Impact of Sodium Zirconium Cyclosilicate Therapy Cessation in Patients with Systolic Heart Failure
Abstract
:1. Background
2. Methods
2.1. Patient Selection
2.2. Study Design
2.3. SZC Therapy
2.4. Study Outcomes
2.5. Data Collection
2.6. Statistics
3. Results
3.1. Baseline Characteristics
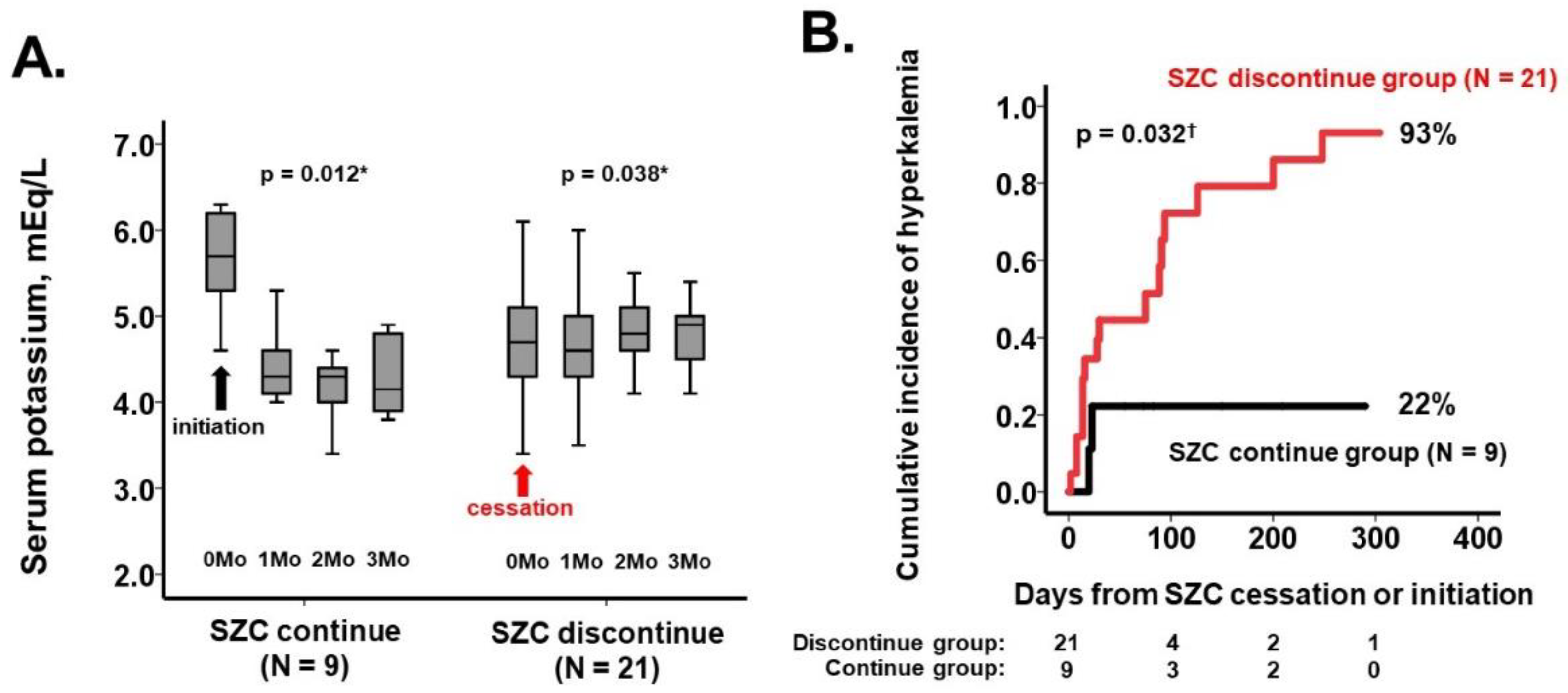
3.2. Occurrence of Hyperkalemia (Primary Outcome)
3.3. Trends in Clinical Parameters (Secondary Outcomes)
3.4. Occurrence of Clinical Events (Secondary Outcomes)
4. Discussion
4.1. SZC Cessation and Recurrence of Hyperkalemia
4.2. SZC Cessation and Doses of RASis and MRAs
4.3. Limitations
4.4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stavros, F.; Yang, A.; Leon, A.; Nuttall, M.; Rasmussen, H.S. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS ONE 2014, 9, e114686. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, N.; Kohsaka, S.; Kanda, E.; Okami, S.; Yajima, T. Hyperkalemia in Real-World Patients under Continuous Medical Care in Japan. Kidney Int. Rep. 2019, 4, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Tafesse, E.; Hurst, M.; Hoskin, L.; Badora, K.; Sugrue, D.; Qin, L.; James, G.; McEwan, P. Risk factors associated with the incidence and recurrence of hyperkalaemia in patients with cardiorenal conditions. Int. J. Clin. Pract. 2021, 75, e13941. [Google Scholar] [CrossRef] [PubMed]
- James, G.; Kim, J.; Mellstrom, C.; Ford, K.L.; Jenkins, N.C.; Tsang, C.; Evans, M.; McEwan, P. Serum potassium variability as a predictor of clinical outcomes in patients with cardiorenal disease or diabetes: A retrospective UK database study. Clin. Kidney J. 2022, 15, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Xu, H.; Trevisan, M.; Dahlstrom, U.; Rossignol, P.; Pitt, B.; Lund, L.H.; Carrero, J.J. Incidence, Predictors, and Outcome Associations of Dyskalemia in Heart Failure with Preserved, Mid-Range, and Reduced Ejection Fraction. JACC Heart Fail. 2019, 7, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Vardeny, O.; Claggett, B.; Anand, I.; Rossignol, P.; Desai, A.S.; Zannad, F.; Pitt, B.; Solomon, S.D. Randomized Aldactone Evaluation Study I. Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ. Heart Fail. 2014, 7, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Hsu, B.G.; Maeda, Y.; Shin, S.K.; Vishneva, E.M.; Rensfeldt, M.; Eklund, S.; Zhao, J. Efficacy and safety of sodium zirconium cyclosilicate for hyperkalaemia: The randomized, placebo-controlled HARMONIZE-Global study. ESC Heart Fail. 2020, 7, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, N.; Yamasaki, Y.; Osonoi, T.; Harada, H.; Shibagaki, Y.; Zhao, J.; Kim, H.; Yajima, T.; Sarai, N. A phase 3 multicenter open-label maintenance study to investigate the long-term safety of sodium zirconium cyclosilicate in Japanese subjects with hyperkalemia. Clin. Exp. Nephrol. 2021, 25, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.; Reaven, N.L.; Funk, S.E.; McGaughey, K.J.; Oestreicher, N.; Knispel, J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am. J. Manag. Care 2015, 21 (Suppl. 11), S212–S220. [Google Scholar] [PubMed]
- Linde, C.; Bakhai, A.; Furuland, H.; Evans, M.; McEwan, P.; Ayoubkhani, D.; Qin, L. Real-World Associations of Renin-Angiotensin-Aldosterone System Inhibitor Dose, Hyperkalemia, and Adverse Clinical Outcomes in a Cohort of Patients with New-Onset Chronic Kidney Disease or Heart Failure in the United Kingdom. J. Am. Heart Assoc. 2019, 8, e012655. [Google Scholar] [CrossRef] [PubMed]


| Total (n = 30) | SZC Discontinue (n = 21) | SZC Continue (n = 9) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 83 (75, 87) | 86 (79, 87) | 78 (63, 78) | 0.019 * |
| Men | 16 (53%) | 11 (52%) | 5 (56%) | 0.87 |
| Body mass index | 21.4 (18.9, 22.8) | 21.7 (18.8, 23.0) | 20.8 (18.9, 22.8) | 0.86 |
| Systolic blood pressure, mmHg | 124 (116, 136) | 122 (114, 138) | 118 (116, 136) | 0.66 |
| Pulse rate, bpm | 72 (65, 78) | 70 (63, 82) | 72 (64, 83) | 0.59 |
| New York Heart Association class (I/II/III/IV) | 0/25/5/0 | 0/19/2/0 | 0/6/3/0 | 0.27 |
| History of heart failure hospitalization | 13 (43%) | 9 (43%) | 4 (44%) | 0.94 |
| Cardiac resynchronization therapy | 2 (7%) | 1 (5%) | 1 (11%) | 0.52 |
| Hospitalized/ambulatory at day 0 | 16/14 | 12/9 | 4/5 | 0.52 |
| Comorbidity | ||||
| Diabetes mellitus | 16 (53%) | 11 (52%) | 5 (56%) | 0.87 |
| Atrial fibrillation | 8 (27%) | 4 (19%) | 4 (44%) | 0.15 |
| Ischemic heart disease | 16 (53%) | 14 (67%) | 2 (22%) | 0.025 * |
| History of stroke | 5 (17%) | 5 (24%) | 0 | 0.11 |
