The Effects of Statin Treatment on Serum Ferritin Levels: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
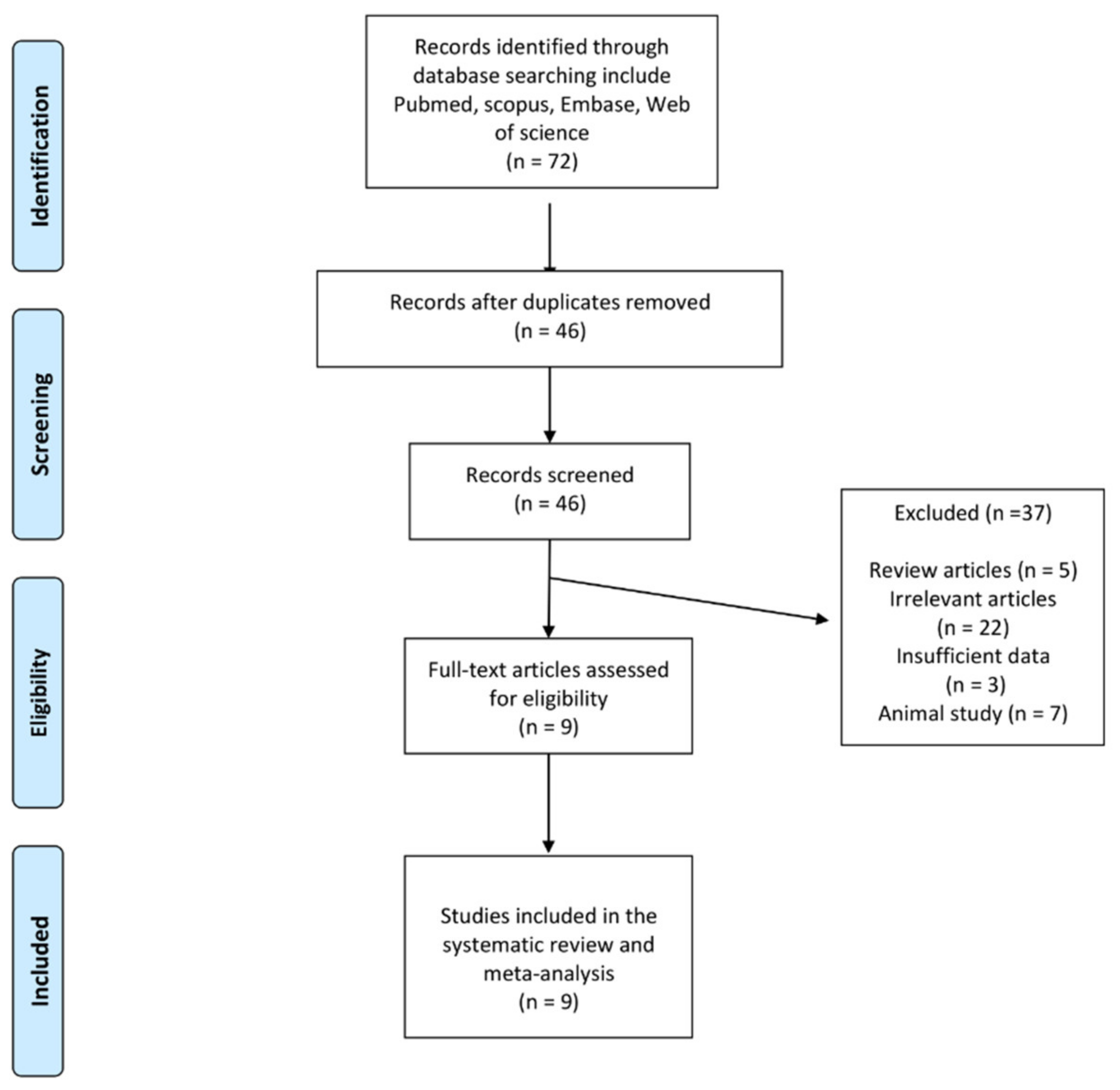
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Quantitative Data Synthesis
2.6. Meta-Regression
2.7. Publication Bias
3. Results
3.1. Risk-of-Bias Assessment of Clinical Trials
3.2. Effect of Statins on Circulating Concentrations of Serum Ferritin
3.3. Meta-Regression
3.4. Effect of Statins on Circulating Concentrations of Serum hs_CRP
3.5. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reiner, Ž. Statins in the primary prevention of cardiovascular disease. Nat. Rev. Cardiol. 2013, 10, 453–464. [Google Scholar] [CrossRef]
- Mk, J.; Ridker, P.M. Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 2005, 4, 977–987. [Google Scholar]
- Shakour, N.; Ruscica, M.; Hadizadeh, F.; Cirtori, C.; Banach, M.; Jamialahmadi, T.; Saheb-kar, A. Statins and C-reactive protein: In silico evidence on direct interaction. Arch. Med. Sci. 2020, 16, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Watts, G.F. New LDL-cholesterol lowering therapies: Pharmacology, clinical trials, and relevance to acute coronary syndromes. Clin. Ther. 2013, 35, 1082–1098. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Watts, G.F. New therapies targeting apoB metabolism for high-risk patients with inherited dyslipidaemias: What can the clinician expect? Cardiovasc. Drugs Ther. 2013, 27, 559–567. [Google Scholar] [CrossRef] [PubMed]
- DePalma, R.G.; Hayes, V.W.; Chow, B.K.; Shamayeva, G.; May, P.E.; Zacharski, L.R. Ferritin levels, inflammatory biomarkers, and mortality in peripheral arterial disease: A substudy of the Iron (Fe) and Atherosclerosis Study (FeAST) Trial. J. Vasc. Surg. 2010, 51, 1498–1503. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jadhav, A.B.; Hassan, A.; Meng, Q.H. Acute phase reactants as novel predictors of cardiovascular disease. ISRN Inflamm. 2012, 2012, 953461. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum ferritin: Past, present and future. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2010, 1800, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Invernizzi, P.; Arosio, P.; Cairo, G. New functions for an iron storage protein: The role of ferritin in immunity and autoimmunity. J. Autoimmun. 2008, 30, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Knovich, M.A.; Storey, J.A.; Coffman, L.G.; Torti, S.V.; Torti, F.M. Ferritin for the clinician. Blood Rev. 2009, 23, 95–104. [Google Scholar] [CrossRef]
- Ruddell, R.G.; Hoang-Le, D.; Barwood, J.M.; Rutherford, P.S.; Piva, T.J.; Watters, D.J.; Santambrogio, P.; Arosio, P.; Ramm, G.A. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology 2009, 49, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Li, L.; Liu, Y.; Liang, Y.; Qi, X. Silencing ferritin alleviates atherosclerosis in mice via regulating the expression levels of matrix metalloproteinases and interleukins. Acta Biochim. Pol. 2021, 68, 705–710. [Google Scholar] [CrossRef]
