Associations between Sleep Characteristics and Cardiovascular Risk Factors in Adolescents Living with Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
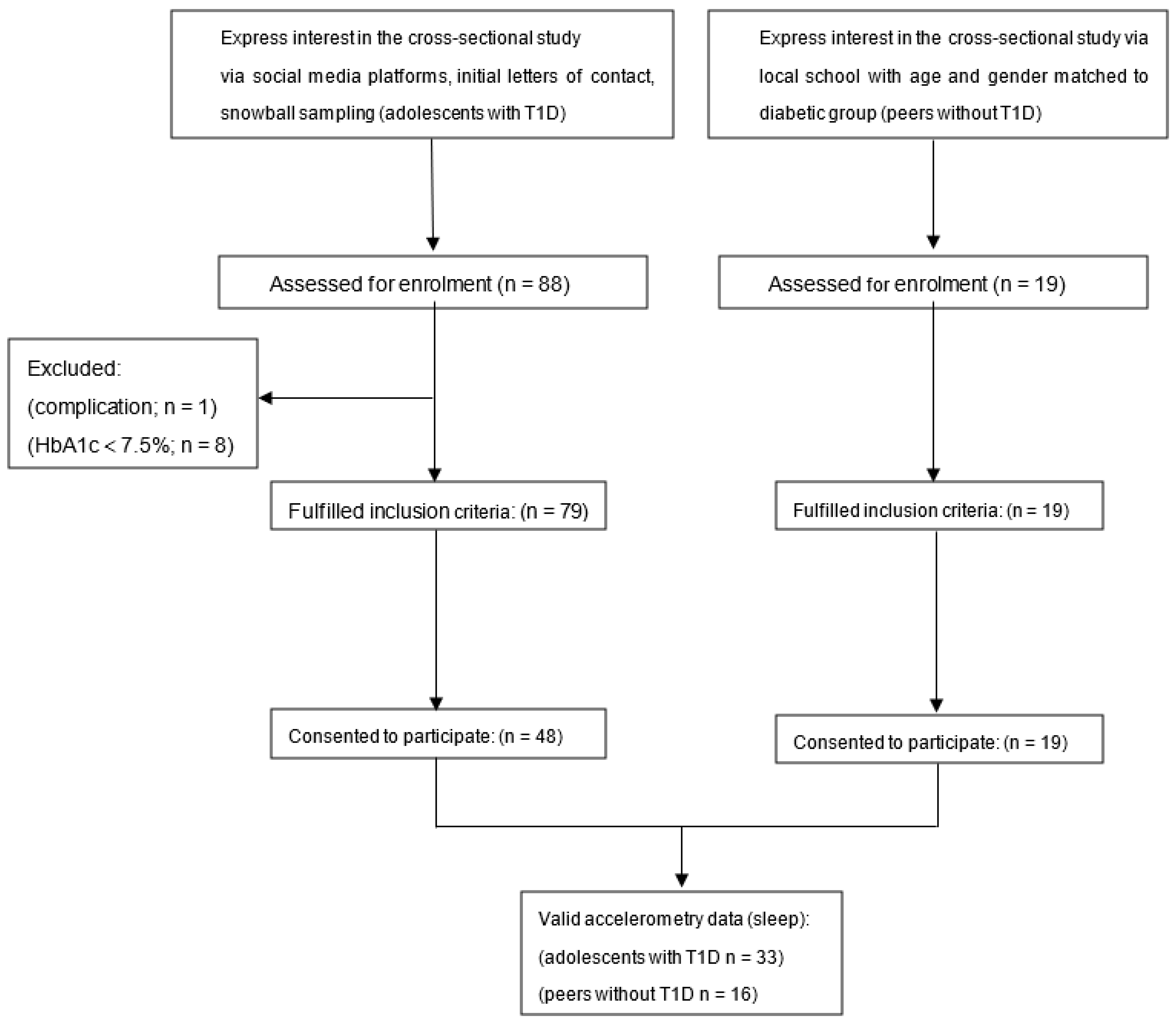
2.1. Participants
2.2. Demographic and Anthropometric Data Collection
2.3. Cardiovascular Outcomes
2.4. Accelerometry Assessment of Composition of the Day
2.5. Statistical Analyses
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bortolotti, S.; Cattazzo, F.; Tagetti, A.; Marcon, D.; Rosin, E.; Minuz, P.; Maffeis, C.; Fava, C. Cardiovascular risk factors in type 1 diabetes mellitus: Any difference between adolescents and young adults for subclinical atherosclerosis? J. Hypertens. 2022, 40, e144. [Google Scholar] [CrossRef]
- Steigleder-Schweiger, C.; Rami-Merhar, B.; Waldhör, T.; Fröhlich-Reiterer, E.; Schwarz, I.; Fritsch, M.; Borkenstein, M.; Schober, E. Prevalence of cardiovascular risk factors in children and adolescents with type 1 diabetes in Austria. Eur. J. Pediatr. 2012, 171, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Skrivarhaug, T.; Bangstad, H.-J.; Stene, L.C.; Sandvik, L.; Hanssen, K.F.; Joner, G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006, 49, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, E.M.; Rifas-Shiman, S.L.; Redline, S.; Gillman, M.W.; Pena, M.-M.; Taveras, E.M. Longitudinal associations of sleep curtailment with metabolic risk in mid-childhood. Obesity 2014, 22, 2586–2592. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Friedman, L.A.; Gray-McGuire, C. Metabolic Syndrome in Childhood Predicts Adult Cardiovascular Disease 25 Years Later: The Princeton Lipid Research Clinics Follow-up Study. Pediatrics 2007, 120, 340–345. [Google Scholar] [CrossRef]
- Matthews, K.A.; Pantesco, E. Sleep characteristics and cardiovascular risk in children and adolescents: An enumerative review. Sleep Med. 2016, 18, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Mullington, J.M.; Haack, M.; Toth, M.; Serrador, J.M.; Meier-Ewert, H.K. Cardiovascular, Inflammatory, and Metabolic Consequences of Sleep Deprivation. Prog. Cardiovasc. Dis. 2009, 51, 294–302. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, W.; Li, X.; Qi, X.; Pan, K.; Xu, W. Association of Sleep Duration, Napping, and Sleep Patterns With Risk of Cardiovascular Diseases: A Nationwide Twin Study. J. Am. Heart Assoc. 2022. [Google Scholar] [CrossRef]
- Perfect, M.M. The Relations of Sleep and Quality of Life to School Performance in Youth With Type 1 Diabetes. J. Appl. Sch. Psychol. 2014, 30, 7–28. [Google Scholar] [CrossRef]
- Perfect, M.M.; Patel, P.G.; Scott, R.E.; Wheeler, M.D.; Patel, C.; Griffin, K.J.; Sorensen, S.T.; Goodwin, J.L.; Quan, S.F. Sleep, Glucose, and Daytime Functioning in Youth with Type 1 Diabetes. Sleep 2012, 35, 81–88. [Google Scholar] [CrossRef]
