Implantation Failure in Endometriosis Patients: Etiopathogenesis
Abstract
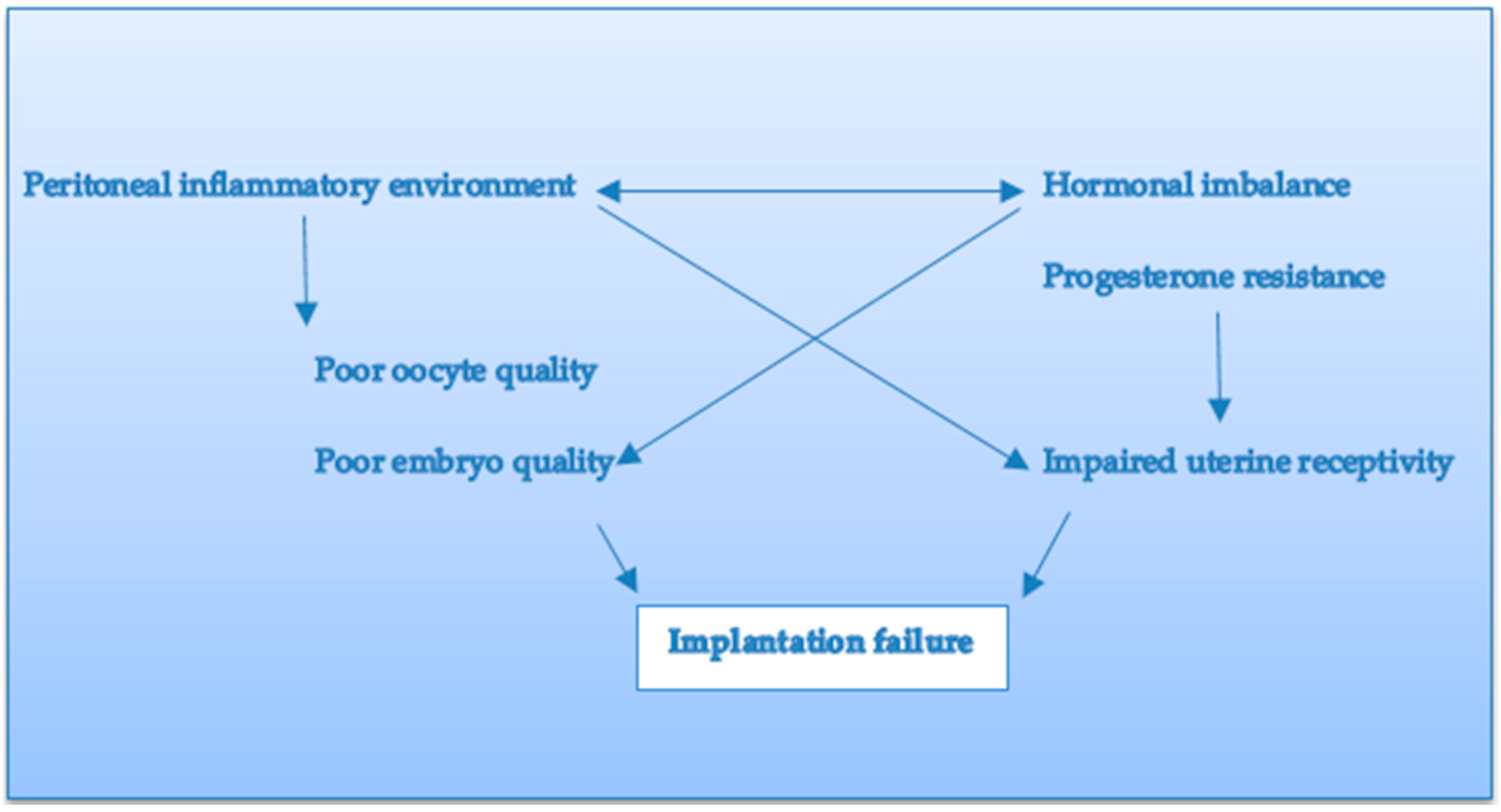
:1. Introduction
2. Materials and Methods
3. Oocyte Alterations
4. Endometrial Microenvironment and Endometrial Receptivity
5. Hormonal Imbalance
6. Progesterone Resistance
7. Pregnancy Outcomes
8. Associated Adenomyosis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Nisolle, M.; Donnez, J. Modern Trends Peritoneal Endometriosis, Ovarian Endometriosis, and Adenomyotic Nodules of the Rectovaginal Septum Are Three Different Entities; Wallach, A.E.E., Ed.; American Society for Reproductive Medicine: Washington, DC, USA, 1997. [Google Scholar]
- Parkin, K.L.; Fazleabas, A.T. Uterine Leukocyte Function and Dysfunction: A Hypothesis on the Impact of Endometriosis. Am. J. Reprod. Immunol. 2016, 75, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, C.; Vandenabeele, B.; Fieuws, S.; Spiessens, C.; Timmerman, D.; D’Hooghe, T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil. Steril. 2009, 92, 68–74. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and Infertility: A Committee Opinion. Fertil. Steril. 2012, 98, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, T.; Hansen, M.V.H.; Hartwell, D.; Lidegaard, Ø. Reproductive prognosis in daughters of women with and without endometriosis. Hum. Reprod. 2013, 28, 2284–2288. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.E.; Ahn, S.H.; Monsanto, S.P.; Khalaj, K.; Koti, M.; Tayade, C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget 2016, 8, 7138–7147. [Google Scholar] [CrossRef]
- Arici, A.; Oral, E.; Bukulmez, O.; Duleba, A.; Olive, D.L.; Jones, E.E. The effect of endometriosis on implantation: Results from the Yale University in vitro fertilization and embryo transfer program. Fertil. Steril. 1996, 65, 603–607. [Google Scholar] [CrossRef]
- Prescott, J.; Farland, L.; Tobias, D.; Gaskins, A.; Spiegelman, D.; Chavarro, J.; Rich-Edwards, J.; Barbieri, R.; Missmer, S. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum. Reprod. 2016, 31, 1475–1482. [Google Scholar] [CrossRef]
- Simón, C.; Gutiérrez, A.; Vidal, A.; de los Santos, M.J.; Tarín, J.J.; Remohí, J.; Pellicer, A. Outcome of patients with endometriosis in assisted reproduction: Results from in-vitro fertilization and oocyte donation. Hum. Reprod. 1994, 9, 725–729. [Google Scholar] [CrossRef]
- Singh, N.; Tiwari, A.; Vanamail, P.; Lata, K.; Malhotra, N.; Naha, M. Effect of endometriosis on implantation rates when compared to tubal factor in fresh non donor in vitro fertilization cycles. J. Hum. Reprod. Sci. 2014, 7, 143–147. [Google Scholar] [CrossRef]
- Lessey, B.A. Implantation Defects in Infertile Women with Endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Lessey, B.A.; Young, S.L. What exactly is endometrial receptivity? Fertil. Steril. 2019, 111, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.; Morin, S.; Jeong, J.-W.; Scott, R.T.; Lessey, B.A. Local and systemic factors and implantation: What is the evidence? Fertil. Steril. 2016, 105, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Freitag, N.; Pour, S.J.; Fehm, T.N.; Toth, B.; Markert, U.R.; Weber, M.; Togawa, R.; Kruessel, J.-S.; Baston-Buest, D.M.; Bielfeld, A.P. Are uterine natural killer and plasma cells in infertility patients associated with endometriosis, repeated implantation failure, or recurrent pregnancy loss? Arch. Gynecol. Obstet. 2020, 302, 1487–1494. [Google Scholar] [CrossRef]