- Sciacqua, A.; Ventura, E.; Tripepi, G.; Cassano, V.; D’Arrigo, G.; Roumeliotis, S.; Maio, R.; Miceli, S.; Perticone, M.; Andreozzi, F.; et al. Ferritin modifies the relationship between inflammation and arterial stiffness in hypertensive patients with different glucose tolerance. Cardiovasc. Diabetol. 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Ha, J.Y.; Kim, M.K.; Kang, S.; Nam, J.S.; Ahn, C.W.; Kim, K.R.; Park, J.S. Serum ferritin levels are associated with arterial stiffness in healthy Korean adults. Vasc. Med. 2016, 21, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Poredoš, P.; Cífková, R.; Maier, J.A.M.; Nemcsik, J.; Šabovič, M.; Jug, B.; Ježovnik, M.K.; Schernthaner, G.H.; Antignani, P.L.; Catalano, M.; et al. Preclinical atherosclerosis and cardiovascular events: Do we have a consensus about the role of preclinical atherosclerosis in the prediction of cardiovascular events? Atherosclerosis 2022, 348, 25–35. [Google Scholar]
- Zacharski, L.R.; DePalma, R.G.; Shamayeva, G.; Chow, B.K. The statin–iron nexus: Anti-inflammatory intervention for arterial disease prevention. Am. J. Public Health 2013, 103, e105–e112. [Google Scholar] [CrossRef]
- Ali, F.; Hamdulay, S.S.; Kinderlerer, A.R.; Boyle, J.J.; Lidington, E.A.; Yamaguchi, T.; Soares, M.P.; Haskard, D.O.; Randi, A.M.; Mason, J.C. Statin-mediated cytoprotection of human vascular endothelial cells: A role for Kruppel-like factor 2-dependent induction of heme oxygenase-1. J. Thromb. Haemost. 2007, 5, 2537–2546. [Google Scholar] [CrossRef]
- Sullivan, J.L. Iron in arterial plaque: A modifiable risk factor for atherosclerosis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 718–723. [Google Scholar] [CrossRef]
- Jamialahmadi, T.; Baratzadeh, F.; Reiner, Ž.; Simental-Mendía, L.E.; Xu, S.; Susekov, A.V.; Santos, R.D.; Sahebkar, A. The Effects of Statin Dose, Lipophilicity, and Combination of Statins plus Ezetimibe on Circulating Oxidized Low-Density Lipoprotein Levels: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Mediat. Inflamm. 2021, 2021, 9661752. [Google Scholar] [CrossRef]
- Sutton, A.J.; Abrams, K.R.; Jones, D.R.; Jones, D.R.; Sheldon, T.A.; Song, F. Methods for Meta-Analysis in Medical Research; Wiley: Chichester, UK, 2000. [Google Scholar]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.; Ga, S.W.; Zello, G. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Oxford: Oxford, UK, 2000. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis, 2nd ed.; Biostat: Englewood, NJ, USA, 2005. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, C.; Ursoniu, S.; Wong, N.D.; Muntner, P.; Graham, I.M.; Mikhailidis, D.P.; Rizzo, M.; Rysz, J.; Sperling, L.S.; et al. Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors-Results from a systematic review and meta-analysis of randomized controlled trials. Int. J. Cardiol. 2015, 189, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Serban, C.; Ursoniu, S.; Rysz, J.; Muntner, P.; Toth, P.P.; Jones, S.R.; Rizzo, M.; Glasser, S.P.; Watts, G.F.; et al. Statin therapy and plasma coenzyme Q10 concentrations—A systematic review and meta-analysis of placebo-controlled trials. Pharmacol. Res. 2015, 99, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Sirken, G.; Kung, S.-C.; Raja, R. Decreased erythropoietin requirements in maintenance hemodialysis patients with statin therapy. Asaio J. 2003, 49, 422–425. [Google Scholar] [CrossRef]
- Dornbrook-Lavender, K.A.; Joy, M.S.; Denu-Ciocca, C.J.; Chin, H.; Hogan, S.L.; Pieper, J.A. Effects of atorvastatin on low-density lipoprotein cholesterol phenotype and C-reactive protein levels in patients undergoing long-term dialysis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2005, 25, 335–344. [Google Scholar] [CrossRef]
- Ukinc, K.; Ersoz, H.O.; Erem, C.; Hacihasanoglu, A.B.; Karti, S.S. Effects of one year simvastatin and atorvastatin treatments on acute phase reactants in uncontrolled type 2 diabetic patients. Endocrine 2009, 35, 380–388. [Google Scholar] [CrossRef]
- Tsouchnikas, I.; Dounousi, E.; Papakonstantinou, S.; Ioannou, K.; Kelesidis, A.; Kotzadamis, N.; Xanthopoulou, K.; Tsakiris, D. Beneficial effect of atorvastatin on erythropoietin responsiveness in maintenance haemodialysis patients. Nephrology 2009, 14, 560–564. [Google Scholar] [CrossRef]
- Li, X.Y.; Chang, J.P.; Su, Z.W.; Li, J.H.; Peng, B.S.; Zhu, S.L.; Cai, A.J.; Zhang, J.; Jiang, Y. How Does Short-Term Low-Dose Simvastatin Influence Serum Prohepcidin Levels in Patients With End-Stage Renal Disease? A Pilot Study. Ther. Apher. Dial. 2010, 14, 308–314. [Google Scholar] [CrossRef]
- Kimura, Y.; Hyogo, H.; Yamagishi, S.I.; Takeuchi, M.; Ishitobi, T.; Nabeshima, Y.; Arihiro, K.; Chayama, K. Atorvastatin decreases serum levels of advanced glycation endproducts (AGEs) in nonalcoholic steatohepatitis (NASH) patients with dyslipidemia: Clinical usefulness of AGEs as a biomarker for the attenuation of NASH. J. Gastroenterol. 2010, 45, 750–757. [Google Scholar] [CrossRef]
- Koc, M.; Dogan, C.; Arinsoy, T.; Tonbul, Z.; Ayli, D.; Cirit, M.; Sever, M.S.; Yilmaz, M.E.; Unsal, A.; Suleymanlar, G.; et al. Statin use is associated with lower inflammation and erythropoietin responsiveness index in hemodialysis patients. Hemodial. Int. 2011, 15, 366–373. [Google Scholar] [CrossRef]