- Reutrakul, S.; Thakkinstian, A.; Anothaisintawee, T.; Chontong, S.; Borel, A.-L.; Perfect, M.M.; Janovsky, C.C.P.S.; Kessler, R.; Schultes, B.; Harsch, I.A.; et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: Systematic review and meta-analysis. Sleep Med. 2016, 23, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.D.M.; Patton, S.R.; Koren, D. Childhood diabetes and sleep. Pediatr. Pulmonol. 2021, 57, 1835–1850. [Google Scholar] [CrossRef]
- Pillar, G.; Schuscheim, G.; Weiss, R.; Malhotra, A.; McCowen, K.C.; Shlitner, A.; Peled, N.; Shehadeh, N. Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. J. Pediatr. 2003, 142, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Matyka, K.A.; Crawford, C.; Wiggs, L.; Dunger, D.; Stores, G. Alterations in sleep physiology in young children with insulin-dependent diabetes mellitus: Relationship to nocturnal hypoglycemia. J. Pediatr. 2000, 137, 233–238. [Google Scholar] [CrossRef]
- Lauderdale, D.S.; Knutson, K.L.; Yan, L.L.; Liu, K.; Rathouz, P.J. Sleep duration: How well do self-reports reflect objective measures? The CARDIA Sleep Study. Epidemiology 2008, 19, 838. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Donga, E.; van Dijk, M.; van Dijk, J.G.; Biermasz, N.R.; Lammers, G.-J.; van Kralingen, K.; Hoogma, R.P.; Corssmit, E.P.; Romijn, J.A. Partial Sleep Restriction Decreases Insulin Sensitivity in Type 1 Diabetes. Diabetes Care 2010, 33, 1573–1577. [Google Scholar] [CrossRef]
- Smith, M.T.; McCrae, C.S.; Cheung, J.; Martin, J.L.; Harrod, C.G.; Heald, J.L.; Carden, K.A. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2018, 14, 1231–1237. [Google Scholar] [CrossRef]
- Withrow, D.; Roth, T.; Koshorek, G.; Roehrs, T. Relation between ambulatory actigraphy and laboratory polysomnography in insomnia practice and research. J. Sleep Res. 2019, 28, e12854. [Google Scholar] [CrossRef]
- Kosmadopoulos, A.; Sargent, C.; Darwent, D.; Zhou, X.; Roach, G. Alternatives to polysomnography (PSG): A validation of wrist actigraphy and a partial-PSG system. Behav. Res. Methods 2014, 46, 1032–1041. [Google Scholar] [CrossRef]
- Mantua, J.; Gravel, N.; Spencer, R.M.C. Reliability of Sleep Measures from Four Personal Health Monitoring Devices Compared to Research-Based Actigraphy and Polysomnography. Sensors 2016, 16, 646. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Bredin, S.S.D.; Jamnik, V.K.; Koehle, M.S.; Guan, Y.; Shellington, E.M.; Li, Y.; Li, J.; Warburton, D.E.R. Association between physical activity level and cardiovascular risk factors in adolescents living with type 1 diabetes mellitus: A cross-sectional study. Cardiovasc. Diabetol. 2021, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Morris, N.M.; Udry, J.R. Validation of a self-administered instrument to assess stage of adolescent development. J. Youth Adolesc. 