Correlations between Morphology, the Functional Properties of Upper Airways, and the Severity of Sleep Apnea
Abstract
:1. Introduction
2. Materials and Methods
3. Results
- -
- Mild OSA in 45 cases, with a medium value of 11.96, SD of 1.729, and 14.46% variability;
- -
- Moderate OSA in 59 cases, with a medium value of 20.54, SD of 4.269, and 20.78% variability;
- -
- Severe OSA in 48 cases, with a medium value of 50.49, SD of 17.65, and 34.96% variability;
- -
- Nasal septum deviation in any degree of severity was recorded in 80% of the cases. In addition, 67% of the cases were associated with inferior nasal turbinate hypertrophy.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mashaqi, S.; Staebler, D.; Mehra, R. Combined nocturnal pulse oximetry and questionnaire-based obstructive sleep apnea screening—A cohort study. Sleep Med. 2020, 72, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Tutar, B.; Ekincioğlu, M.E.; Berkiten, G.; Yılmazbaş, P.; Karaketir, S.; Arkan, M.E.; Saltürk, Z.; Göker, A.E.; Uyar, Y. The Effect of Pre-operative Obstructive Sleep Apnea (OSA) Severity on the Change of Sleep Patterns in Children Undergoing Adenotonsillectomy. Indian J. Pediatrics 2020, 87, 955. [Google Scholar] [CrossRef] [PubMed]
- Migueis, D.P.; Thuler, L.C.S.; de Andrade Lemes, L.N.; Moreira, C.S.S.; Joffily, L.; de Araujo-Melo, M.H. Systematic review: The influence of nasal obstruction on sleep apnea. Braz. J. Otorhinolaryngol. 2016, 82, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.C.; Min, H.J.; Kim, K.S. Refractory sleep apnea caused by tubal tonsillar hypertrophy. Int. J. Pediatric Otorhinolaryngol. 2017, 95, 84–86. [Google Scholar] [CrossRef]
- Möller, A. Atemstörungen im Schlaf [Sleep-disordered Breathing]. Pneumologie 2020, 74, 222–229. (In German) [Google Scholar] [CrossRef]
- Tan, H.L.; Kaditis, A.G. Phenotypic variance in pediatric obstructive sleep apnea. Pediatric Pulmonol. 2021, 56, 1754–1762. [Google Scholar] [CrossRef]
- Perri, R.A.; Kairaitis, K.; Cistulli, P.; Wheatley, J.R.; Amis, T.C. Surface cephalometric and anthropometric variables in OSA patients: Statistical models for the OSA phenotype. Sleep Breath. 2013, 18, 39–52. [Google Scholar] [CrossRef]
- Bertuzzi, F.; Santagostini, A.; Pollis, M.; Meola, F.; Segù, M. The Interaction of Craniofacial Morphology and Body Mass Index in Obstructive Sleep Apnea. Dent. J. 2022, 10, 136. [Google Scholar] [CrossRef]
- Ahmed-Nusrath, A.; Tong, J.L.; Smith, J.E. Pathways through the nose for nasal intubation: A comparison of three endotracheal tubes. Br. J. Anaesth. 2008, 100, 269–274. [Google Scholar] [CrossRef]
- Watanabe, T.; Isono, S.; Tanaka, A.; Tanzawa, H.; Nishino, T. Contribution of Body Habitus and Craniofacial Characteristics to Segmental Closing Pressures of the Passive Pharynx in Patients with Sleep-Disordered Breathing. Am. J. Respir. Crit. Care Med. 2002, 165, 260–265. [Google Scholar] [CrossRef]