- Hyogo, H.; Yamagishi S-i Maeda, S.; Kimura, Y.; Ishitobi, T.; Chayama, K. Atorvastatin improves disease activity of nonalcoholic steatohepatitis partly through its tumour necrosis factor-α-lowering property. Dig. Liver Dis. 2012, 44, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Nand, N.; Mittal, A. Evaluation of Effect of Statins on Erythropoietin Resistance in Patients of Chronic Kidney Disease on Maintenance Haemodialysis. J. Assoc. Physicians India 2018, 66, 29–32. [Google Scholar]
- Reyes, C.; Pons, N.A.; Reñones, C.R.; Gallisà, J.B.; Val, V.A.; Tebé, C.; Mateo, G.F. Association between serum ferritin and acute coronary heart disease: A population-based cohort study. Atherosclerosis 2020, 293, 69–74. [Google Scholar] [CrossRef]
- Egbuche, O.; Millard, H.R.; Renelus, B.; Maihemuti, A.; Musani, S.K.; Fox, E.R.; Liu, J.; Taylor, H.A.; Bidulescu, A. Serum ferritin levels in blacks without known cardiovascular disease (from the Jackson heart study). Am. J. Cardiol. 2017, 120, 1533–1540. [Google Scholar] [CrossRef]
- Zacharski, L.R.; Chow, B.K.; Howes, P.S.; Shamayeva, G.; Baron, J.A.; Dalman, R.L.; Malenka, D.J.; Ozaki, C.K.; Lavori, P.W. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: A randomized controlled trial. JAMA 2007, 297, 603–610. [Google Scholar] [CrossRef] [Green Version]




| Study, Year | Study Design | Follow-Up | Treatment | Control | Clinical Outcome | Patients | No. of Patients |
|---|---|---|---|---|---|---|---|
| Sirken et al., 2003 [29] | retrospective study | mean of 4.7 months | statin therapy | non-statin therapy | significant decrease in serum ferritin level | dialysis patients | 19 |
| Dornbrook-Lavender et al., 2005 [30] | randomized, parallel-group substudy | 8 months | A at 10 mg/day | no statins | no significant changes in serum ferritin level | hemodialysis patients | 13 |
| Ukinc et al., 2009 [31] | open-label, randomized study | 12 months | A at 10 to 20 mg/day | - | significant decrease in serum ferritin level | patients with type 2 diabetes | 50 |
| S at 10 to 20 mg/day | - | significant decrease in serum ferritin level | |||||
| Tsouchnikas et al., 2009 [32] | nonrandomized clinical trial | 9 months | A at 20 to 40 mg/day | - | no significant changes in serum ferritin level | hemodialysis patients with low-density lipoprotein cholesterol (LDL) >32.58 mmol/L | 25 |
| Li et al., 2010 [33] | nonrandomized clinical trial | 2 months | S at 20 mg/day | control | no significant changes in serum ferritin level | hemodialysis patients | 26 |
| Kimura et al., 2010 [34] | nonrandomized clinical trial | 12 months | A at 10 mg/day | - | significant decrease in serum ferritin level | NASH patients with dyslipidemia | 43 |
| Mehmet KOC et al., 2011 [35] | retrospective study | 3 months | statin | statin nonusers | no significant changes in serum ferritin level | hemodialysis patients | 1363 |
