Analysis of Predictive Factors for Successful Vascular Anastomoses in a Sheep Uterine Transplantation Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. The Team
2.3. Animals and Experimental Protocol
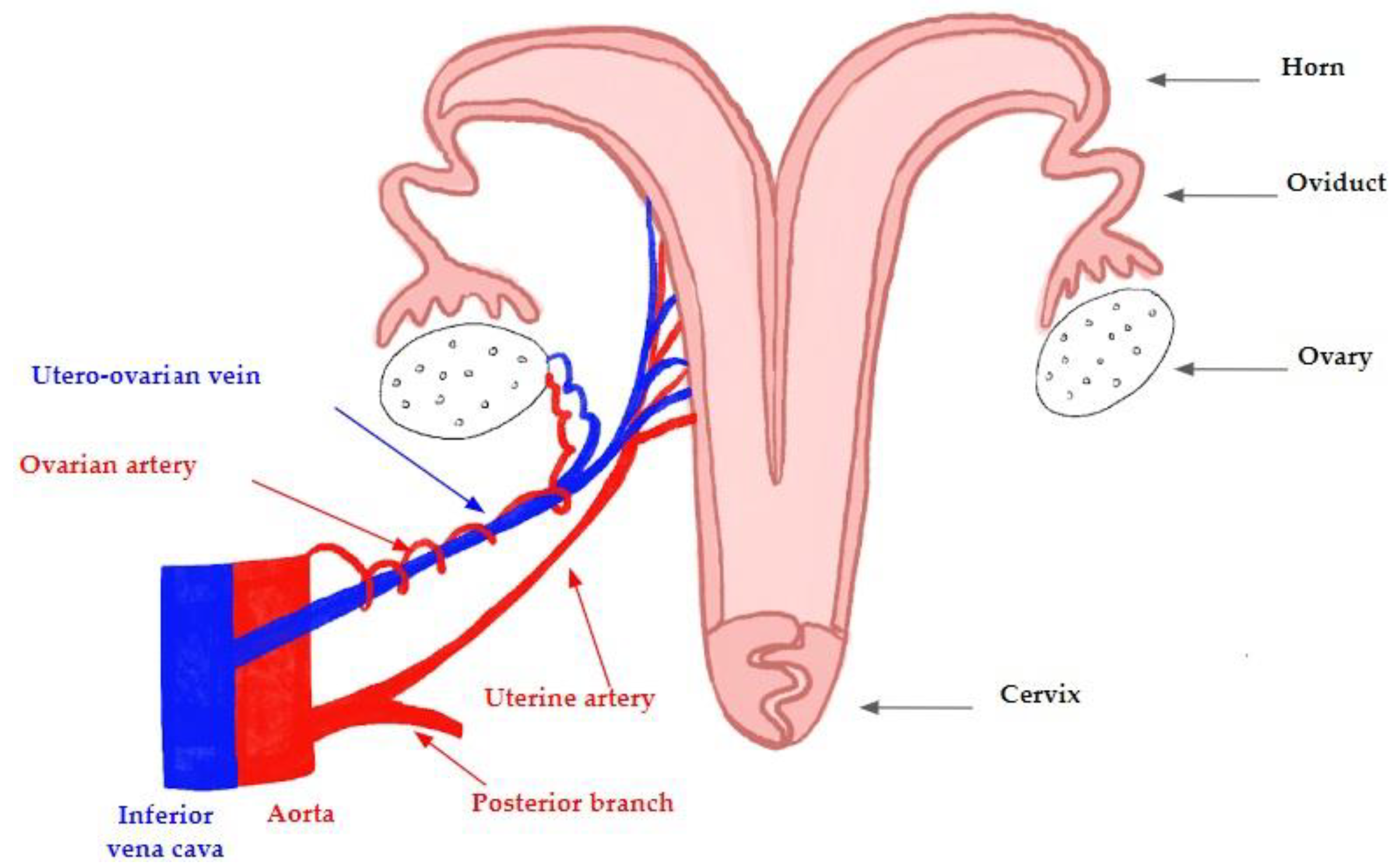
2.4. Anatomy of the Ovine Model
2.5. Anesthesia
2.6. Surgical Technique
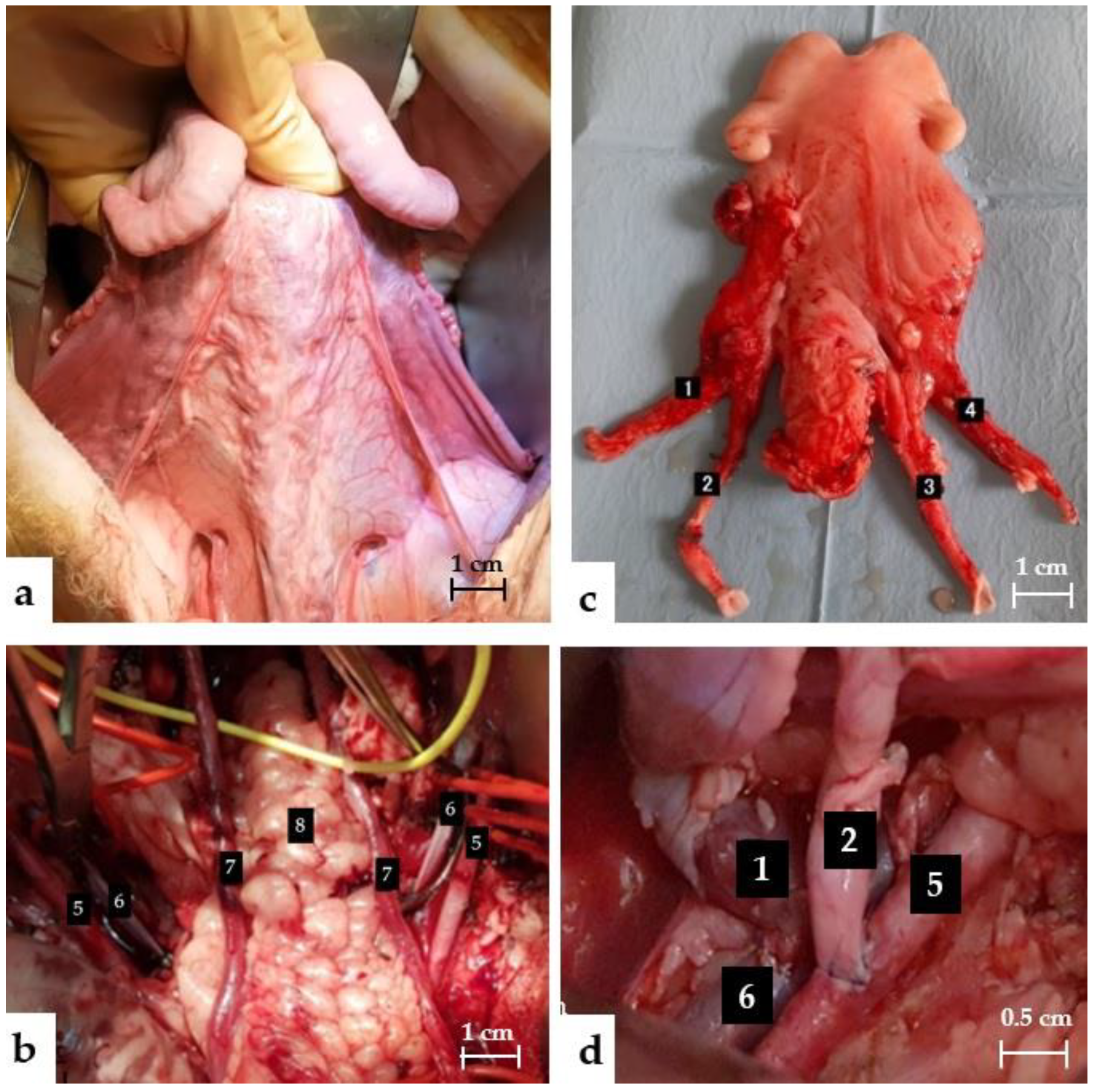
2.6.1. Uterine Dissection
2.6.2. Back Table
2.6.3. Transplantation
2.7. Postoperative Monitoring
2.8. Criteria for Evaluating Success
2.9. Collected Data
2.10. Statistical Analysis
3. Results
3.1. Immediate Success Criteria
3.1.1. Arterial Anastomoses
3.1.2. Venous Anastomoses
3.2. Short-Term Success Criteria
3.2.1. Arterial Anastomoses
3.2.2. Venous Anastomoses
3.2.3. Biologic Parameters
3.3. Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johannesson, L.; Järvholm, S. Uterus Transplantation: Current Progress and Future Prospects. Int. J. Women’s Health 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- American Society for Reproductive Medicine Position Statement on Uterus Transplantation: A Committee Opinion—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30196945/ (accessed on 31 May 2022).