| Echocardiography data | ||||
| Left ventricular end-diastolic diameter, mm | 51 (48, 58) | 51 (47, 57) | 53 (50, 63) | 0.42 |
| Left ventricular ejection fraction, % | 42 (31, 48) | 37 (29, 48) | 44 (36, 47) | 0.19 |
| Left atrial diameter, mm | 43 (37, 46) | 43 (37, 49) | 43 (41, 45) | 0.97 |
| E/e’ ratio | 13.3 (10.7, 16.0) | 13.7 (11.5, 17.0) | 11.5 (10.2, 15.2) | 0.32 |
| Mitral valve regurgitation mild or greater | 6 (20%) | 3 (14%) | 3 (33%) | 0.23 |
| Tricuspid valve regurgitation mild or greater | 2 (7%) | 2 (10%) | 0 | 0.34 |
| Laboratory data | ||||
| Hemoglobin, g/dL | 10.9 (9.7, 11.8) | 10.3 (9.7, 11.8) | 11.7 (10.6, 13.7) | 0.21 |
| Serum sodium, mEq/L | 140 (137, 141) | 140 (138, 141) | 138 (135, 141) | 0.26 |
| Serum potassium, mEq/L | 4.9 (4.4, 5.5) | 4.7 (4.1, 5.1) | 5.7 (5.3, 6.2) | 0.001 * |
| Estimated glomerular filtration ratio, mL/min/1.73 m2 | 32.2 (20.3, 44.0) | 32.9 (21.8, 46.6) | 29.7 (13.9, 36.0) | 0.28 |
| Plasma B-type natriuretic peptide, pg/mL | 254 (135, 316) | 254 (123, 341) | 243 (198, 300) | 0.56 |
| Medications | ||||
| Beta-blocker dose, mg/day | 5.0 (2.5, 10.0) | 2.5 (2.5, 10) | 5.0 (2.5, 15) | 0.14 |
| Renin-angiotensin system inhibitors dose, mg/dL | 2.5 (1.25, 2.5) | 2.5 (1.25, 2.5) | 2.5 (2.5, 2.5) | 0.48 |
| Mineralocorticoid receptor antagonists dose, mg/dL | 0 (0, 12.5) | 0 (0, 12.5) | 12.5 (0, 25) | 0.30 |
| Furosemide dose, mg/dL | 20 (0, 20) | 20 (0, 20) | 10 (0, 20) | 0.65 |
| SGLT2 inhibitor | 9 (30%) | 5 (24%) | 4 (44%) | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, T.; Narang, N.; Kinugawa, K. Impact of Sodium Zirconium Cyclosilicate Therapy Cessation in Patients with Systolic Heart Failure. J. Clin. Med. 2022, 11, 5330. https://doi.org/10.3390/jcm11185330
Imamura T, Narang N, Kinugawa K. Impact of Sodium Zirconium Cyclosilicate Therapy Cessation in Patients with Systolic Heart Failure. Journal of Clinical Medicine. 2022; 11(18):5330. https://doi.org/10.3390/jcm11185330
Chicago/Turabian StyleImamura, Teruhiko, Nikhil Narang, and Koichiro Kinugawa. 2022. "Impact of Sodium Zirconium Cyclosilicate Therapy Cessation in Patients with Systolic Heart Failure" Journal of Clinical Medicine 11, no. 18: 5330. https://doi.org/10.3390/jcm11185330