1980, 9, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.P.; Sung, R.Y.; Kong, A.P.; Goggins, W.B.; So, H.K.; Nelson, E.A.S. Reliability of pubertal self-assessment in Hong Kong Chinese children. J. Paediatr. Child Health 2008, 44, 353–358. [Google Scholar] [CrossRef]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Ferreira, C.E.D.S.; França, C.N.; Correr, C.J.; Zucker, M.L.; Andriolo, A.; Scartezini, M. Clinical correlation between a point-of-care testing system and laboratory automation for lipid profile. Clin. Chim. Acta 2015, 446, 263–266. [Google Scholar] [CrossRef]
- Jiang, F.; Hou, X.; Lu, J.; Zhou, J.; Lu, F.; Kan, K.; Tang, J.; Bao, Y.; Jia, W. Assessment of the Performance of A1CNow+ and Development of an Error Grid Analysis Graph for Comparative Hemoglobin A1c Measurements. Diabetes Technol. Ther. 2014, 16, 363–369. [Google Scholar] [CrossRef]
- Carter, A.W. An Analysis of the Assessment of Glycated Hemoglobin Using A1cNow+™ Point-of-Care Device Compared to Central Laboratory Testing—An Important Addition to Pharmacist-Managed Diabetes Programs? J. Diabetes Sci. Technol. 2008, 2, 828–830. [Google Scholar] [CrossRef]
- Panz, V.R.; Raal, F.J.; Paiker, J.; Immelman, R.; Miles, H. Performance of the CardioChekTM PA and Cholestech LDX (R) point-of-care analysers compared to clinical diagnostic laboratory methods for the measurement of lipids: Cardiovascular topic. Cardiovasc. J. S. Afr. 2005, 16, 112–116. [Google Scholar]
- Choi, S.J.; Kang, M.-R.; Sung, M.J.; Joo, E.Y. Discordant sleep parameters among actigraphy, polysomnography, and perceived sleep in patients with sleep-disordered breathing in comparison with patients with chronic insomnia disorder. Sleep Breath. 2017, 21, 837–843. [Google Scholar] [CrossRef]
- Sadeh, A.; Sharkey, K.M.; Carskadon, M.A. Activity-Based Sleep-Wake Identification: An Empirical Test of Methodological Issues. Sleep 1994, 17, 201–207. [Google Scholar] [CrossRef]
- Kostkova, M.; Durdik, P.; Ciljakova, M.; Vojtková, J.; Sujanska, A.; Pozorciakova, K.; Snahnicanova, Z.; Jancinova, M.; Banovcin, P. Short-term metabolic control and sleep in children and adolescents with type 1 diabetes mellitus. J. Diabetes Complicat. 2018, 32, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, G.C.; Galland, B.C.; Boucher, S.E.; Wiltshire, E.J.; Haszard, J.J.; Campbell, A.J.; Black, S.M.; Smith, C.; Elder, D.; Wheeler, B.J. Impact of type 1 diabetes mellitus, glucose levels, and glycemic control on sleep in children and adolescents: A case–control study. Sleep 2020, 43, zsz226. [Google Scholar] [CrossRef] [PubMed]
- Manin, G.; Pons, A.; Baltzinger, P.; Moreau, F.; Iamandi, C.; Wilhelm, J.M.; Lenoble, P.; Kessler, L. Obstructive sleep apnoea in people with Type 1 diabetes: Prevalence and association with micro- and macrovascular complications. Diabet. Med. 2015, 32, 90–96. [Google Scholar] [CrossRef]