- Morcel, K.; Camborieux, L.; Guerrier, D.; Programme de Recherches sur les Aplasies Müllériennes. Mayer-Rokitansky-Küster-Hauser (MRKH) Syndrome. Orphanet J. Rare Dis. 2007, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.P.; Ranaei-Zamani, N.; Vali, S.; Williams, N.; Saso, S.; Thum, M.; Al-Memar, M.; Dixon, N.; Rose, G.; Testa, G.; et al. Options for Acquiring Motherhood in Absolute Uterine Factor Infertility; Adoption, Surrogacy and Uterine Transplantation. Obstet. Gynaecol. 2021, 23, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M.; Johannesson, L.; Bokström, H.; Kvarnström, N.; Mölne, J.; Dahm-Kahler, P.; Enskog, A.; Milenkovic, M.; Ekberg, J.; Diaz-Garcia, C.; et al. Livebirth after uterus transplantation. Lancet 2015, 385, 607–616. [Google Scholar] [CrossRef]
- Brännström, M.; Johannesson, L.; Dahm-Kähler, P.; Enskog, A.; Mölne, J.; Kvarnström, N.; Diaz-Garcia, C.; Hanafy, A.; Lundmark, C.; Marcickiewicz, J.; et al. First Clinical Uterus Transplantation Trial: A Six-Month Report. Fertil. Steril. 2014, 101, 1228–1236. [Google Scholar] [CrossRef]
- Brännström, M.; Belfort, M.A.; Ayoubi, J.M. Uterus Transplantation Worldwide: Clinical Activities and Outcomes. Curr. Opin. Organ Transplant. 2021, 26, 616–626. [Google Scholar] [CrossRef]
- Richards, E.G.; Farrell, R.M.; Ricci, S.; Perni, U.; Quintini, C.; Tzakis, A.; Falcone, T. Uterus Transplantation: State of the Art in 2021. J. Assist. Reprod. Genet. 2021, 38, 2251–2259. [Google Scholar] [CrossRef]
- Dahm-Kähler, P.; Diaz-Garcia, C.; Brännström, M. Human Uterus Transplantation in Focus. Br. Med. Bull. 2016, 117, 69–78. [Google Scholar] [CrossRef]
- Johannesson, L.; Diaz-Garcia, C.; Leonhardt, H.; Dahm-Kähler, P.; Marcickiewicz, J.; Olausson, M.; Brännström, M. Vascular Pedicle Lengths After Hysterectomy: Toward Future Human Uterus Transplantation. Obstet. Gynecol. 2012, 119, 1219–1225. [Google Scholar] [CrossRef]
- Puntambekar, S.; Puntambekar, S.; Telang, M.; Kulkarni, P.; Date, S.; Panse, M.; Sathe, R.; Agarkhedkar, N.; Warty, N.; Kade, S.; et al. Novel Anastomotic Technique for Uterine Transplant Using Utero-Ovarian Veins for Venous Drainage and Internal Iliac Arteries for Perfusion in Two Laparoscopically Harvested Uteri. J. Minim. Invasive Gynecol. 2019, 26, 628–635. [Google Scholar] [CrossRef]
