, 76, 1078–1084. [Google Scholar] [CrossRef]
- Klein, E.Y.; Monteforte, B.; Gupta, A.; Jiang, W.; May, L.; Hsieh, Y.-H.; Dugas, A. The frequency of influenza and bacterial coinfection: A systematic review and meta-analysis. Influenza Other Respir. Viruses 2016, 10, 394–403. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, C.R.; Chughtai, A.A.; Barnes, M.; Ridda, I.; Seale, H.; Toms, R.; Heywood, A. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect. Dis. 2018, 18, 637. [Google Scholar] [CrossRef]
- Rice, T.W.; Rubinson, L.; Uyeki, T.M.; Vaughn, F.L.; John, B.B.; Miller, R.R., II; Higgs, E.; Randolph, A.G.; Smoot, B.E.; Thompson, B.T.; et al. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit. Care Med. 2012, 40, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Esper, F.P.; Spahlinger, T.; Zhou, L. Rate and influence of respiratory virus co-infection on pandemic (H1N1) influenza disease. J. Infect. 2011, 63, 260–266. [Google Scholar] [CrossRef]
- Cox, M.J.; Loman, N.; Bogaert, D.; O’Grady, J. Co-infections: Potentially lethal and unexplored in COVID-19. Lancet Microbe 2020, 1, E11. [Google Scholar] [CrossRef]
- Getahun, H.; Smith, I.; Trivedi, K.; Paulin, S.; Balkhy, H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull World Health Organ. 2020, 98, 442. [Google Scholar] [CrossRef]
- Ginsburg, A.S.; Klugman, K.P. COVID-19 pneumonia and the appropriate use of antibiotics. Lancet Glob. Health 2020, 8, E1453–E1454. [Google Scholar] [CrossRef]
- Huttner, B.D.; Catho, G.; Pano-Pardo, J.R.; Pulcini, C.; Schouten, J. COVID-19: Don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020, 26, 808–810. [Google Scholar] [CrossRef]
- Meintrup, D.; Borgmann, S.; Seidl, K.; Stecher, M.; Jakob, C.E.M.; Pilgram, L.; Spinner, C.D.; Rieg, S.; Isberner, N.; Hower, M.; et al. Specific Risk Factors for Fatal Outcome in Critically Ill COVID-19 Patients: Results from a European Multicenter Study. J. Clin. Med. 2021, 10, 3855. [Google Scholar] [CrossRef]
- Jakob, C.E.M.; Kohlmayer, F.; Meurers, T.; Vehreschild, J.J.; Prasser, F. Design and evaluation of a data anonymization pipeline to promote Open Science on COVID-19. Sci. Data 2020, 7, 435. [Google Scholar] [CrossRef]
- Jakob, C.E.M.; Borgmann, S.; Duygu, F.; Behrends, U.; Hower, M.; Merle, U.; Friedrichs, A.; Tometten, L.; Hanses, F.; Jung, N.; et al. First results of the Lean European Open Survey on SARS-CoV-2-Infected Patients (LEOSS). Infection 2021, 49, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Johnson, S.; Maine, G.; Garcia, M.H.; Nimmagadda, S.; Qu, L.; Chen, N.-W. Vaccination reduces need for emergency care in breakthrough COVID-19 infections: A multicenter cohort study. Lancet Reg Health Am. 2021, 4, 100065. [Google Scholar] [CrossRef] [PubMed]
- Peghin, M.; Vena, A.; Graziano, E.; Giacobbe, D.R.; Tascini, C.; Bassetti, M. Improving management and antimicrobial stewardship for bacterial and fungal infections in hospitalized patients with COVID-19. Ther. Adv. Infect. Dis. 2022, 9, 28. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Alshawi, A.M.; Alomran, S.A.; Almuhanna, M.S.; Almuslim, A.A.; Bu Shafia, A.H.; Alotaibi, A.M.; Ahmed, G.Y.; et al. Coinfections with Bacteria, Fungi, and Respiratory Viruses in Patients with SARS-CoV-2: A Systematic Review and Meta-Analysis. Pathogens 2021, 10, 809. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Leung, V.; Raybardhan, S.; Lo, J.; Kan, T.; Leung, F.; Westwood, D.; Daneman, N.; MacFadden, D.R.; et al. Predictors and microbiology of respiratory and bloodstream bacterial infection in patients with COVID-19: Living rapid review update and meta-regression. Clin. Microbiol. Infect. 2022, 28, 491–501. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin. Infect. Dis. 2020, 73, e1634–e1644. [Google Scholar] [CrossRef]
- Silva, D.L.; Lima, C.M.; Magalhaes, V.C.R.; Baltazar, L.M.; Peres, N.T.A.; Caligiorne, R.B.; Moura, A.S.; Fereguetti, T.; Martins, J.C.; Rabelo, L.F.; et al. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. J. Hosp. Infect. 2021, 113, 145–154. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestana, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Europ. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Wolrd Health Organization. Living Guidance for Clinical Management of COVID-19: Living Guidance, 23 November 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf (accessed on 4 August 2022).




