Mobile-Bearing Total Ankle Replacement In Vivo Kinematic Assessment: A Prospective Study Protocol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participant Recruitment
2.3. Inclusion and Exclusion Criteria
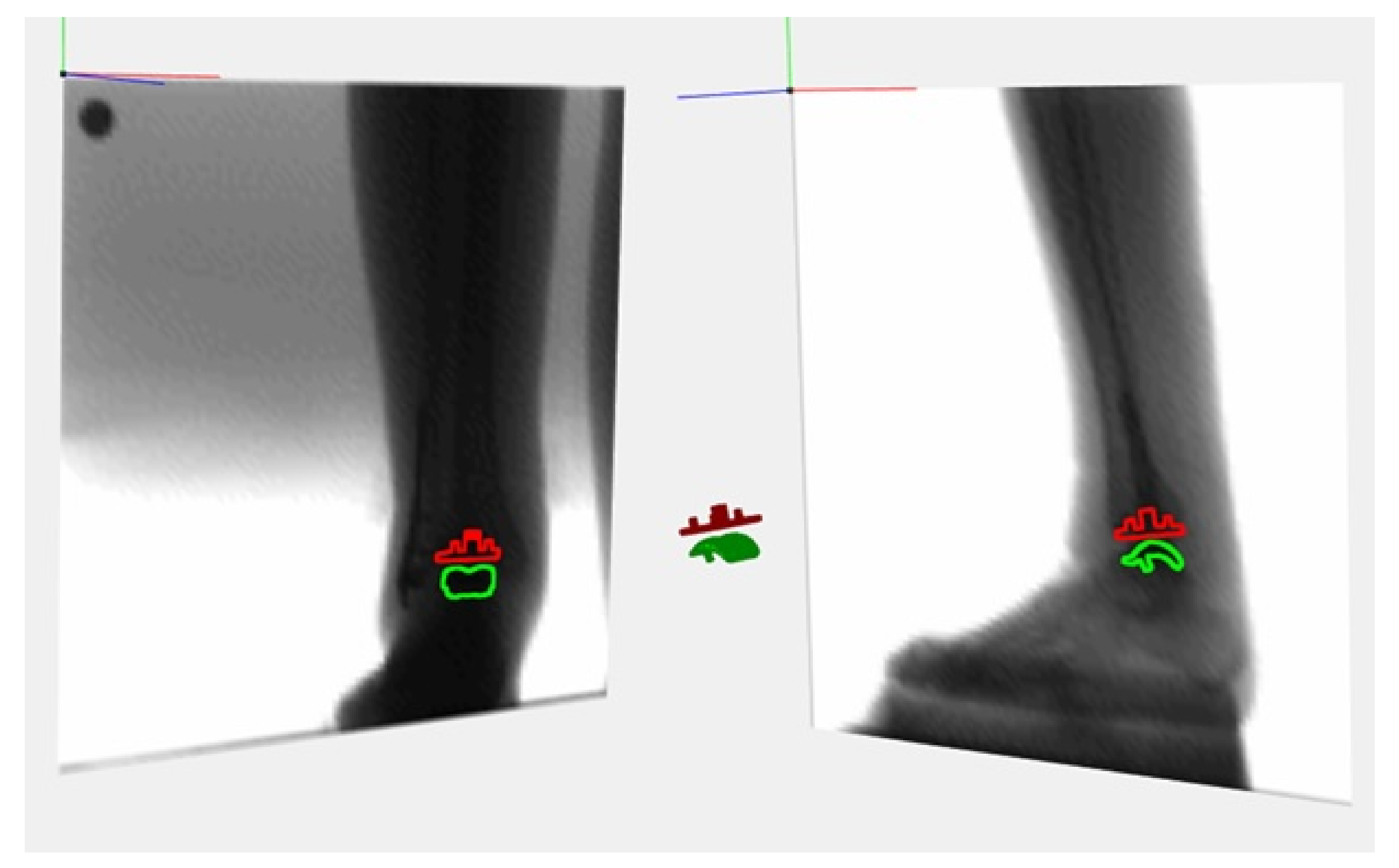
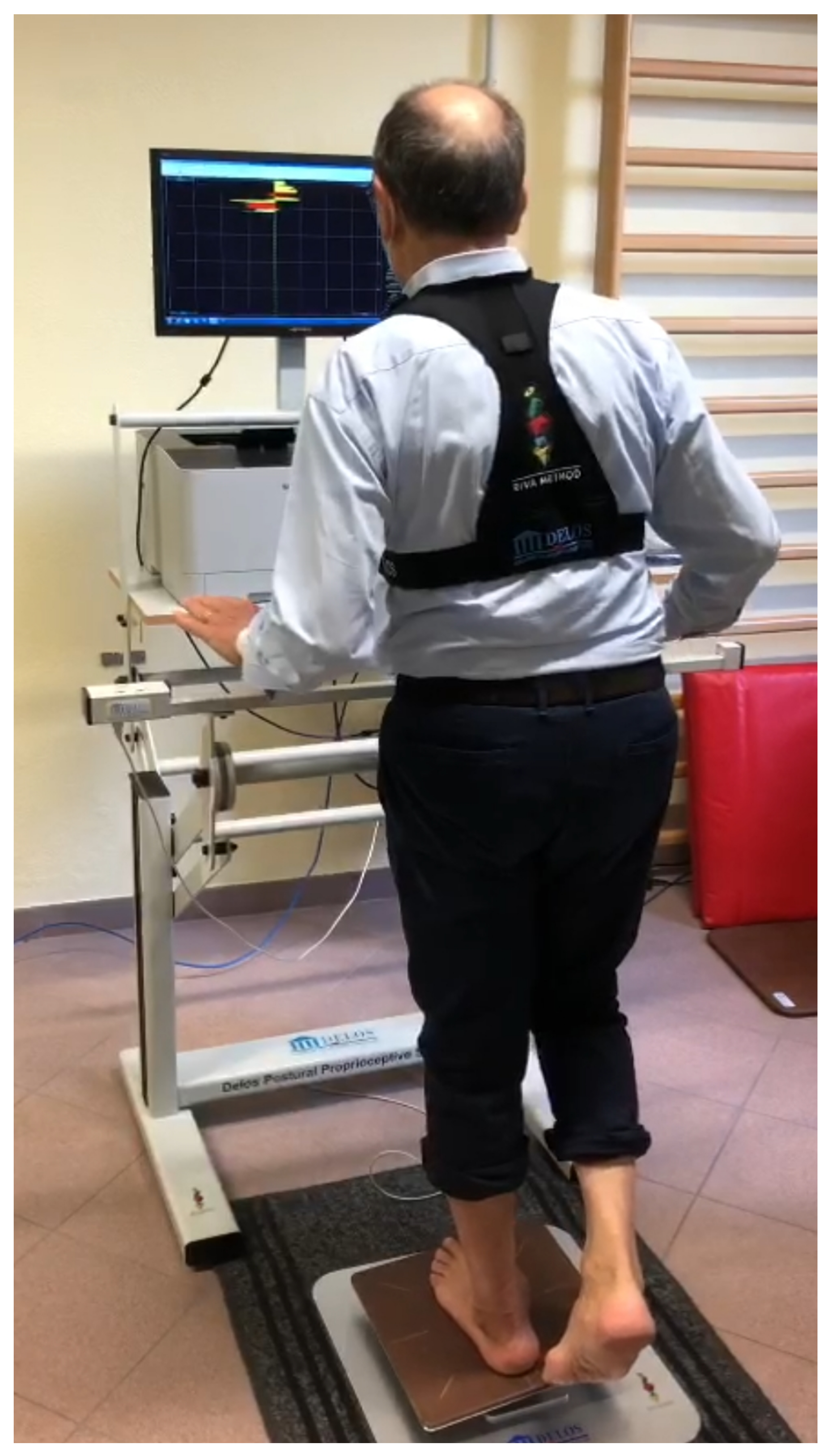
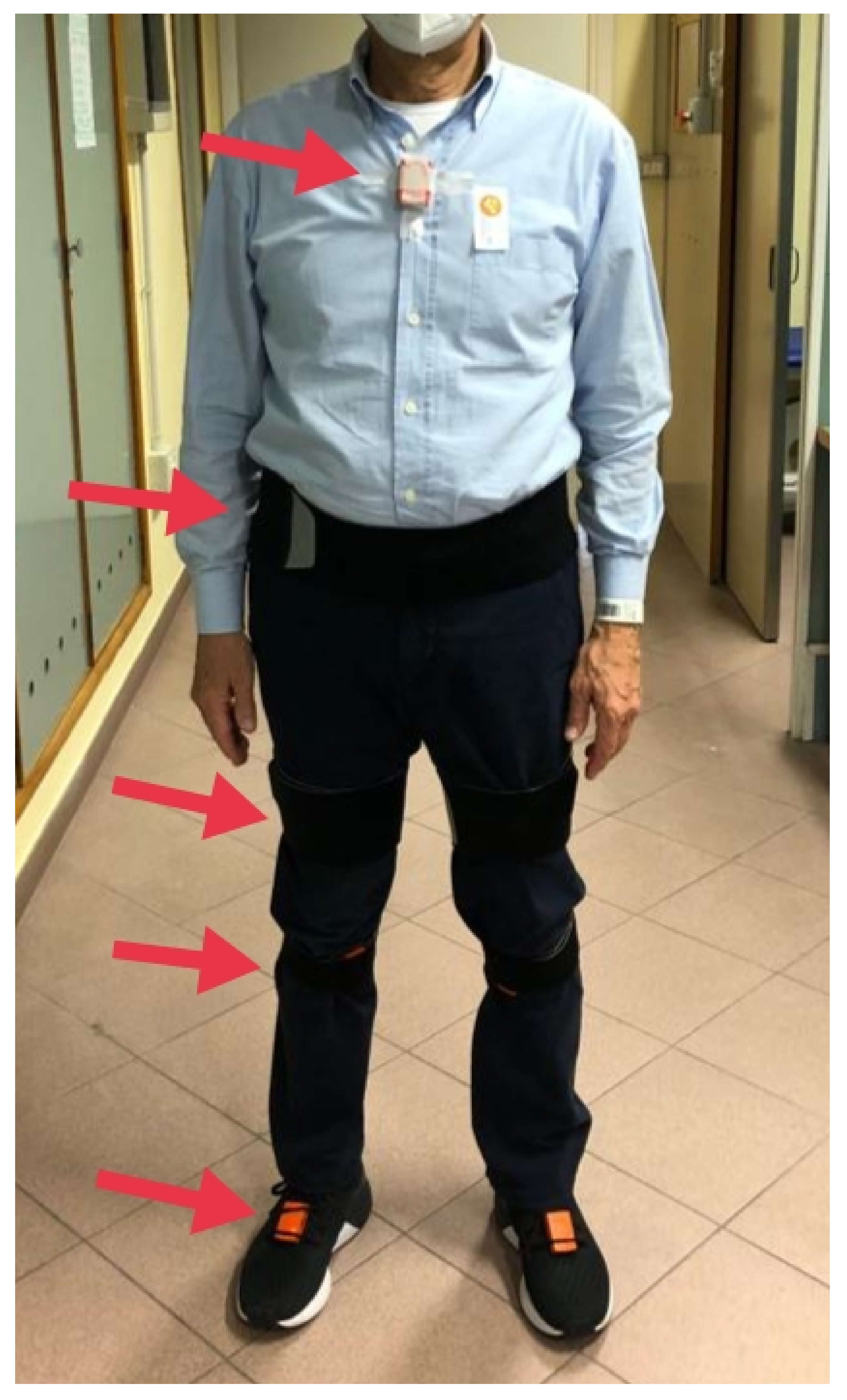
2.4. Data Collection and Measures
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valderrabano, V.; Horisberger, M.; Russell, I.; Dougall, H.; Hintermann, B. Etiology of ankle osteoarthritis. Clin. Orthop. Relat. Res. 2009, 467, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, M. End-stage ankle arthritis: Magnitude of the problem and solutions. Instr. Course Lect. 2010, 59, 359–365. [Google Scholar] [PubMed]
- Glazebrook, M.; Daniels, T.; Younger, A.; Foote, C.J.; Penner, M.; Wing, K.; Lau, J.; Leighton, R.; Dunbar, M. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J. Bone Joint Surg. Am. 2008, 90, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Malterud, K.; Siersma, V.D.; Guassora, A.D. Sample size in qualitative interview studies: Guided by information power. Qual. Health Res. 2016, 26, 1753–1760. [Google Scholar] [CrossRef]
- Nilsson, K.G.; Kärrholm, J.; Carlsson, L.; Dalén, T. Hydroxyapatite coating versus cemented fixation of the tibial component in total knee arthroplasty: Prospective randomized comparison of hydroxyapatite-coated and cemented tibial components with 5-year follow-up using radiostereometry. J. Arthroplasty 1999, 14, 9–20. [Google Scholar] [CrossRef]
- Nilsson, K.G.; Kärrholm, J.; Ekelund, L.; Magnusson, P. Evaluation of micromotion in cemented vs uncemented knee arthroplasty in osteoarthrosis and rheumatoid arthritis. Randomized study using roentgen stereophotogrammetric analysis. J. Arthroplasty 1991, 6, 265–278. [Google Scholar] [CrossRef]