- Wei, L.; Xue, T.; Tao, K.-S.; Zhang, G.; Zhao, G.-Y.; Yu, S.-Q.; Cheng, L.; Yang, Z.-X.; Zheng, M.-J.; Li, F.; et al. Modified Human Uterus Transplantation Using Ovarian Veins for Venous Drainage: The First Report of Surgically Successful Robotic-Assisted Uterus Procurement and Follow-up for 12 Months. Fertil. Steril. 2017, 108, 346–356.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.W.; Liu, X.Y.; Cui, Z.Y.; Zheng, Y.H.; Jiang, H.W.; Yang, H.Y.; Lin, Y.Z.; Zhu, Y.; Wang, Y.; Li, X.X.; et al. Surgical technique and outcomes of uteri retrieval from brain-dead multi-organ donors: A preclinical research of human uterine transplantation. Zhonghua Yi Xue Za Zhi 2018, 98, 3178–3182. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M.; Dahm Kähler, P.; Greite, R.; Mölne, J.; Díaz-García, C.; Tullius, S.G. Uterus Transplantation: A Rapidly Expanding Field. Transplantation 2018, 102, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Ayoubi, J.M.; Carbonnel, M.; Pirtea, P.; Kvarnström, N.; Brännström, M.; Dahm-Kähler, P. Laparotomy or Minimal Invasive Surgery in Uterus Transplantation: A Comparison. Fertil. Steril. 2019, 112, 11–18. [Google Scholar] [CrossRef]
- Testa, G.; Koon, E.C.; Johannesson, L.; McKenna, G.J.; Anthony, T.; Klintmalm, G.B.; Gunby, R.T.; Warren, A.M.; Putman, J.M.; de Prisco, G.; et al. Living Donor Uterus Transplantation: A Single Center’s Observations and Lessons Learned From Early Setbacks to Technical Success. Am. J. Transplant. 2017, 17, 2901–2910. [Google Scholar] [CrossRef]
- Favre-Inhofer, A.; Carbonnel, M.; Revaux, A.; Sandra, O.; Mougenot, V.; Bosc, R.; Gélin, V.; Rafii, A.; Hersant, B.; Vialard, F.; et al. Critical Steps for Initiating an Animal Uterine Transplantation Model in Sheep: Experience from a Case Series. Int. J. Surg. 2018, 60, 245–251. [Google Scholar] [CrossRef]
- Saso, S.; Petts, G.; Thum, M.-Y.; Corless, D.; Boyd, M.; Noakes, D.; Del Priore, G.; Ghaem-Maghami, S.; Smith, J.R. Achieving Uterine Auto-Transplantation in a Sheep Model Using Iliac Vessel Anastomosis: A Short-Term Viability Study. Acta Obstet. Gynecol. Scand. 2015, 94, 245–252. [Google Scholar] [CrossRef]
- Ayoubi, J.M.; Carbonnel, M.; Kvarnström, N.; Revaux, A.; Poulain, M.; Vanlieferinghen, S.; Coatantiec, Y.; Le Marchand, M.; Tourne, M.; Pirtea, P.; et al. Case Report: Post-Partum SARS-CoV-2 Infection After the First French Uterus Transplantation. Front. Surg. 2022, 9, 854225. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Carbonnel, M.; Cornet, N.; Revaux, A.; Favre-Inhofer, A.; Galio, L.; Raliou, M.; Couturier-Tarrade, A.; Giraud-Delville, C.; Charpigny, G.; Gelin, V.; et al. Analysis of Blood Parameters and Molecular Endometrial Markers during Early Reperfusion in Two Ovine Models of Uterus Transplantation. PLoS ONE 2021, 16, e0251474. [Google Scholar] [CrossRef]
- Solomonov, E.; Marcus Braun, N.; Siman-Tov, Y.; Ben-Shachar, I. Team Preparation for Human Uterus Transplantation: Autologous Transplantation in Sheep Model. Clin. Transplant. 2017, 31, e13137. [Google Scholar] [CrossRef]