- Lee, X.K.; Chee, N.I.; Ong, J.L.; Teo, T.B.; Van Rijn, E.; Lo, J.C.; Chee, M.W. Validation of a Consumer Sleep Wearable Device With Actigraphy and Polysomnography in Adolescents Across Sleep Opportunity Manipulations. J. Clin. Sleep Med. 2019, 15, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Paruthi, S.; Brooks, L.J.; D’Ambrosio, C.; Hall, W.; Kotagal, S.; Lloyd, R.M.; Malow, B.A.; Maski, K.; Nichols, C.; Quan, S.F.; et al. Recommended Amount of Sleep for Pediatric Populations: A Consensus Statement of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2016, 12, 785–786. [Google Scholar] [CrossRef]
- Consensus Conference Panel; Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.; et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep 2015, 38, 1161–1183. [Google Scholar] [CrossRef]
- Luo, J.; Cao, M.; Sun, F.; Shi, B.; Wang, X.; Jing, J. Association between outdoor activity and insufficient sleep in Chinese school-aged children. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e921617-1–e921617-10. [Google Scholar] [CrossRef]
- Spilsbury, J.C.; Storfer-Isser, A.; Drotar, D.; Rosen, C.L.; Kirchner, L.H.; Benham, H.; Redline, S. Sleep Behavior in an Urban US Sample of School-aged Children. Arch. Pediatr. Adolesc. Med. 2004, 158, 988–994. [Google Scholar] [CrossRef]
- Sluggett, L.; Wagner, S.L.; Hardy, C.; Harris, R.L. Associations between Sleep Duration and Indicators of Cardiometabolic Disease in Canadian Children and Adolescents: Analyses of the 2007–2009 Canadian Health Measures Survey. Child. Obes. 2016, 12, 325–333. [Google Scholar] [CrossRef]
- Loessl, B.; Valerius, G.; Kopasz, M.; Hornyak, M.; Riemann, D.; Voderholzer, U. Are adolescents chronically sleep-deprived? An investigation of sleep habits of adolescents in the Southwest of Germany. Child Care Health Dev. 2008, 34, 549–556. [Google Scholar] [CrossRef]
- Olds, T.; Maher, C.; Blunden, S.; Matricciani, L. Normative data on the sleep habits of Australian children and adolescents. Sleep 2010, 33, 1381–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beebe, D.W. Cognitive, Behavioral, and Functional Consequences of Inadequate Sleep in Children and Adolescents. Pediatr. Clin. N. Am. 2011, 58, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Taggart, F.M.; Kandala, N.-B.; Currie, A.; Peile, E.; Stranges, S.; Miller, M.A. Meta-Analysis of Short Sleep Duration and Obesity in Children and Adults. Sleep 2008, 31, 619–626. [Google Scholar] [CrossRef]
- Reilly, J.J.; Armstrong, J.; Dorosty-Motlagh, A.-R.; Emmett, P.; Ness, A.; Rogers, I.; Steer, C.; Sherriff, A. Early life risk factors for obesity in childhood: Cohort study. BMJ 2005, 330, 1357. [Google Scholar] [CrossRef] [PubMed]
