Lung Ultrasound Findings and Endothelial Perturbation in a COVID-19 Low-Intensity Care Unit
Abstract
:1. Introduction
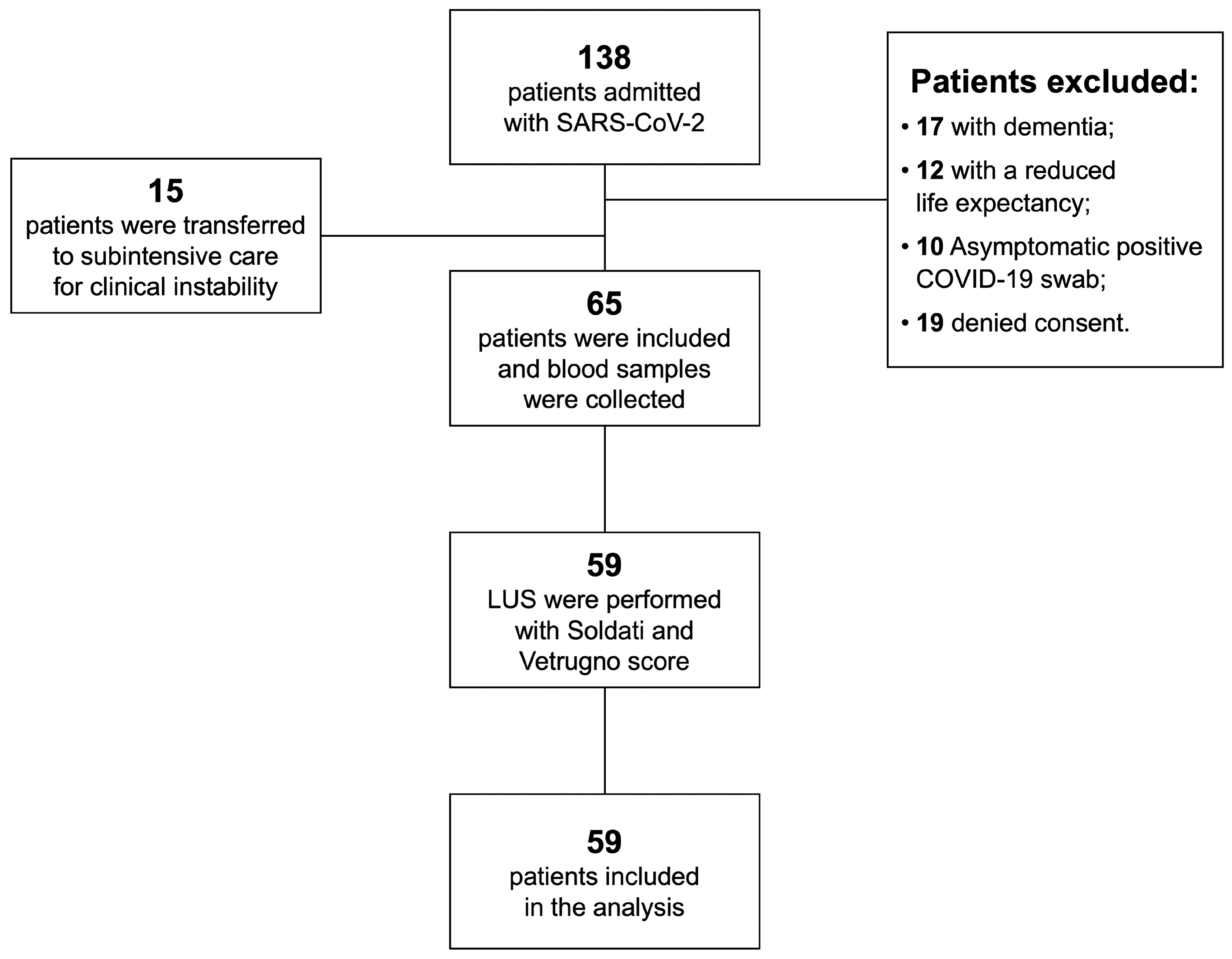
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Variables and Outcomes
2.4. Compressive Ultrasound (CUS) Protocol
2.5. Lung Ultrasound (LUS) Protocol
2.6. Humoral Biomarkers
2.7. Statistical Analysis
2.8. Sample Size Calculation
2.9. Potential Bias
3. Results
3.1. Patients
3.2. Outcome Data
3.3. CUS Results
3.4. LUS Results
3.5. Humoral Biomarkers
3.6. Univariate and Multivariate Regression Analysis
4. Discussion
Limitations and Bias
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Liao, B.; Guo, Y.; Li, F.; Lei, C.; Zhang, F.; Cai, W.; Hong, W.; Zeng, Y.; Qiu, S.; et al. Clinical Characteristics of Patients Infected with the Novel 2019 Coronavirus (SARS-CoV-2) in Guangzhou, China. Open Forum Infectious Diseases 2020, 7, ofaa187. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, W.; Xie, X.; Zhong, Z.; Shi, F.; Ma, T.; Liu, J.; Shen, D. Severity assessment of COVID-19 using CT image features and laboratory indices. Phys. Med. Biol. 2021, 66, 035015. [Google Scholar] [CrossRef] [PubMed]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef]
- Cugno, M.; Meroni, P.L.; Gualtierotti, R.; Griffini, S.; Grovetti, E.; Torri, A.; Lonati, P.; Grossi, C.; Borghi, M.O.; Novembrino, C.; et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J. Autoimmun. 2021, 116, 102560. [Google Scholar] [CrossRef]
- Lichter, Y.; Topilsky, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Oz, A.G.; Vine, J.; Goren, O.; Cohen, B.; et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensiv. Care Med. 2020, 46, 1873–1883. [Google Scholar] [CrossRef]
- Bouhemad, B.; Mongodi, S.; Via, G.; Rouquette, I. Ultrasound for “Lung Monitoring” of Ventilated Patients. Anesthesiology 2015, 122, 437–447. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Is There a Role for Lung Ultrasound During the COVID-19 Pandemic? J. Ultrasound Med. 2020, 39, 1459–1462. [Google Scholar] [CrossRef]
- Volpicelli, G.; Gargani, L. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J. 2020, 12, 1–3. [Google Scholar] [CrossRef]
- Maggi, L.; Biava, A.M.; Fiorelli, S.; Coluzzi, F.; Ricci, A.; Rocco, M. Lung Ultrasound: A Diagnostic Leading Tool for SARS-CoV-2 Pneumonia: A Narrative Review. Diagnostics 2021, 11, 2381. [Google Scholar] [CrossRef]