- Dahm-Kähler, P.; Wranning, C.; Lundmark, C.; Enskog, A.; Mölne, J.; Marcickiewicz, J.; El-Akouri, R.R.; McCracken, J.; Brännström, M. Transplantation of the Uterus in Sheep: Methodology and Early Reperfusion Events. J. Obstet. Gynaecol. Res. 2008, 34, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Moore, F.D. Ethical Problems Special to Surgery: Surgical Teaching, Surgical Innovation, and the Surgeon in Managed Care. Arch. Surg. Chic. 2000, 135, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Catsanos, R.; Rogers, W.; Lotz, M. The Ethics of Uterus Transplantation. Bioethics 2013, 27, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M.; Diaz-Garcia, C.; Hanafy, A.; Olausson, M.; Tzakis, A. Uterus Transplantation: Animal Research and Human Possibilities. Fertil. Steril. 2012, 97, 1269–1276. [Google Scholar] [CrossRef]
- Favre-Inhofer, A.; Carbonnel, M.; Domert, J.; Cornet, N.; Chastant, S.; Coscas, R.; Vialard, F.; Gelin, V.; Galio, L.; Richard, C.; et al. Involving Animal Models in Uterine Transplantation. Front. Surg. 2022, 9, 830826. [Google Scholar] [CrossRef]
- Wranning, C.A.; El-Akouri, R.R.; Lundmark, C.; Dahm-Kähler, P.; Mölne, J.; Enskog, A.; Brännström, M. Auto-Transplantation of the Uterus in the Domestic Pig (Sus Scrofa): Surgical Technique and Early Reperfusion Events. J. Obstet. Gynaecol. Res. 2006, 32, 358–367. [Google Scholar] [CrossRef]
- Urofrance. Intérêt du Dosage des Lactates Pendant la Transplantation Rénale; Urofrance: Paris, Franch, 2001. [Google Scholar]
- Wranning, C.A.; Marcickiewicz, J.; Enskog, A.; Dahm-Kähler, P.; Hanafy, A.; Brännström, M. Fertility after Autologous Ovine Uterine-Tubal-Ovarian Transplantation by Vascular Anastomoses to the External Iliac Vessels. Hum. Reprod. 2010, 25, 1973–1979. [Google Scholar] [CrossRef]
- Asayama, Y.; Yoshimitsu, K.; Aibe, H.; Nishie, A.; Kakihira, D.; Irie, H.; Tajima, T.; Matake, K.; Nakayama, T.; Ohishi, Y.; et al. MDCT of the Gonadal Veins in Females with Large Pelvic Masses: Value in Differentiating Ovarian versus Uterine Origin. Am. J. Roentgenol. 2006, 186, 440–448. [Google Scholar] [CrossRef]
- Kisu, I.; Banno, K.; Mihara, M.; Hara, H.; Umene, K.; Adachi, M.; Nogami, Y.; Aoki, D. A Surgical Technique Using the Ovarian Vein in Non-Human Primate Models of Potential Living-Donor Surgery of Uterus Transplantation. Acta Obstet. Gynecol. Scand. 2015, 94, 942–948. [Google Scholar] [CrossRef]