- Spruyt, K.; Molfese, D.L.; Gozal, D. Sleep Duration, Sleep Regularity, Body Weight, and Metabolic Homeostasis in School-aged Children. Pediatrics 2011, 127, e345–e352. [Google Scholar] [CrossRef] [PubMed]
- Sung, V.; Beebe, D.W.; VanDyke, R.; Fenchel, M.C.; Crimmins, N.A.; Kirk, S.; Hiscock, H.; Amin, R.; Wake, M. Does Sleep Duration Predict Metabolic Risk in Obese Adolescents Attending Tertiary Services? A Cross-Sectional Study. Sleep 2011, 34, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Hitze, B.; Bosy-Westphal, A.; Bielfeldt, F.; Settler, U.; Plachta-Danielzik, S.; Pfeuffer, M.; Schrezenmeir, J.; Mönig, H.; Müller, M.J. Determinants and impact of sleep duration in children and adolescents: Data of the Kiel Obesity Prevention Study. Eur. J. Clin. Nutr. 2008, 63, 739–746. [Google Scholar] [CrossRef]
- Narang, I.; Manlhiot, C.; Davies-Shaw, J.; Gibson, D.; Chahal, N.; Stearne, K.; Fisher, A.; Dobbin, S.; McCrindle, B.W. Sleep disturbance and cardiovascular risk in adolescents. Can. Med. Assoc. J. 2012, 184, E913–E920. [Google Scholar] [CrossRef]
- A Hazen, R.; Fehr, K.K.; Fidler, A.; Cousino, M.K.; A MacLeish, S.; Gubitosi-Klug, R. Sleep disruption in adolescents with Type 1 diabetes mellitus: Relationships with adherence and diabetes control. Diabetes Manag. 2015, 5, 257–265. [Google Scholar] [CrossRef]
- Von Schnurbein, J.; Boettcher, C.; Brandt, S.; Karges, B.; Dunstheimer, D.; Galler, A.; Denzer, C.; Denzer, F.; Vollbach, H.; Wabitsch, M.; et al. Sleep and glycemic control in adolescents with type 1 diabetes. Pediatr. Diabetes 2018, 19, 143–149. [Google Scholar] [CrossRef]
- Jarrin, D.C.; McGrath, J.J.; Drake, C.L. Beyond sleep duration: Distinct sleep dimensions are associated with obesity in children and adolescents. Int. J. Obes. 2013, 37, 552–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| T1D (n = 48) | Peers without T1D (n = 19) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |
| Sex (male/female) (%) | 37.5/62.5 | 42.1/57.9 | 0.129 | ||
| Pubertal stage (n) | 0.543 | ||||
| Stage 1 (Not started) | 18 | 7 | |||
| Stage 2 (Barely started) | 8 | 1 | |||
| Stage 3 (Definitely underway) | 12 | 6 | |||
| Stage 4 (Seems completed) | 5 | 1 | |||
| Stage 5 (Completed) | 5 | 4 | |||
| Ages (y) | 14.02 | 2.89 | 13.58 | 3.46 | 0.601 |
| Body mass (kg) | 49.48 | 12.56 | 52.34 | 15.45 | 0.452 |
| Height (m) | 1.60 | 0.13 | 1.59 | 0.13 | 0.772 |
| BMI (kg∙m−2) | 18.97 | 3.10 | 20.38 | 3.32 | 0.119 |
| Body fat (%) | 29.28 | 9.54 | 28.42 | 6.61 | 0.765 |
| Waist circumference (cm) | 67.63 | 7.96 | 73.65 | 8.22 | 0.055 |
| Systolic blood pressure (mmHg) | 106.07 | 16.72 | 107.07 | 15.45 | 0.837 |
| Diastolic blood pressure (mmHg) | 65.24 | 11.65 | 65.75 | 9.88 | 0.878 |
| Total cholesterol (mmol∙L−1) | 4.03 | 0.81 | 3.14 | 0.67 | 0.001 * |
| HDL-C (mmol∙L−1) | 1.48 | 0.28 | 1.29 | 0.42 | 0.078 |