- Onsten, I.; Nordqvist, A.; Carlsson, A.S.; Besjakov, J.; Shott, S. Hydroxyapatite augmentation of the porous coating improves fixation of tibial components. A randomised RSA study in 116 patients. J. Bone Joint Surg. Br. 1998, 80, 417–425. [Google Scholar] [CrossRef]
- Kaptein, B.L.; Valstar, E.R.; Stoel, B.C.; Rozing, P.M.; Reiber, J.H. A new model-based RSA method validated using CAD models and models from reversed engineering. J. Biomech. 2003, 36, 873–882. [Google Scholar] [CrossRef]
- Kitaoka, H.B.; Alexander, I.J.; Adelaar, R.S.; Nunley, J.A.; Myerson, M.S.; Sanders, M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994, 15, 349–353. [Google Scholar] [CrossRef]
- Jenkinson, C.; Layte, R.; Jenkinson, D.; Lawrence, K.; Petersen, S.; Paice, C.; Stradling, J. A shorter form health survey: Can the SF-12 replicate results from the SF-36 in longitudinal studies? J. Public Health Med. 1997, 19, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Chimera, N.J.; Larson, M. Predicting Lower Quarter Y-Balance Test Performance From Foot Characteristics. J. Sport Rehabil. 2020, 30, 16–21. [Google Scholar] [CrossRef]
- De Carli, P.; Patrizi, M.; Pepe, L.; Cavaniglia, G.; Riva, D.; D’Ottavi, L.R. Postural control and risk of falling in bipodalic and monopodalic stabilometric tests of healthy subjects before, after visuo-proprioceptive vestibulo-postural rehabilitation and at 3 months thereafter: Role of the proprioceptive system. Acta Otorhinolaryngol. Ital. 2010, 30, 182–189. [Google Scholar]
- Bragonzoni, L.; Barone, G.; Benvenuti, F.; Canal, V.; Ripamonti, C.; Marini, S.; Dallolio, L. A Randomized Clinical Trial to Evaluate the Efficacy and Safety of the ACTLIFE Exercise Program for Women with Post-menopausal Osteoporosis: Study Protocol. Int. J. Environ. Res. Public Health 2020, 17, 809. [Google Scholar] [CrossRef]
- Lins, L.; Carvalho, F.M. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016, 4, 2050312116671725. [Google Scholar] [CrossRef]