- Patwa, A.; Shah, A. Anatomy and physiology of respiratory system relevant to anaesthesia. Indian J. Anaesth. 2015, 59, 533. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.; Wu, T. Large Hypopharyngeal Tongue: A Shared Anatomic Abnormality for Difficult Mask Ventilation, Difficult Intubation, and Obstructive Sleep Apnea? Anesthesiology 2001, 94, 936–937. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Guha, R. A morphometric study of human subcarinal angle in different age groups in both sexes and its clinical implications. J. Anat. Soc. India 2017, 66, S14. [Google Scholar] [CrossRef]
- Gropper, M.A.; Miller, R.D.; Eriksson, L.I.; Fleisher, L.A.; Wiener-Kronish, J.P.; Cohen, N.H.; Leslie, K. Miller’s Anesthesia; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Cross, M.E.; Plunkett, E.V.E. Physics, Pharmacology and Physiology for Anaesthetists: Key Concepts for the FRCA; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Dunham-Snary, K.J.; Wu, D.; Sykes, E.A.; Thakrar, A.; Parlow, L.R.; Mewburn, J.D.; Parlow, J.L.; Archer, S.L. Hypoxic Pulmonary Vasoconstriction. Chest 2017, 151, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Lutfi, M.F. The physiological basis and clinical significance of lung volume measurements. Multidiscip. Respir. Med. 2017, 12. [Google Scholar] [CrossRef]
- Kholdani, C.; Fares, W.H.; Mohsenin, V. Pulmonary Hypertension in Obstructive Sleep Apnea: Is it Clinically Significant? a Critical Analysis of the Association and Pathophysiology. Pulm. Circ. 2015, 5, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.S.; D’Ambrosio, C.M. Pathophysiology of Pediatric Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 253–262. [Google Scholar] [CrossRef]
- Schwengel, D.A.; Dalesio, N.M.; Stierer, T.L. Pediatric Obstructive Sleep Apnea. Anesthesiol. Clin. 2014, 32, 237–261. [Google Scholar] [CrossRef]
- Isono, S. Developmental changes of pharyngeal airway patency: Implications for pediatric anesthesia. Pediatric Anesth. 2006, 16, 109–122. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Sleep Disordered Breathing in Children: A Comprehensive Clinical Guide to Evaluation and Treatment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Vasu, T.; Grewal, R.; Doghramji, K. Obstructive sleep apnea syndrome and perioperative complications: A systematic review of the literature. J. Clin. Sleep Med. 2012, 8, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Karen, S.; David, G. REM and NREM sleep-state distribution of respiratory events in habitually snoring school-aged community children. Sleep Med. 2011, 13, 178–184. [Google Scholar]
- Davies, R.J.; Ali, N.J.; Stradling, J.R. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax 1992, 47, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Black, J.E.; Palombini, L.; Ohayon, M. A clinical investigation of obstructive sleep apnea syndrome (OSAS) and upper airway resistance syndrome (UARS) patients. Sleep Med. 2000, 1, 51–56. [Google Scholar] [CrossRef]