- Sega, F.V.D.; Fortini, F.; Spadaro, S.; Ronzoni, L.; Zucchetti, O.; Manfrini, M.; Mikus, E.; Fogagnolo, A.; Torsani, F.; Pavasini, R.; et al. Time course of endothelial dysfunction markers and mortality in COVID-19 patients: A pilot study. Clin. Transl. Med. 2021, 11, e283. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Lensing, A.W.; Prandoni, P.; Brandjes, D.; Huisman, P.M.; Vigo, M.; Tomasella, G.; Krekt, J.; Cate, J.W.T.; Huisman, M.V.; Büller, H.R. Detection of Deep-Vein Thrombosis by Real-Time B-Mode Ultrasonography. N. Engl. J. Med. 1989, 320, 342–345. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Proposal for International Standardization of the Use of Lung Ultrasound for Patients with COVID-19: A Simple, Quantitative, Reproducible Method. J. Ultrasound Med. 2020, 39, 1413–1439. [Google Scholar] [CrossRef] [PubMed]
- Vetrugno, L.; Bove, T.; Orso, D.; Bassi, F.; Boero, E.; Ferrari, G. Lung Ultrasound and the COVID-19 “Pattern”: Not All That Glitters Today Is Gold Tomorrow. J. Ultrasound Med. 2020, 39, 2281–2282. [Google Scholar] [CrossRef] [PubMed]
- Rouby, J.-J.; Arbelot, C.; Gao, Y.; Zhang, M.; Lv, J.; Wang, C.; Chunyao, W.; Bin, D.; Barbas, C.S.V.; Neto, F.L.D.; et al. Training for Lung Ultrasound Score Measurement in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2018, 198, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Bressollette, L.; Nonent, M.; Oger, E.; Garcia, J.F.; Larroche, P.; Guias, B.; Scarabin, P.Y.; Mottier, D. Diagnostic accuracy of compression ultrasonography for the detection of asymptomatic deep venous thrombosis in medical patients--the TADEUS project. Thromb. Haemost. 2001, 86, 529–533. [Google Scholar] [CrossRef]
- Giorgi-Pierfranceschi, M.; Paoletti, O.; Pan, A.; De Gennaro, F.; Nardecchia, A.L.; Morandini, R.; Dellanoce, C.; Lombi, S.; Tala, M.; Cancelli, V.; et al. Prevalence of asymptomatic deep vein thrombosis in patients hospitalized with SARS-CoV-2 pneumonia: A cross-sectional study. Intern. Emerg. Med. 2020, 15, 1425–1433. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Z.; Liu, J.; Song, Y.; Qiao, S.; Duan, Y.; Cao, H.; Xie, Y.; Wang, R.; Zhang, W.; et al. Lung Ultrasound Score as a Predictor of Mortality in Patients with COVID-19. Front. Cardiovasc. Med. 2021, 8, 633539. [Google Scholar] [CrossRef]
- Senter, R.; Capone, F.; Pasqualin, S.; Cerruti, L.; Molinari, L.; Basso, E.F.; Zanforlin, N.; Previato, L.; Toffolon, A.; Sensi, C.; et al. Lung Ultrasound Patterns and Clinical-Laboratory Correlates during COVID-19 Pneumonia: A Retrospective Study from North East Italy. J. Clin. Med. 2021, 10, 1288. [Google Scholar] [CrossRef]