| LDL-C (mmol∙L−1) | 2.31 | 0.72 | 1.74 | 0.38 | 0.005 * |
| Triglycerides (mmol∙L−1) | 0.89 | 0.31 | 0.60 | 0.40 | 0.012 * |
| Diabetes duration (y) | 3.64 | 2.29 | |||
| MDI (n) | 30 | ||||
| CSII (n) | 18 | ||||
| CGM (n) | 26 | ||||
| SMBG (n) | 22 | ||||
| HbA1c (%) | 7.70 | 2.47 | |||
| Insulin dose (unit∙kg−1∙day−1) | 0.87 | 0.29 | |||
| Sleep Parameters | T1D (n = 33) Mean (SD) | Peers without Diabetes (n = 16) | p-Value |
|---|---|---|---|
| Sleep-onset time (hh:mm) § | 22:51 (22:06–23:06) | 22:42 (22:08–23:12) | 0.932 |
| Sleep-offset time (hh:mm) § | 6:54 (6:33–7:30) | 6:54 (6:33–7:30) | 0.757 |
| Sleep latency (min) § | 1.78 (0.86–5.29) | 2.17 (1.08–3.74) | 0.662 |
| Sleep efficiency (%) | 86.30 (4.70) | 86.43 (6.00) | 0.933 |
| Total sleep time (min) | 421.30 (62.30) | 432.02 (38.05) | 0.534 |
| Wake after sleep onset (min) | 62.23 (24.10) | 66.71 (32.49) | 0.589 |
| Number of awakenings (n) | 20.82 (5.94) | 21.21 (8.34) | 0.848 |
| Length of awakenings (min) | 62.23 (24.10) | 69.62 (39.64) | 0.422 |
| Dependent Variable: LDL-C | ||||||
|---|---|---|---|---|---|---|
| Independent Variables | β | 95% CI | p | Adjusted R2 | R2 | |
| Sleep efficiency (%) | ||||||
| Step 1 (unadjusted) | −0.0554 | −0.07 | −0.005 | 0.026 | 0.258 | 0.307 |
| Step 2 (adjusted †) | −0.04 | −0.084 | 0.004 | 0.071 | 0.175 | 0.395 |
| Total sleep time (min∙day−1) | ||||||
| Step 1 (unadjusted) | −0.001 | −0.003 | 0.001 | 0.201 | 0.027 | 0.065 |
| Step 2 (adjusted †) | −0.002 | −0.003 | 0.000 | 0.060 | 0.383 | 0.597 |
| Independent Variables: Sleep Efficiency | ||||||
|---|---|---|---|---|---|---|
| Dependent Variable | β | 95% CI | p | Adjusted R2 | R2 | |
| LDL-C (mmol·L−1) | ||||||
| Step 2 (adjusted) | −0.045 | −0.082 | −0.008 | 0.018 | 0.120 | 0.230 |
| Triglycerides (mmol·L−1) | ||||||
| Step 2 (adjusted ††) | −0.027 | −0.048 | −0.006 | 0.012 | 0.111 | 0.222 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.; Jamnik, V.K.; Koehle, M.S.; Guan, Y.; Li, Y.; Kaufman, K.; Warburton, D.E.R. Associations between Sleep Characteristics and Cardiovascular Risk Factors in Adolescents Living with Type 1 Diabetes. J. Clin. Med. 2022, 11, 5295. https://doi.org/10.3390/jcm11185295
Wu N, Jamnik VK, Koehle MS, Guan Y, Li Y, Kaufman K, Warburton DER. Associations between Sleep Characteristics and Cardiovascular Risk Factors in Adolescents Living with Type 1 Diabetes. Journal of Clinical Medicine. 2022; 11(18):5295. https://doi.org/10.3390/jcm11185295
Chicago/Turabian StyleWu, Nana, Veronica K. Jamnik, Michael S. Koehle, Yanfei Guan, Yongfeng Li, Kai Kaufman, and Darren E. R. Warburton. 2022. "Associations between Sleep Characteristics and Cardiovascular Risk Factors in Adolescents Living with Type 1 Diabetes" Journal of Clinical Medicine 11, no. 18: 5295. https://doi.org/10.3390/jcm11185295