- Chervin, R.D. Sleepiness, Fatigue, Tiredness, and Lack of Energy in Obstructive Sleep Apnea. Chest 2000, 118, 372–379. [Google Scholar] [CrossRef]
- Geer, J.H.; Hilbert, J. Gender Issues in Obstructive Sleep Apnea. Yale J. Biol. Med. 2021, 94, 487–496. [Google Scholar] [PubMed]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire to Identify Patients at Risk for the Sleep Apnea Syndrome. Ann. Intern. Med. 1999, 131, 485. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.; Yegneswaran, B.; Liao, P.; Chung, S.A.; Vairavanathan, S.; Islam, S.; Khajehdehi, A.; Shapiro, C.M. STOP Questionnaire. Anesthesiology 2008, 108, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Walker, N.A.; Sunderram, J.; Zhang, P.; Lu, S.E.; Scharf, M.T. Clinical utility of the Epworth sleepiness scale. Sleep Breath. 2020, 24, 1759–1765. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tufik, S.; Santos-Silva, R.; Taddei, J.A.; Bittencourt, L.R.A. Obstructive Sleep Apnea Syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010, 11, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Gläser, S.; Glos, M.; Schmidt, C.O.; Stubbe, B.; et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—Results of SHIP-Trend. J. Sleep Res. 2018, 28, e12770. [Google Scholar] [CrossRef] [PubMed]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Smardz, J.; Wieckiewicz, M.; Wojakowska, A.; Michalek-Zrabkowska, M.; Poreba, R.; Gac, P.; Mazur, G.; Martynowicz, H. Incidence of Sleep Bruxism in Different Phenotypes of Obstructive Sleep Apnea. J. Clin. Med. 2022, 11, 4091. [Google Scholar] [CrossRef]
- Jonas, D.E.; Amick, H.R.; Feltner, C.; Weber, R.P.; Arvanitis, M.; Stine, A.; Lux, L.; Harris, R.P. Screening for Obstructive Sleep Apnea in Adults. JAMA 2017, 317, 415. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- El Shayeb, M.; Topfer, L.-A.; Stafinski, T.; Pawluk, L.; Menon, D. Diagnostic accuracy of level 3 portable sleep tests versus level 1 polysomnography for sleep-disordered breathing: A systematic review and meta-analysis. Can. Med. Assoc. J. 2013, 186, E25–E51. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea. JAMA 2020, 323, 1389. [Google Scholar] [CrossRef]
- Goyal, M.M.D.; Johnson, J.D.O. Obstructive Sleep Apnea Diagnosis and Management. Mo. Med. 2017, 114, 120–124. [Google Scholar]