| Hyogo et al., 2012 [36] | nonrandomized clinical trial | 12 months | A at 10 mg/day | - | no significant changes in serum ferritin level | NASH patients with dyslipidemia (males) | 28 |
| significant change in serum ferritin level | NASH patients with dyslipidemia (females) | 14 | |||||
| Nand et al., 2018 [37] | prospective randomized controlled study | 4 months | A at 20 mg/day | control | significant change in serum ferritin level | hemodialysis patients | 30 |
| Study | Selection | Comparability | Exposure | |||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur? | Adequacy of Follow-Up of Cohorts | |
| Sirken et al., 2003 [29] | * | * | * | - | * | * | - | - |
| Mehmet KOC et al., 2011 [35] | * | * | * | - | * | * | - | - |
| Confounding | Participant Selection | Interventions Classification | Intended Interventions Deviations | Missing Data | Outcome Measurement | Reported Results Classification | ||
| Hyogo et al., 2012 [36] | serious | low | low | low | low | low | low | |
| Tsouchnikas et al., 2007 [32] | serious | low | low | low | serious | low | low | |
| Xiang-Yang Li et al., 2010 [33] | low | serious | low | low | serious | low | low | |
| Kimura et al., 2010 [34] | critical | low | low | low | low | low | low | |
| Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other Bias | |||
| Random Sequence Generation | Allocation Concealment | |||||||
| Dornbrook-Lavender et al., 2005 [30] | high | high | high | high | low | low | low | |
| Ukinc et al., 2009 [31] | high | high | high | high | low | low | unclear | |
| Nand et al., 2017 [37] | high | high | high | high | low | low | low | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamialahmadi, T.; Abbasifard, M.; Reiner, Ž.; Rizzo, M.; Eid, A.H.; Sahebkar, A. The Effects of Statin Treatment on Serum Ferritin Levels: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5251. https://doi.org/10.3390/jcm11175251
Jamialahmadi T, Abbasifard M, Reiner Ž, Rizzo M, Eid AH, Sahebkar A. The Effects of Statin Treatment on Serum Ferritin Levels: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(17):5251. https://doi.org/10.3390/jcm11175251
Chicago/Turabian StyleJamialahmadi, Tannaz, Mitra Abbasifard, Željko Reiner, Manfredi Rizzo, Ali H. Eid, and Amirhossein Sahebkar. 2022. "The Effects of Statin Treatment on Serum Ferritin Levels: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 17: 5251. https://doi.org/10.3390/jcm11175251
APA StyleJamialahmadi, T., Abbasifard, M., Reiner, Ž., Rizzo, M., Eid, A. H., & Sahebkar, A. (2022). The Effects of Statin Treatment on Serum Ferritin Levels: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(17), 5251. https://doi.org/10.3390/jcm11175251