- Corachán, A.; Pellicer, N.; Pellicer, A.; Ferrero, H. Novel therapeutic targets to improve IVF outcomes in endometriosis patients: A review and future prospects. Hum. Reprod. Updat. 2021, 27, 923–972. [Google Scholar] [CrossRef]
- Kolanska, K.; Alijotas-Reig, J.; Cohen, J.; Cheloufi, M.; Selleret, L.; D’Argent, E.; Kayem, G.; Valverde, E.E.; Fain, O.; Bornes, M.; et al. Endometriosis with infertility: A comprehensive review on the role of immune deregulation and immunomodulation therapy. Am. J. Reprod. Immunol. 2020, 85, e13384. [Google Scholar] [CrossRef]
- Squillace, A.L.; Simonian, D.S.; Allegro, M.C.; Júnior, E.B.; Bianchi, P.H.D.M.; Bibancos, M. Adenomyosis and in vitro fertilization impacts—A literature review. JBRA Assist. Reprod. 2021, 25, 303–309. [Google Scholar] [CrossRef]
- Soave, I.; Wenger, J.-M.; Pluchino, N.; Marci, R. Treatment options and reproductive outcome for adenomyosis-associated infertility. Curr. Med Res. Opin. 2017, 34, 839–849. [Google Scholar] [CrossRef]
- Ticconi, C.; Di Simone, N.; Campagnolo, L.; Fazleabas, A. Clinical consequences of defective decidualization. Tissue Cell 2021, 72, 101586. [Google Scholar] [CrossRef]
- Miravet-Valenciano, J.; Ruiz-Alonso, M.; Gómez, E.; Garcia-Velasco, J.A. Endometrial receptivity in eutopic endometrium in patients with endometriosis: It is not affected, and let me show you why. Fertil. Steril. 2017, 108, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Blank, C.; Deboever, C.; Decroos, E.; DeCroo, I.; Tilleman, K.; De Sutter, P.; Mischi, M.; Schoot, B.C. Impaired implantation in endometriosis compared with couples with male subfertility after transfer of equal quality embryos: A matched cohort study. Reprod. Biomed. Online 2021, 42, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Cerrillo, M.; Cruz, M.; Cecchino, G.; Garcia-Velasco, J. Early Pregnancy Outcomes in Fresh Versus Deferred Embryo Transfer Cycles for Endometriosis-Associated Infertility: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Dueholm, M. Uterine adenomyosis and infertility, review of reproductive outcome after in vitro fertilization and surgery. Acta Obstet. Gynecol. Scand. 2017, 96, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Kokcu, A. Possible effects of endometriosis-related immune events on reproductive function. Arch. Gynecol. Obstet. 2013, 287, 1225–1233. [Google Scholar] [CrossRef]
- Patel, B.; Lessey, B. Clinical Assessment and Management of the Endometrium in Recurrent Early Pregnancy Loss. Semin. Reprod. Med. 2011, 29, 491–506. [Google Scholar] [CrossRef]
- Borges, E., Jr.; Braga, D.P.A.F.; Setti, A.S.; Vingris, L.S.; Figueira, R.C.S.; Iaconelli, A., Jr. Endometriosis Affects Oocyte Morphology in Intracytoplasmic Sperm Injection Cycles. JBRA Assist. Reprod. 2015, 19, 235–240. [Google Scholar] [CrossRef]