- Neelapu, B.C.; Kharbanda, O.P.; Sardana, H.K.; Balachandran, R.; Sardana, V.; Kapoor, P.; Gupta, A.; Vasamsetti, S. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep Med. Rev. 2017, 31, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Moon, I.J.; Han, D.H.; Kim, J.W.; Rhee, C.S.; Sung, M.W.; Park, J.W.; Kim, D.S.; Lee, C.H. Sleep magnetic resonance imaging as a new diagnostic method in obstructive sleep apnea syndrome. Laryngoscope 2010, 120, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, K.; Van de Perck, E.; Wellman, A.; Kazemeini, E.; Willemen, M.; Verbraecken, J.; Vanderveken, O.M.; Vena, D.; Op de Beeck, S. Comparison of Drug-Induced Sleep Endoscopy and Natural Sleep Endoscopy in the Assessment of Upper Airway Pathophysiology During Sleep: Protocol and Study Design. Front. Neurol. 2021, 12, 768973. [Google Scholar] [CrossRef]
- Ryu, H.H.; Kim, C.H.; Cheon, S.M.; Bae, W.Y.; Kim, S.H.; Koo, S.K.; Kim, M.S.; Kim, B.J. The usefulness of cephalometric measurement as a diagnostic tool for obstructive sleep apnea syndrome: A retrospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Miles, P.G.; Vig, P.S.; Weyant, R.J.; Forrest, T.D.; Rockette, H.E., Jr. Craniofacial structure and obstructive sleep apnea syndrome—A qualitative analysis and meta-analysis of the literature. Am. J. Orthod. Dentofac. Orthop. 1996, 109, 163–172. [Google Scholar] [CrossRef]
- Gungor, A.Y.; Turkkahraman, H.; Yilmaz, H.H.; Yariktas, M. Cephalometric comparison of obstructive sleep apnea patients and healthy controls. Eur. J. Dent. 2013, 7, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Takeuchi, S.; Takanami, T.; Ishimoto, S. Evaluation of the Role of Palatal Tonsil Hypertrophy in Sleep Apnea Patients: Cephalometric Analysis. Open J. Stomatol. 2016, 6, 164–169. [Google Scholar] [CrossRef]
- Solyom, R.; Csiszer, I.; Neagos, A. Tonsillar hypertrophy implications in sleep disorders in adults and children. Rom. J. Morphol. Embryol. 2014, 55 (Suppl. 2), 603–606. [Google Scholar]
- Bouloukaki, I.; Tsiligianni, I.; Schiza, S. Evaluation of Obstructive Sleep Apnea in Female Patients in Primary Care: Time for Improvement? Med. Princ. Pract. 2021, 30, 508–514. [Google Scholar] [CrossRef]
- Barrera, J.E.; Pau, C.Y.; Forest, V.-I.; Holbrook, A.B.; Popelka, G.R. Anatomic measures of upper airway structures in obstructive sleep apnea. World J. Otorhinolaryngol. Head Neck Surg. 2017, 3, 85–91. [Google Scholar] [CrossRef]
- Tepedino, M.; Illuzzi, G.; Laurenziello, M.; Perillo, L.; Taurino, A.M.; Cassano, M.; Guida, L.; Burlon, G.; Ciavarella, D. Craniofacial morphology in patients with obstructive sleep apnea: Cephalometric evaluation. Braz. J. Otorhinolaryngol. 2022, 88, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Elbassiouny, A.M. Uvula in Snoring and Obstructive Sleep Apnea: An Approach to Surgical Intervention. In Recent Developments in Medicine and Medical Research; Book Publisher International (A Part of SCIENCEDOMAIN International): West Bengal, India, 2021; Volume 1, pp. 17–23. [Google Scholar] [CrossRef]