- Kin, Y.; Katsumori, T.; Kasahara, T.; Nozaki, T.; Ito, H.; Nishimura, T. Hemodynamics of Ovarian Veins: MR Angiography in Women with Uterine Leiomyomata. Eur. J. Radiol. 2007, 63, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Shockley, M.; Arnolds, K.; Beran, B.; Rivas, K.; Escobar, P.; Tzakis, A.; Falcone, T.; Sprague, M.L.; Zimberg, S. Uterine Viability in the Baboon after Ligation of Uterine Vasculature: A Pilot Study to Assess Alternative Perfusion and Venous Return for Uterine Transplantation. Fertil. Steril. 2017, 107, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; McKenna, G.J.; Gunby, R.T., Jr.; Anthony, T.; Koon, E.C.; Warren, A.M.; Putman, J.M.; Zhang, L.; de Prisco, G.; Mitchell, J.M.; et al. First Live Birth after Uterus Transplantation in the United States. Am. J. Transplant. 2018, 18, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.H. Bilateral Oophorectomy versus Ovarian Conservation: Effects on Long-Term Women’s Health. J. Minim. Invasive Gynecol. 2010, 17, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, T.; Bertin, F.; Fourcade, L.; Maubon, A.; Saint Marcoux, F.; Piver, P.; Marquet, P.; Pommepuy, I.; Plainard, X.; Couquet, C.; et al. Uterine Allotransplantation in Ewes Using an Aortocava Patch. Hum. Reprod. 2011, 26, 3028–3036. [Google Scholar] [CrossRef]
- Carbonnel, M.; Dahm-Kähler, P.; Revaux, A.; Brännström, M.; Ayoubi, J.-M. Adapting Surgical Skills from Robotic-Assisted Radical Hysterectomy in Cervical Cancer to Uterine Transplantation: A Look to an Optimistic Future! J. Robot. Surg. 2020, 14, 841–847. [Google Scholar] [CrossRef]
- Brännström, M.; Dahm-Kähler, P.; Kvarnström, N. Robotic-Assisted Surgery in Live-Donor Uterus Transplantation. Fertil. Steril. 2018, 109, 256–257. [Google Scholar] [CrossRef]
- Brännström, M.; Dahm-Kähler, P.; Kvarnström, N.; Akouri, R.; Rova, K.; Olausson, M.; Groth, K.; Ekberg, J.; Enskog, A.; Sheikhi, M.; et al. Live Birth after Robotic-Assisted Live Donor Uterus Transplantation. Acta Obstet. Gynecol. Scand. 2020, 99, 1222–1229. [Google Scholar] [CrossRef]
- Díaz-García, C.; Akhi, S.N.; Martínez-Varea, A.; Brännström, M. The Effect of Warm Ischemia at Uterus Transplantation in a Rat Model. Acta Obstet. Gynecol. Scand. 2013, 92, 152–159. [Google Scholar] [CrossRef]




| Variable | Success n = 59 (78.7%) | Failure n = 16 (21.3%) | p-Value | ||
|---|---|---|---|---|---|
| n or Mean ± SD | Percentage or Range | n or Mean ± SD | Percentage or Range | ||
| Ewe characteristic | |||||
| Age (month) | 39.6 ± 6.5 | 29.1–70.0 | 45.4 ± 11.7 | 33.4–70.0 | 0.01 |
| Weight | 70.9 ± 11.6 | 52.0–97.0 | 71.8 ± 12.9 | 55.0–94.0 | 0.78 |
| Rank of ewes | |||||
| 1–10 | 9 | 15.3 | 7 | 43.8 | 0.002 |
| >10 | 50 | 84.7 | 9 | 56.3 | |
| Anatomical data | |||||
| Caliber (mm) | 5.3 ± 2.1 | 1.0–10.0 | 4.7 ± 1.9 | 2.0–10.0 | 0.32 |