- Tremellen, K.P.; Russell, P. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. II: Adenomyosis and macrophages. J. Reprod. Immunol. 2011, 93, 58–63. [Google Scholar] [CrossRef]
- Lédée, N.; Petitbarat, M.; Prat-Ellenberg, L.; Dray, G.; Cassuto, G.-N.; Chevrier, L.; Kazhalawi, A.; Vezmar, K.; Chaouat, G. The uterine immune profile: A method for individualizing the management of women who have failed to implant an embryo after IVF/ICSI. J. Reprod. Immunol. 2020, 142, 103207. [Google Scholar] [CrossRef]
- Giuliani, E.; Parkin, K.L.; Lessey, B.A.; Young, S.L.; Fazleabas, A.T. Characterization of Uterine NK Cells in Women with Infertility or Recurrent Pregnancy Loss and Associated Endometriosis. Am. J. Reprod. Immunol. 2014, 72, 262–269. [Google Scholar] [CrossRef]
- Krishnamurthy, M. Integrins and extracellular matrices in pancreatic tissue engineering. Front. Biosci. 2009, 1, 477. [Google Scholar] [CrossRef]
- Prins, J.R.; Marissen, L.M.; Scherjon, S.A.; Hoek, A.; Cantineau, A.E.P. Is there an immune modulating role for follicular fluid in endometriosis? A narrative review. Reproduction 2020, 159, R45–R54. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, H.; Hernandes, C.; Piccinato, C.A.; Podgaec, S. Interleukin in endometriosis-associated infertility-pelvic pain: Systematic review and meta-analysis. Reproduction 2019, 158, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Simopoulou, M.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Tsioulou, P.; Maziotis, E.; Tzanakaki, D.; Triantafyllidou, O.; Kalampokas, T.; Siristatidis, C.; et al. Getting to Know Endometriosis-Related Infertility Better: A Review on How Endometriosis Affects Oocyte Quality and Embryo Development. Biomedicines 2021, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Juneau, C.; Kraus, E.; Werner, M.; Franasiak, J.; Morin, S.; Patounakis, G.; Molinaro, T.; de Ziegler, D.; Scott, R.T. Patients with endometriosis have aneuploidy rates equivalent to their age-matched peers in the in vitro fertilization population. Fertil. Steril. 2017, 108, 284–288. [Google Scholar] [CrossRef]
- Bishop, L.A.; Gunn, J.; Jahandideh, S.; Devine, K.; Decherney, A.H.; Hill, M.J. Endometriosis does not impact live-birth rates in frozen embryo transfers of euploid blastocysts. Fertil. Steril. 2020, 115, 416–422. [Google Scholar] [CrossRef]
- Prapas, Y.; Goudakou, M.; Matalliotakis, I.; Kalogeraki, A.; Matalliotaki, C.; Panagiotidis, Y.; Ravanos, K.; Prapas, N. History of endometriosis may adversely affect the outcome in menopausal recipients of sibling oocytes. Reprod. Biomed. Online 2012, 25, 543–548. [Google Scholar] [CrossRef]
- Lessey, B.A.; Kim, J.J. Endometrial receptivity in the eutopic endometrium of women with endometriosis: It is affected, and let me show you why. Fertil. Steril. 2017, 108, 19–27. [Google Scholar] [CrossRef]
- Lessey, B.A.; Lebovic, D.I.; Taylor, R.N. Eutopic Endometrium in Women with Endometriosis: Ground Zero for the Study of Implantation Defects. Semin. Reprod. Med. 2013, 31, 109–124. [Google Scholar] [CrossRef]