- Campello, E.; Bulato, C.; Spiezia, L.; Boscolo, A.; Poletto, F.; Cola, M.; Gavasso, S.; Simion, C.; Radu, C.M.; Cattelan, A.; et al. Thrombin generation in patients with COVID-19 with and without thromboprophylaxis. Clin. Chem. Lab. Med. (CCLM) 2021, 59, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Poor, H.D. Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest 2021, 160, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef]
- Bois, M.C.; Boire, N.A.; Layman, A.J.; Aubry, M.-C.; Alexander, M.P.; Roden, A.C.; Hagen, C.E.; Quinton, R.A.; Larsen, C.; Erben, Y.; et al. COVID-19–Associated Nonocclusive Fibrin Microthrombi in the Heart. Circulation 2021, 143, 230–243. [Google Scholar] [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Rapkiewicz, A.V.; Mai, X.; Carsons, S.E.; Pittaluga, S.; Kleiner, D.E.; Berger, J.S.; Thomas, S.; Adler, N.M.; Charytan, D.M.; Gasmi, B.; et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. eClinicalMedicine 2020, 24, 100434. [Google Scholar] [CrossRef]
- Vassiliou, A.; Keskinidou, C.; Jahaj, E.; Gallos, P.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S. ICU Admission Levels of Endothelial Biomarkers as Predictors of Mortality in Critically Ill COVID-19 Patients. Cells 2021, 10, 186. [Google Scholar] [CrossRef]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.E.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef]
- Francischetti, I.M.B.; Toomer, K.; Zhang, Y.; Jani, J.; Siddiqui, Z.; Brotman, D.J.; Hooper, J.E.; Kickler, T.S. Upregulation of pulmonary tissue factor, loss of thrombomodulin and immunothrombosis in SARS-CoV-2 infection. EClinicalMedicine 2021, 39, 101069. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Long, H.; Sun, J.; Li, H.; He, Y.; Wang, Q.; Pan, K.; Tong, Y.; Wang, B.; Wu, Q.; et al. New laboratory evidence for the association between endothelial dysfunction and COVID-19 disease progression. J. Med. Virol. 2022, 94, 3112–3120. [Google Scholar] [CrossRef]
- Spadaro, S.; Fogagnolo, A.; Campo, G.; Zucchetti, O.; Verri, M.; Ottaviani, I.; Tunstall, T.; Grasso, S.; Scaramuzzo, V.; Murgolo, F.; et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit. Care 2021, 25, 74. [Google Scholar] [CrossRef] [PubMed]
- Tufa, A.; Gebremariam, T.H.; Manyazewal, T.; Getinet, T.; Webb, D.-L.; Hellström, P.M.; Genet, S. Inflammatory mediators profile in patients hospitalized with COVID-19: A comparative study. Front. Immunol. 2022, 13, 964179. [Google Scholar] [CrossRef]