| Total Cohort | Subcohort: No Ventilation | Subcohort: Non-Invasive Ventilation | Subcohort: Invasive Ventilation | Subcohort: ECMO | |
|---|---|---|---|---|---|
| Patient count | 840 | 147 | 87 | 492 | 114 |
| Age range (years) | <1 to >85 | <1 to >85 | 36 to >85 | 9 to >85 | 26 to 85 |
| Gender distribution (male/female) | 602/238 | 92/55 | 60/27 | 357/135 | 93/21 |
| Number of comorbidities | 0 to 14 | 0 to 14 | 0 to 11 | 0 to 12 | 0 to 7 |
| Length of stay in ICU (weeks) | 0 to 10 | 0 to 10 | 0 to 6 | 0 to 10 | 0 to 10 |
| Length of ventilation (weeks) | up to 9 | - | up to 6 | up to 9 | up to 9 |
| Mortality rate (%) | 46.0 | 53.7 | 39.1 | 41.1 | 62.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Hesselle, M.L.; Borgmann, S.; Rieg, S.; Vehreshild, J.J.; Spinner, C.D.; Koll, C.E.M.; Hower, M.; Stecher, M.; Ebert, D.; Hanses, F.; et al. Invasiveness of Ventilation Therapy Is Associated to Prevalence of Secondary Bacterial and Fungal Infections in Critically Ill COVID-19 Patients. J. Clin. Med. 2022, 11, 5239. https://doi.org/10.3390/jcm11175239
de Hesselle ML, Borgmann S, Rieg S, Vehreshild JJ, Spinner CD, Koll CEM, Hower M, Stecher M, Ebert D, Hanses F, et al. Invasiveness of Ventilation Therapy Is Associated to Prevalence of Secondary Bacterial and Fungal Infections in Critically Ill COVID-19 Patients. Journal of Clinical Medicine. 2022; 11(17):5239. https://doi.org/10.3390/jcm11175239
Chicago/Turabian Stylede Hesselle, Marie Louise, Stefan Borgmann, Siegbert Rieg, Jörg Janne Vehreshild, Christoph D. Spinner, Carolin E. M. Koll, Martin Hower, Melanie Stecher, Daniel Ebert, Frank Hanses, and et al. 2022. "Invasiveness of Ventilation Therapy Is Associated to Prevalence of Secondary Bacterial and Fungal Infections in Critically Ill COVID-19 Patients" Journal of Clinical Medicine 11, no. 17: 5239. https://doi.org/10.3390/jcm11175239
APA Stylede Hesselle, M. L., Borgmann, S., Rieg, S., Vehreshild, J. J., Spinner, C. D., Koll, C. E. M., Hower, M., Stecher, M., Ebert, D., Hanses, F., Schumann, J., & on behalf of the SAREL Investigators. (2022). Invasiveness of Ventilation Therapy Is Associated to Prevalence of Secondary Bacterial and Fungal Infections in Critically Ill COVID-19 Patients. Journal of Clinical Medicine, 11(17), 5239. https://doi.org/10.3390/jcm11175239