- Riva, D.; Mamo, C.; Fanì, M.; Saccavino, P.; Rocca, F.; Momenté, M.; Fratta, M. Single stance stability and proprioceptive control in older adults living at home: Gender and age differences. J. Aging Res. 2013, 2013, 561695. [Google Scholar] [CrossRef]
- Doets, H.C.; van Middelkoop, M.; Houdijk, H.; Nelissen, R.G.; Veeger, H.E. Gait analysis after successful mobile bearing total ankle replacement. Foot Ankle Int. 2007, 28, 313–322. [Google Scholar] [CrossRef]
- Gougoulias, N.E.; Khanna, A.; Maffulli, N. History and evolution in total ankle arthroplasty. Br. Med. Bull. 2009, 89, 111–151. [Google Scholar] [CrossRef]
- Coester, L.M.; Saltzman, C.L.; Leupold, J.; Pontarelli, W. Long-term results following ankle arthrodesis for post-traumatic arthritis. J. Bone Joint Surg. Am. 2001, 83, 219–228. [Google Scholar] [CrossRef]
- Myerson, M.; Won, H. Primary and revision total ankle replacement using custom-designed prosthesis. Foot Ankle Clin. N. Am. 2008, 13, 521–538. [Google Scholar] [CrossRef]
- Rohlfing, F.I.; Wiebking, U.; O’Loughlin, P.F.; Krettek, C.; Gaulke, R. Clinical and Radiological Mid-to-Long-term Outcomes Following Ankle Arthrolysis. In Vivo 2019, 33, 535–542. [Google Scholar] [CrossRef]
- Lord, G.; Marotte, J.H. Total ankle prosthesis, Technic and 1st results. Apropos of 12 cases. Rev. Chir. Orthop. Reparatrice Appar. Mot. 1973, 59, 139–151. [Google Scholar]
- Lord, G.; Marotte, J.H. Total ankle replacement (author’s translation). Rev. Chir. Orthop. Reparatrice Appar. Mot. 1980, 66, 527–530. [Google Scholar]
- Giannini, S.; Romagnoli, M.; O’Connor, J.J.; Malerba, F.; Leardini, A. Total ankle replacement compatible with ligament function produces mobility, good clinical scores, and low complication rates: An early clinical assessment. Clin. Orthop. Relat. Res. 2010, 468, 2746–2753. [Google Scholar] [CrossRef] [Green Version]