- Chang, E.T.; Baik, G.; Torre, C.; Brietzke, S.E.; Camacho, M. The relationship of the uvula with snoring and obstructive sleep apnea: A systematic review. Sleep Breath. 2018, 22, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Shigeta, Y.; Ogawa, T.; Tomoko, I.; Clark, G.T.; Enciso, R. Soft palate length and upper airway relationship in OSA and non-OSA subjects. Tex. Dent. J. 2013, 130, 203–211. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, G.; Li, Y.; Zang, H.; Wang, T.; Wang, D.; Han, D. Apnea–hypopnea index decreased significantly after nasal surgery for obstructive sleep apnea. Medicine 2017, 96, e6008. [Google Scholar] [CrossRef]
- Yeom, S.W.; Kim, M.G.; Lee, E.J.; Chung, S.K.; Kim, D.H.; Noh, S.J.; Lee, M.H.; Yang, Y.N.; Lee, C.M.; Kim, J.S. Association between septal deviation and OSA diagnoses: A nationwide 9-year follow-up cohort study. J. Clin. Sleep Med. 2021, 17, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Thukral, H.; Gupta, G.; Sinha, S.; Garg, R. Anthropometric Correlation with Pathophysiology of Obstructive Sleep Apnea (OSA): A Review. Sleep Vigil. 2020, 4, 95–103. [Google Scholar] [CrossRef]
- Pollis, M.; Lobbezoo, F.; Aarab, G.; Ferrari, M.; Marchese-Ragona, R.; Manfredini, D. Correlation between Apnea Severity and Sagittal Cephalometric Features in a Population of Patients with Polysomnographically Diagnosed Obstructive Sleep Apnea. J. Clin. Med. 2022, 11, 4572. [Google Scholar] [CrossRef]
- Baudouin, R.; Blumen, M.; Chaufton, C.; Chabolle, F. Adult sleep apnea and tonsil hypertrophy: Should pharyngoplasty be associated with tonsillectomy? Sleep Breath. 2019, 23, 917–923. [Google Scholar] [CrossRef]
- Leclere, J.C.; Marianowski, R.; Monteyrol, P.J.; Akkari, M.; Chalumeau, F.; Fayoux, P.; Leboulanger, N.; Franco, P.; Couloigner, V.; Mondain, M. Guidelines of the French Society of Otorhinolaryngology. Role of the ENT specialist in the diagnosis of childhood obstructive sleep apnea-hypopnea syndrome (OSAHS). Part 1: Interview and physical examination. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019, 136, 301–305. [Google Scholar] [CrossRef]
- Leclere, J.C.; Marianowski, R.; Monteyrol, P.J.; Akkari, M.; Chalumeau, F.; Fayoux, P.; Leboulanger, N.; Franco, P.; Couloigner, V.; Mondain, M. Guidelines of the French Society of Otorhinolaryngology. Role of the ENT specialist in the diagnosis of obstructive sleep apnea-hypopnea syndrome (OSAHS) in children. Part 2: Diagnostic investigations apart from sleep studies. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019, 136, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Eldaboosy, S.A.M.; Eldosky, I.; Nour, S.O. Modified Mallampati Score as a Predictor for the Presence and the Severity of Obstructive Sleep Apnea in Snoring Patients. La Prensa Med. Argent. 2021, 107, 3. [Google Scholar]