- Lessey, B.A.; Castelbaum, A.J.; Sawin, S.W.; Buck, C.A.; Schinnar, R.; Bilker, W.; Strom, B.L. Aberrant integrin expression in the endometrium of women with endometriosis. J. Clin. Endocrinol. Metab. 1994, 79, 643–649. [Google Scholar] [CrossRef]
- Muteshi, C.M.; Ohuma, E.O.; Child, T.; Becker, C.M. The effect of endometriosis on live birth rate and other reproductive outcomes in ART cycles: A cohort study. Hum. Reprod. Open 2018, 2018, hoy016. [Google Scholar] [CrossRef] [Green Version]
- Bourdon, M.; Santulli, P.; Maignien, C.; Gayet, V.; Pocate-Cheriet, K.; Marcellin, L.; Chapron, C. The deferred embryo transfer strategy improves cumulative pregnancy rates in endometriosis-related infertility: A retrospective matched cohort study. PLoS ONE 2018, 13, e0194800. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, X.; Huang, J.; Kuang, Y.; Wang, Y. Fertility and Neonatal Outcomes of Freeze-All vs. Fresh Embryo Transfer in Women with Advanced Endometriosis. Front. Endocrinol. 2019, 10, 770. [Google Scholar] [CrossRef] [PubMed]
- Lessey, B.A.; Palomino, W.A.; Apparao, K.B.C.; Young, S.L.; Lininger, R.A. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reprod. Biol. Endocrinol. 2006, 4 (Suppl. S1), S9. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Velasco, J.A.; Fassbender, A.; Ruiz-Alonso, M.; Blesa, D.; D’Hooghe, T.; Simon, C. Is endometrial receptivity transcriptomics affected in women with endometriosis? A pilot study. Reprod. Biomed. Online 2015, 31, 647–654. [Google Scholar] [CrossRef] [PubMed]
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]
- Vercellini, P.; Parazzini, F.; Pietropaolo, G.; Cipriani, S.; Frattaruolo, M.P.; Fedele, L. Pregnancy outcome in women with peritoneal, ovarian and rectovaginal endometriosis: A retrospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1538–1543. [Google Scholar] [CrossRef]
- Stephansson, O.; Kieler, H.; Granath, F.; Falconer, H. Endometriosis, assisted reproduction technology, and risk of adverse pregnancy outcome. Hum. Reprod. 2009, 24, 2341–2347. [Google Scholar] [CrossRef]
- Healy, D.; Breheny, S.; Halliday, J.; Jaques, A.; Rushford, D.; Garrett, C.; Talbot, J.; Baker, H. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia. Hum. Reprod. 2009, 25, 265–274. [Google Scholar] [CrossRef]
- Shmueli, A.; Salman, L.; Hiersch, L.; Ashwal, E.; Hadar, E.; Wiznitzer, A.; Yogev, Y.; Aviram, A. Obstetrical and neonatal outcomes of pregnancies complicated by endometriosis. J. Matern. Neonatal Med. 2017, 32, 845–850. [Google Scholar] [CrossRef]
- Fujii, T.; Wada-Hiraike, O.; Nagamatsu, T.; Harada, M.; Hirata, T.; Koga, K.; Fujii, T.; Osuga, Y. Assisted reproductive technology pregnancy complications are significantly associated with endometriosis severity before conception: A retrospective cohort study. Reprod. Biol. Endocrinol. 2016, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Zullo, F.; Spagnolo, E.; Saccone, G.; Acunzo, M.; Xodo, S.; Ceccaroni, M.; Berghella, V. Endometriosis and obstetrics complications: A systematic review and meta-analysis. Fertil. Steril. 2017, 108, 667–672.