- Selvik, G. Roentgen stereophotogrammetry. A method for the study of the kinematics of the skeletal system. Acta Orthop. Scand. 1989, 232, 1–51. [Google Scholar] [CrossRef]
- Christensen, R.; Petersen, E.T.; Jürgens-Lahnstein, J.; Rytter, S.; Lindgren, L.; de Raedt, S.; Brüel, A.; Stilling, M. Assessment of knee kinematics with dynamic radiostereometry: Validation of an automated model-based method of analysis using bone models. J. Orthop. Res. 2021, 39, 597–608. [Google Scholar] [CrossRef]
- Hansen, L.; de Raedt, S.; Jørgensen, P.B.; Mygind-Klavsen, B.; Kaptein, B.; Stilling, M. Dynamic radiostereometric analysis for evaluation of hip joint pathomechanics. J. Exp. Orthop. 2017, 4, 20. [Google Scholar] [CrossRef]
- Ten Brinke, B.; Beumer, A.; Koenraadt, K.L.M.; Eygendaal, D.; Kraan, G.A.; Mathijssen, N.M.C. The accuracy and precision of radiostereometric analysis in upper limb arthroplasty. Acta Orthop. 2017, 88, 320–325. [Google Scholar] [CrossRef]
- Kibsgård, T.J. Radiostereometric analysis of sacroiliac joint movement and outcomes of pelvic joint fusion. Acta Orthop. Suppl. 2015, 86, 1–43. [Google Scholar] [CrossRef]
- Hansen, L.; De Raedt, S.; Jørgensen, P.B.; Mygind-Klavsen, B.; Kaptein, B.; Stilling, M. Marker free model-based radiostereometric analysis for evaluation of hip joint kinematics: A validation study. Bone Joint Res. 2018, 7, 379–387. [Google Scholar] [CrossRef]
- Alesi, D.; Marcheggiani Muccioli, G.M.; Roberti di Sarsina, T.; Bontempi, M.; Pizza, N.; Zinno, R.; Di Paolo, S.; Zaffagnini, S.; Bragonzoni, L. In vivo femorotibial kinematics of medial-stabilized total knee arthroplasty correlates to post-operative clinical outcomes. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 491–497. [Google Scholar] [CrossRef]