| Length (mm) | 83.4 ± 17.4 | 50–120 | 80.6 ± 24.5 | 45–150 | 0.61 |
| Transplantation | |||||
| Operating time (min) | 479.7 ± 86.5 | 353–678 | 472.9 ± 100.7 | 350–675 | 0.79 |
| Time of uterine dissection | 77.7 ± 16.6 | 50–118 | 74.0 ± 15.5 | 50–92 | 0.43 |
| Cold ischemia | 52.6 ± 31.3 | 20–150 | 59.7 ± 31.7 | 29–122 | 0.42 |
| Warm ischemia | 106.6 ± 30.2 | 75–197 | 135.3 ± 73.8 | 85–314 | 0.02 |
| <120 min | 51 | 86.4 | 8 | 50.0 | |
| >120 min | 8 | 13.6 | 8 | 50.0 | 0.002 |
| Anastomoses | |||||
| Anastomoses time per vessel | 24.0 ± 13.0 | 8–76 | 30.2 ± 14.7 | 12–66 | 0.1 |
| Operator | |||||
| Non-vascular surgeon | 17 | 28.8 | 9 | 56.3 | 0.04 |
| Vascular surgeon | 42 | 71.2 | 7 | 43.8 | |
| Suture technique | |||||
| Separate points | 8 | 13.6 | 5 | 31.3 | 0.1 |
| Continuous suture | 51 | 86.4 | 11 | 68.8 | |
| Posterior branch | |||||
| Yes | 48 | 81.4 | 9 | 56.3 | 0.04 |
| No | 11 | 18.6 | 7 | 43.8 | |
| Papaverine | |||||
| Yes | 6 | 10.2 | 3 | 21.4 | 0.25 |
| No | 53 | 89.8 | 11 | 78.6 | |
| Missing | 2 | ||||
| Anastomoses complications * | |||||
| Yes | 3 | 5.4 | 3 | 27.3 | 0.02 |
| No | 53 | 94.6 | 8 | 72.7 | |
| Missing | 3 | 5 | |||
| Remedial points | |||||
| Yes | 15 | 26.8 | 4 | 33.3 | 0.65 |
| No | 41 | 73.2 | 8 | 66.7 | |
| Missing | 3 | 4 | |||
| Variable | Success n = 63 (82.9%) | Failure n = 13 (17.1%) | p-Value | ||
|---|---|---|---|---|---|
| n or Mean ± SD | Percentage or Range | n or Mean ± SD | Percentage or Range | ||
| Ewe characteristic | |||||
| Age (month) | 39.7 ± 6.6 | 29.1–70.0 | 49.4 ± 11.7 | 35.0–70.0 | <0.0001 |
| <40 | 43 | 68.3 | 3 | 23.1 | |
| >40 | 20 | 31.7 | 10 | 76.9 | 0.002 |
| Weight | 70.4 ± 11.2 | 52.0–97.0 | 73.6 ± 14.2 | 57.0–94.0 | 0.37 |
| Rank of ewes | |||||
| 1–10 | 8 | 12.7 | 9 | 69.2 | <0.0001 |
| >10 | 55 | 87.3 | 4 | 30.8 | |
| Caliber (mm) | 5.93 ± 2.52 | 2.0–13.0 | 6.58 ± 2.84 | 3.0–13.0 | 0.42 |
| Length (mm) | 77.95 ± 22.4 | 35–130 | 68.33 ± 26.4 | 35–135 | 0.19 |
| Transplantation | |||||
| Operating time (min) | 467.9 ± 80.8 | 353–678 | 525.8 ± 119.9 | 350–678 | 0.03 |
| Time of uterine dissection | 76.5 ± 16.3 | 50–118 | 75.3 ± 18.7 | 52–118 | 0.82 |
| Dissection time of the external iliac vessels | 12.3 ± 7.3 | 2–36 | 14.9 ± 6.4 | 5–24 | 0.23 |
| Cold ischemia | 50.0 ± 28.8 | 20–150 | 67.0 ± 35.6 | 30–150 | 0.07 |
| Warm ischemia | 104.2 ± 27.7 | 75–197 | 163.7 ± 76.5 | 88–314 | <0.0001 |
| <120 min | 55 | 87.3 | 4 | 30.8 | |
| >120 min | 8 | 12.7 | 9 | 69.2 | <0.0001 |
| Anastomoses | |||||
| Anastomoses time | 26.0 ± 11.1 | 13–82 | 52.2 ± 34.3 | 20–129 | <0.0001 |
| <30 min | 49 | 77.8 | 3 | 23.1 | 0.0001 |
| >30 min | 14 | 22.2 | 10 | 76.9 | |