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Q.; Deng, B.; Chen, F.; Liu, X.; Cheng, J. Pregnancy outcomes of Chinese women undergoing IVF with embryonic cryopreservation as compared to natural conception. BMC Pregnancy Childbirth 2021, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Benaglia, L.; Candotti, G.; Papaleo, E.; Pagliardini, L.; Leonardi, M.; Reschini, M.; Quaranta, L.; Munaretto, M.; Vigano, P.; Candiani, M.; et al. Pregnancy outcome in women with endometriosis achieving pregnancy with IVF. Hum. Reprod. 2016, 31, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Rombauts, L.; Motteram, C.; Berkowitz, E.; Fernando, S. Risk of placenta praevia is linked to endometrial thickness in a retrospective cohort study of 4537 singleton assisted reproduction technology births. Hum. Reprod. 2014, 29, 2787–2793. [Google Scholar] [CrossRef]
- Fernando, S.; Breheny, S.; Jaques, A.M.; Halliday, J.L.; Baker, G.; Healy, D. Preterm birth, ovarian endometriomata, and assisted reproduction technologies. Fertil. Steril. 2009, 91, 325–330. [Google Scholar] [CrossRef]
- Santulli, P.; Marcellin, L.; Menard, S.; Thubert, T.; Khoshnood, B.; Gayet, V.; Goffinet, F.; Ancel, P.-Y.; Chapron, C. Increased rate of spontaneous miscarriages in endometriosis-affected women. Hum. Reprod. 2016, 31, 1014–1023. [Google Scholar] [CrossRef]
- Horton, J.; Sterrenburg, M.; Lane, S.; Maheshwari, A.; Li, T.C.; Cheong, Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: A systematic review and meta-analysis. Hum. Reprod. Updat. 2019, 25, 593–633. [Google Scholar] [CrossRef]
- Takemura, Y.; Osuga, Y.; Fujimoto, A.; Oi, N.; Tsutsumi, R.; Koizumi, M.; Yano, T.; Taketani, Y. Increased risk of placenta previa is associated with endometriosis and tubal factor infertility in assisted reproductive technology pregnancy. Gynecol. Endocrinol. 2012, 29, 113–115. [Google Scholar] [CrossRef]
- Gasparri, M.L.; Nirgianakis, K.; Taghavi, K.; Papadia, A.; Mueller, M.D. Placenta previa and placental abruption after assisted reproductive technology in patients with endometriosis: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2018, 298, 27–34. [Google Scholar] [CrossRef]
- Roque, M.; Valle, M.; Sampaio, M.; Geber, S. Obstetric outcomes after fresh versus frozen-thawed embryo transfers: A systematic review and meta-analysis. JBRA Assist. Reprod. 2018, 22, 253–260. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Ueda, Y.; Nagase, Y.; Matsuzaki, S.; Kakuda, M.; Kakuda, S.; Sakaguchi, H.; Hisa, T.; Kamiura, S. Placenta Accreta Spectrum Disorder Complicated with Endometriosis: Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Conejero, J.A.; Morgan, M.; Montesinos, M.; Fortuño, S.; Meseguer, M.; Simón, C.; Horcajadas, J.A.; Pellicer, A. Adenomyosis does not affect implantation, but is associated with miscarriage in patients undergoing oocyte donation. Fertil. Steril. 2011, 96, 943–950.e1. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.C.; McElin, T.W.; Manalo-Estrella, P. The elusive adenomyosis of the uterus—Revisited. Am. J. Obstet. Gynecol. 1972, 112, 583–593. [Google Scholar] [CrossRef]
- Bergeron, C.; Amant, F.; Ferenczy, A. Pathology and physiopathology of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 511–521. [Google Scholar] [CrossRef]