- Di Sarsina, T.R.; Alesi, D.; di Paolo, S.; Zinno, R.; Pizza, N.; Muccioli, G.M.M.; Zaffagnini, S.; Bragonzoni, L. In vivo kinematic comparison between an ultra-congruent and a posterior-stabilized total knee arthroplasty design by RSA. Knee Surg. Sports Traumatol. Arthrosc. 2021, 30, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Hopfgartner, A.J.; Tymofiyeva, O.; Ehses, P.; Rottner, K.; Boldt, J.; Richter, E.-J.; Jakob, P.M. Dynamic MRI of the TMJ under physical load. Dentomaxillofac. Radiol. 2013, 42, 20120436. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Borotikar, B.; Garetier, M.; Acosta, O.; Brochard, S.; Ben Salem, D.; Rousseau, F. 4D in vivo quantification of ankle joint space width using dynamic MRI. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 2115–2118. [Google Scholar]
- Cenni, F.; Leardini, A.; Belvedere, C.; Bugané, F.; Cremonini, K.; Miscione, M.T.; Giannini, S. Kinematics of the three components of a total ankle replacement: In vivo fluoroscopic analysis. Foot Ankle Int. 2012, 33, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, L.; Leonardi, L.; De Carli, P.; Mannocchi, M.L.; Pozzilli, C. Visuo-proprioceptive training reduces risk of falls in patients with multiple sclerosis. Mult. Scler. 2010, 16, 491–499. [Google Scholar] [CrossRef]
- Mosca, M.; Caravelli, S.; Massimi, S.; Fuiano, M.; Catanese, G.; Barone, G.; Bragonzoni, L.; Benedetti, M.G. Evaluation of proprioception and postural control at a minimum 1 year follow-up after ankle capsuloligamentous lateralplasty with Brostrom technique: A cohort study. Medicine 2020, 99, e19862. [Google Scholar] [CrossRef]
- Śliwowski, R.; Marynowicz, J.; Jadczak, Ł.; Grygorowicz, M.; Kalinowski, P.; Paillard, T. The relationships between knee extensors/flexors strength and balance control in elite male soccer players. PeerJ 2021, 9, e12461. [Google Scholar] [CrossRef]
- ISO 5725-6:1994; Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 6: Use in Practice of Accuracy Values. International Organization for Standardization: Geneva, Switzerland, 1994.