| Operator | |||||
| Non-vascular surgeon | 15 | 23.8 | 11 | 84.6 | |
| Vascular surgeon | 48 | 76.2 | 2 | 15.4 | <0.0001 |
| Suture technique | |||||
| Separate points | 2 | 3.2 | 3 | 23.1 | 0.008 |
| Continuous suture | 61 | 96.8 | 10 | 76.9 | |
| Anastomoses complications * | |||||
| Yes | 3 | 5.0 | 3 | 42.9 | 0.001 |
| No | 57 | 95.0 | 4 | 57.1 | |
| Missing | 3 | 6 | |||
| Remedial points | |||||
| Yes | 11 | 18.3 | 2 | 28.6 | 0.52 |
| No | 49 | 81.7 | 5 | 71.4 | |
| Missing | 3 | 6 | |||
| Variable | OR | IC95 | p-Value |
|---|---|---|---|
| Age of ewes (month) | |||
| <40 | 1 | ||
| >40 | 0.88 | 0.11–7.14 | 0.91 |
| Rank of ewes | 1 | ||
| 1–10 | 1 | ||
| >10 | 4.17 | 0.14–100 | 0.41 |
| Operator | |||
| Non-vascular surgeons | 1 | ||
| Vascular surgeon | 29.3 | 1.17–731.91 | 0.04 |
| Warm ischemia | |||
| <120 | 1 | ||
| >120 | 0.05 | 0.003–0.88 | 0.04 |
| Time of dissection | |||
| <30 min | 1 | ||
| >30 min | 0.19 | 0.02–1.52 | 0.12 |
| Suture technique | |||
| Continuous suture | 1 | ||
| Separate stitch | 0.78 | 0.08–8.33 | 0.84 |
| Anastomotic complications | |||
| No | 1 | ||
| Yes | 0.06 | 0.003–0.99 | 0.049 |
| Rank of Ewes | Status | Lactates T1 | Lactates T2 | Delta |
|---|---|---|---|---|
| 17 | F | 1.3 | - | - |
| 21 | F | 1.33 | 5.53 | 4.2 |
| 24 | S | 1.1 | 2.29 | 1.19 |
| 26 | S | 0.56 | 2.43 | 1.87 |
| 27 | S | 1.17 | 2.2 | 1.03 |
| 28 | F | 0.95 | 3.67 | 2.72 |
| 29 | S | 0.77 | 1.48 | 0.71 |
| 32 | S | 1.17 | 4 | 2.83 |
| 33 | F | 0.42 | 1.75 | 1.33 |
| 35 | S | 138 | 1.84 | 0.46 |
| 36 | S | 1.53 | 3.15 | 1.62 |
| 39 | F | 1.36 | 743 | 6.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Gal, C.; Carbonnel, M.; Balaya, V.; Richard, C.; Gelin, V.; Galio, L.; Sandra, O.; Hersant, B.; Bosc, R.; Charton, J.; et al. Analysis of Predictive Factors for Successful Vascular Anastomoses in a Sheep Uterine Transplantation Model. J. Clin. Med. 2022, 11, 5262. https://doi.org/10.3390/jcm11185262
Le Gal C, Carbonnel M, Balaya V, Richard C, Gelin V, Galio L, Sandra O, Hersant B, Bosc R, Charton J, et al. Analysis of Predictive Factors for Successful Vascular Anastomoses in a Sheep Uterine Transplantation Model. Journal of Clinical Medicine. 2022; 11(18):5262. https://doi.org/10.3390/jcm11185262
Chicago/Turabian StyleLe Gal, Claire, Marie Carbonnel, Vincent Balaya, Christophe Richard, Valerie Gelin, Laurent Galio, Olivier Sandra, Barbara Hersant, Romain Bosc, Johanna Charton, and et al. 2022. "Analysis of Predictive Factors for Successful Vascular Anastomoses in a Sheep Uterine Transplantation Model" Journal of Clinical Medicine 11, no. 18: 5262. https://doi.org/10.3390/jcm11185262