- García-Solares, J.; Donnez, J.; Donnez, O.; Dolmans, M.-M. Pathogenesis of uterine adenomyosis: Invagination or metaplasia? Fertil. Steril. 2018, 109, 371–379. [Google Scholar] [CrossRef]
- Maruyama, S.; Imanaka, S.; Nagayasu, M.; Kimura, M.; Kobayashi, H. Relationship between adenomyosis and endometriosis; Different phenotypes of a single disease? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 191–197. [Google Scholar] [CrossRef]
- Chapron, C.; Vannuccini, S.; Santulli, P.; Abrão, M.S.; Carmona, F.; Fraser, I.S.; Gordts, S.; Guo, S.-W.; Just, P.-A.; Noël, J.-C.; et al. Diagnosing adenomyosis: An integrated clinical and imaging approach. Hum. Reprod. Update 2020, 26, 392–411. [Google Scholar] [CrossRef]
- Szubert, M.; Koziróg, E.; Olszak, O.; Krygier-Kurz, K.; Kazmierczak, J.; Wilczynski, J. Adenomyosis and Infertility—Review of Medical and Surgical Approaches. Int. J. Environ. Res. Public Health 2021, 18, 1235. [Google Scholar] [CrossRef]
- Bosch, T.V.D.; De Bruijn, A.M.; De Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Bourne, T.; Timmerman, D.; Huirne, J.A.F. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet. Gynecol. 2019, 53, 576–582. [Google Scholar] [CrossRef]
- Munro, M.G. Classification and Reporting Systems for Adenomyosis. J. Minim. Invasive Gynecol. 2019, 27, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Campo, S.; Campo, V.; Benagiano, G. Infertility and Adenomyosis. Obstet. Gynecol. Int. 2011, 2012, 1–8. [Google Scholar] [CrossRef]
- Vercellini, P.; Consonni, D.; Dridi, D.; Bracco, B.; Frattaruolo, M.P.; Somigliana, E. Uterine adenomyosis and in vitro fertilization outcome: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Piver, P. Facteurs utérins limitant la prise en charge en AMP. Journal de Gynécologie Obstétrique et Biologie de la Reproduction 2005, 34, 30–33. [Google Scholar] [CrossRef]
- Vercellini, P.; Consonni, D.; Barbara, G.; Buggio, L.; Frattaruolo, M.P.; Somigliana, E. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: A systematic review and meta-analysis. Reprod. Biomed. Online 2014, 28, 704–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boucher, A.; Brichant, G.; Gridelet, V.; Nisolle, M.; Ravet, S.; Timmermans, M.; Henry, L. Implantation Failure in Endometriosis Patients: Etiopathogenesis. J. Clin. Med. 2022, 11, 5366. https://doi.org/10.3390/jcm11185366
Boucher A, Brichant G, Gridelet V, Nisolle M, Ravet S, Timmermans M, Henry L. Implantation Failure in Endometriosis Patients: Etiopathogenesis. Journal of Clinical Medicine. 2022; 11(18):5366. https://doi.org/10.3390/jcm11185366
Chicago/Turabian StyleBoucher, Astrid, Géraldine Brichant, Virginie Gridelet, Michelle Nisolle, Stéphanie Ravet, Marie Timmermans, and Laurie Henry. 2022. "Implantation Failure in Endometriosis Patients: Etiopathogenesis" Journal of Clinical Medicine 11, no. 18: 5366. https://doi.org/10.3390/jcm11185366
APA StyleBoucher, A., Brichant, G., Gridelet, V., Nisolle, M., Ravet, S., Timmermans, M., & Henry, L. (2022). Implantation Failure in Endometriosis Patients: Etiopathogenesis. Journal of Clinical Medicine, 11(18), 5366. https://doi.org/10.3390/jcm11185366