- Cielo, C.M.; Duffy, K.A.; Vyas, A.; Taylor, J.A.; Kalish, J.M. Obstructive sleep apnoea and the role of tongue reduction surgery in children with Beckwith-Wiedemann syndrome. Paediatr. Respir. Rev. 2018, 25, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-F.; Lee, C.-H.; Hsueh, W.-Y.; Lin, M.-T.; Kang, K.-T. Prevalence of Obstructive Sleep Apnea in Children With Down Syndrome: A Meta-Analysis. J. Clin. Sleep Med. 2018, 14, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Van Loenhout, L.; van der Zeijden, H. An Unusual Cause of CPAP Intolerance. J. Clin. Sleep Med. 2019, 15, 1535–1537. [Google Scholar] [CrossRef]
- Mihai, V.; Rusu, G.; Mihăescu, T. Care sunt diferenţele demografice, clinice si polisomnografice intre femei si bărbaţi? [Demographic, clinical and polysomnographic differences between men and women]. Pneumologia 2010, 59, 64–67. [Google Scholar]
- McFarlane, S.I. Obstructive sleep apnea and obesity: Implications for public health. Sleep Med. Disord. Int. J. 2017, 1, 00019. [Google Scholar] [CrossRef]
- Stival, C.; Lugo, A.; Odone, A.; van den Brandt, P.A.; Fernandez, E.; Tigova, O.; Soriano, J.B.; López, M.J.; Scaglioni, S.; Gallus, S. TackSHS Project Investigators. Prevalence and correlates of overweight and obesity in 12 European Countries in 2017–2018. Obes. Facts. 2022. [Google Scholar] [CrossRef]
- Ciavarella, D.; Tepedino, M.; Chimenti, C.; Troiano, G.; Mazzotta, M.; Foschino Barbaro, M.P.; Lo Muzio, L.; Cassano, M. Correlation between body mass index and obstructive sleep apnea severity indexes—A retrospective study. Am. J. Otolaryngol. 2018, 39, 388–391. [Google Scholar] [CrossRef]
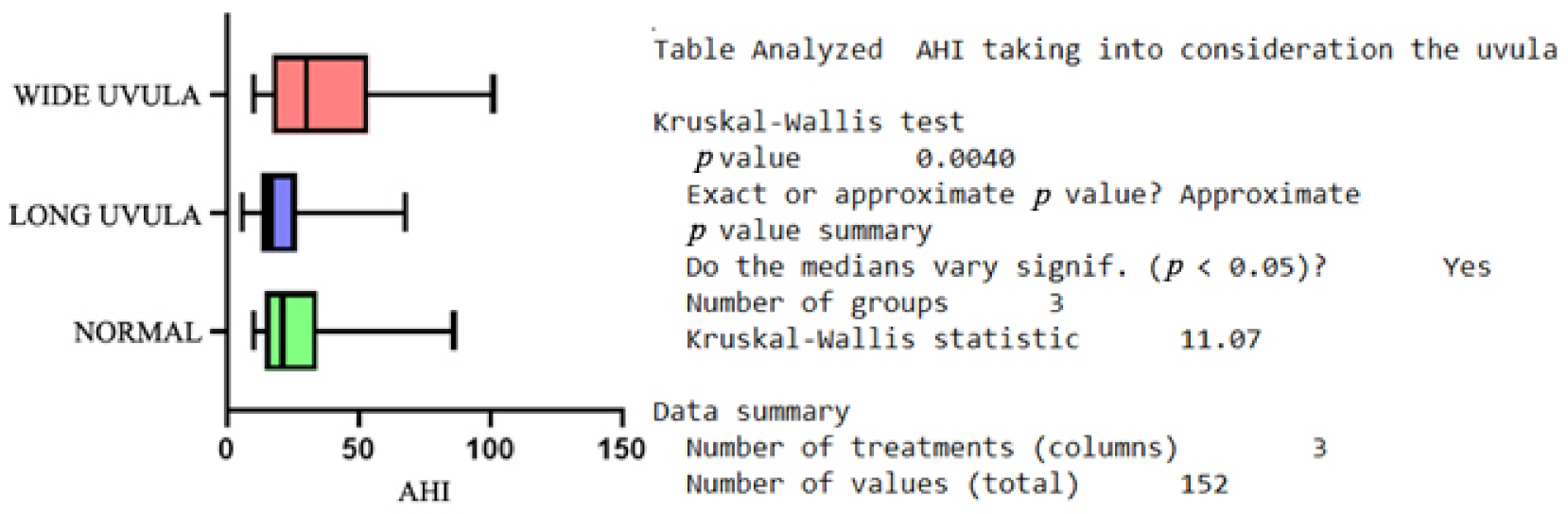
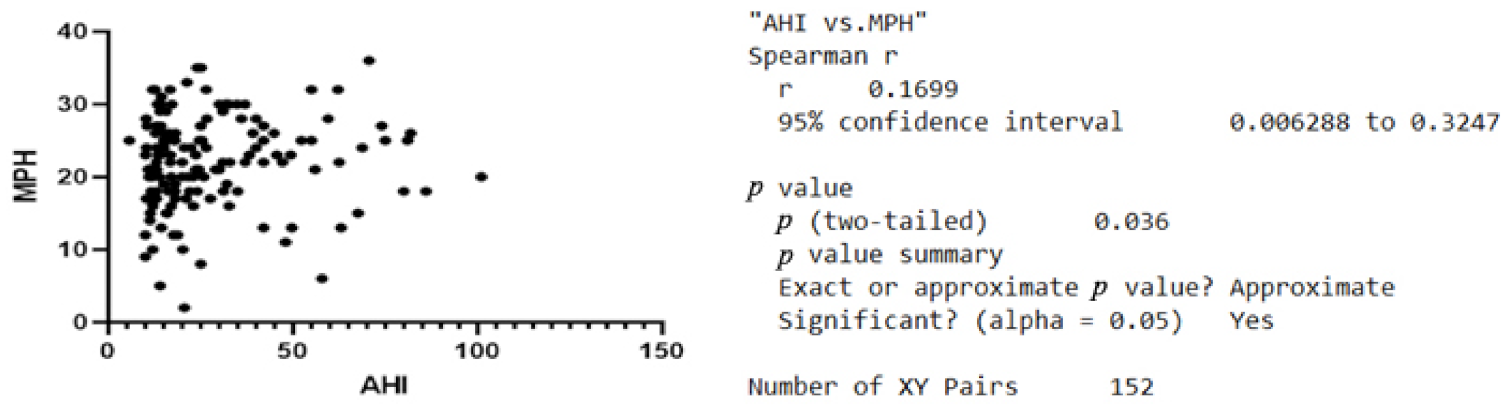
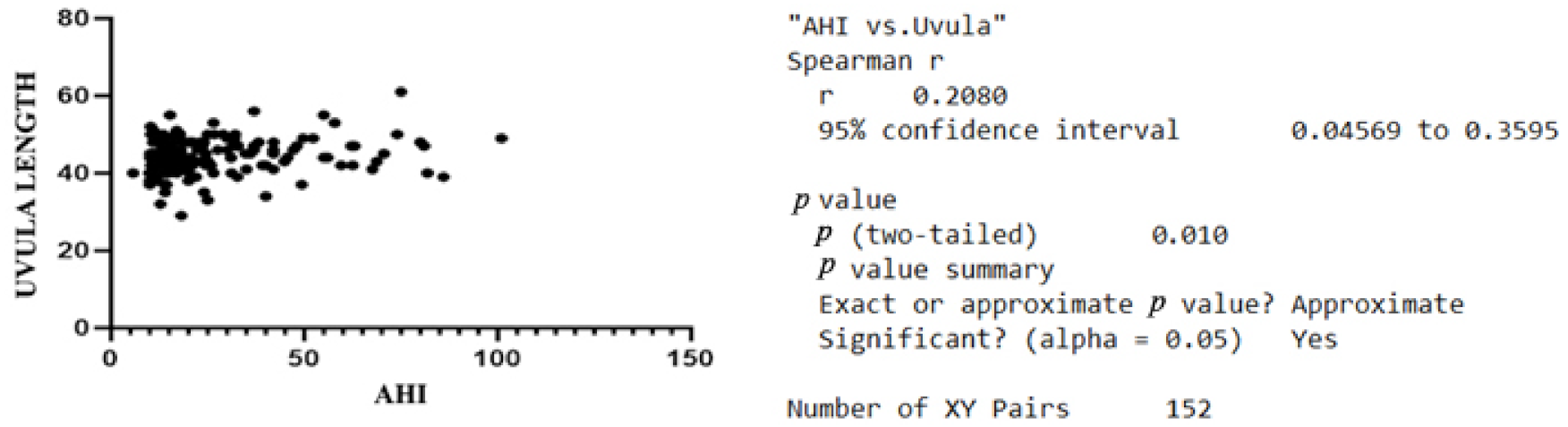
| Variable | Minimum | Maximum | Median | SD | Variability |
|---|---|---|---|---|---|
| MPH | 2 | 36 | 21.85 | 6.367 | 29.14% |
| PAS | 1 | 18 | 10.49 | 3.152 | 30.04% |
| Uvula length | 29 | 61 | 44.32 | 4.758 | 10.74% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagos, A.; Dumitru, M.; Neagos, C.M.; Mitroi, M.; Vrinceanu, D. Correlations between Morphology, the Functional Properties of Upper Airways, and the Severity of Sleep Apnea. J. Clin. Med. 2022, 11, 5347. https://doi.org/10.3390/jcm11185347
Neagos A, Dumitru M, Neagos CM, Mitroi M, Vrinceanu D. Correlations between Morphology, the Functional Properties of Upper Airways, and the Severity of Sleep Apnea. Journal of Clinical Medicine. 2022; 11(18):5347. https://doi.org/10.3390/jcm11185347
Chicago/Turabian StyleNeagos, Adriana, Mihai Dumitru, Cristian Mircea Neagos, Mihaela Mitroi, and Daniela Vrinceanu. 2022. "Correlations between Morphology, the Functional Properties of Upper Airways, and the Severity of Sleep Apnea" Journal of Clinical Medicine 11, no. 18: 5347. https://doi.org/10.3390/jcm11185347
APA StyleNeagos, A., Dumitru, M., Neagos, C. M., Mitroi, M., & Vrinceanu, D. (2022). Correlations between Morphology, the Functional Properties of Upper Airways, and the Severity of Sleep Apnea. Journal of Clinical Medicine, 11(18), 5347. https://doi.org/10.3390/jcm11185347