- Bonanzinga, T.; Signorelli, C.; Bontempi, M.; Russo, A.; Zaffagnini, S.; Marcacci, M.; Bragonzoni, L. Evaluation of RSA set-up from a clinical biplane fluoroscopy system for 3D joint kinematic analysis. Joints 2016, 4, 121–125. [Google Scholar] [CrossRef]
- Angelomenos, V.; Mohaddes, M.; Itayem, R.; Shareghi, B. Precision of low-dose CT-based micromotion analysis technique for the assessment of early acetabular cup migration compared with gold standard RSA: A prospective study of 30 patients up to 1 year. Acta Orthop. 2022, 93, 459–465. [Google Scholar] [CrossRef]
- Peiffer, M.; Belvedere, C.; Clockaerts, S.; Leenders, T.; Leardini, A.; Audenaert, E.; Victor, J.; Burssens, A.; WBCT ISG. Three-dimensional displacement after a medializing calcaneal osteotomy in relation to the osteotomy angle and hindfoot alignment. Foot Ankle Surg. 2020, 26, 78–84. [Google Scholar] [CrossRef]
- Faict, S.; Burssens, A.; Van Oevelen, A.; Maeckelbergh, L.; Mertens, P.; Buedts, K. Correction of ankle varus deformity using patient-specific dome-shaped osteotomy guides designed on weight-bearing CT: A pilot study. Arch. Orthop. Trauma Surg. 2021. Published online ahead of print. [Google Scholar] [CrossRef]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Outcome Assessment | Baseline (T0) | 10 Months (T1) |
|---|---|---|
| MB Radio-stereometric Analysis | X | |
| Delos Balance Assessment | X | X |
| Y-Balance Test | X | X |
| AOFAS score | X | X |
| SF-36 | X | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caravelli, S.; Bragonzoni, L.; Vocale, E.; Zinno, R.; Di Paolo, S.; Barone, G.; De Blasiis, P.; Benedetti, M.G.; Zaffagnini, S.; Mosca, M. Mobile-Bearing Total Ankle Replacement In Vivo Kinematic Assessment: A Prospective Study Protocol. J. Clin. Med. 2022, 11, 5328. https://doi.org/10.3390/jcm11185328
Caravelli S, Bragonzoni L, Vocale E, Zinno R, Di Paolo S, Barone G, De Blasiis P, Benedetti MG, Zaffagnini S, Mosca M. Mobile-Bearing Total Ankle Replacement In Vivo Kinematic Assessment: A Prospective Study Protocol. Journal of Clinical Medicine. 2022; 11(18):5328. https://doi.org/10.3390/jcm11185328
Chicago/Turabian StyleCaravelli, Silvio, Laura Bragonzoni, Emanuele Vocale, Raffaele Zinno, Stefano Di Paolo, Giuseppe Barone, Paolo De Blasiis, Maria Grazia Benedetti, Stefano Zaffagnini, and Massimiliano Mosca. 2022. "Mobile-Bearing Total Ankle Replacement In Vivo Kinematic Assessment: A Prospective Study Protocol" Journal of Clinical Medicine 11, no. 18: 5328. https://doi.org/10.3390/jcm11185328