- Motloch, L.J.; Jirak, P.; Gareeva, D.; Davtyan, P.; Gumerov, R.; Lakman, I.; Tataurov, A.; Zulkarneev, R.; Kabirov, I.; Cai, B.; et al. Cardiovascular Biomarkers for Prediction of in-hospital and 1-Year Post-discharge Mortality in Patients with COVID-19 Pneumonia. Front. Med. 2022, 9, 906665. [Google Scholar] [CrossRef]
| Soldati Scoring System | Vetrugno Scoring System | |
|---|---|---|
| Score 0 | Normal (A-lines) | Normal (A-lines) |
| Score 1 | Jagged pleural line with few B-lines departing (B1 pattern) | B1 pattern with at least 3 B lines |
| Score 2 | Presence of lung consolidations | B2 pattern with coalescent B lines |
| Score 3 | Presence of dense and fused B lines in the shape of B2 pattern “white lung” | Presence of supra-centimetre consolidations |
| All Patients (N = 65) | Survivors (N = 57) | Non-Survivors (N = 8) | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years), mean (SD) | 69.7 (15.1) | 68.2 (15.5) | 79.8 (5.6) * |
| Age, range | 32–92 | 32–92 | 72–90 |
| Male, n (%) | 37 (56.9) | 34 (59.6) | 3 (37.5) |
| Charlson Comorbidity Index #, mean (SD) | 4.1 (2.2) | 3.8 (2.0) | 6.8 (1.7) ** |
| Drugs | |||
| Angiotensin converting enzyme inhibitor/receptor blocker, n (%) | 23 (35.4) | 20 (35.0) | 3 (37.5) |
| Anticoagulant (VKA, DOAC), n (%) | 4 (6.2) | 4 (7.0) | 0 (0.0) |
| Heparin, n (%) | 14 (21.5) | 12 (21.0) | 2 (25.0) |
| Steroids, n (%) | 19 (29.2) | 17 (29.8) | 2 (25.0) |
| All Patients (N = 65) | Survivors (N = 57) | Non-Survivors (N = 8) | p Value | |
|---|---|---|---|---|
| Arterial blood gas analysis examination | ||||
| SatO2 (%), mean (SD) | 97.0 (2.1) | 97.3 (1.9) | 96.4 (2.8) | 0.304 |
| pH, mean (SD) | 7.5 (0.04) | 7.5 (0.04) | 7.5 (0.05) | 0.867 |
| PaO2, mmHg, mean (SD) | 81.6 (14.7) | 81.9 (15.1) | 79.4 (12.5) | 0.650 |
| PaCO2, mmHg, mean (SD) | 33.7 (4.3) | 34.0 (4.3) | 31.4 (4.2) | 0.104 |
| PaO2/FiO2, mmHg, mean (SD) | 308 (76.3) | 310 (73.9) | 389 (94.7) | 0.464 |
| Complete blood count | ||||
| White blood cells, ×106/L, mean (SD) | 6769 (3245.8) | 6612 (2913) | 7887 (5175.7) | 0.302 |
| Lymphocytes, ×109/L, mean (SD) | 1212.3 (1432) | 1026.3 (551) | 2515.0 (3723.8) | 0.005 |
| Haemoglobin, g/dL, mean (SD) | 12.6 (2.2) | 12.7 (2.2) | 12.4 (1.9) | 0.772 |
| Platelets, ×109/L, mean (SD) | 194.1 (91.4) | 192.4 (90.6) | 206.8 (102.7) | 0.680 |
| Biochemistry | ||||
| C-reactive Protein, mg/dL, mean (SD) | 5.8 (5.2) | 5.8 (5.1) | 5.9 (5.9) | 0.948 |
| LDH, U/L, mean (SD) | 271.6 (83.2) | 267.2 (82) | 305.3 (89.2) | 0.257 |
| ALT, U/L, mean (SD) | 31.9 (28.1) | 32.5 (29.9) | 27.8 (8.9) | 0.657 |
| Creatinine, mg/dL, mean (SD) | 1.1 (0.8) | 0.98 (0.35) | 1.99 (2) | 0.001 |
| PT ratio, mean (SD) | 1.1 (0.2) | 1.1 (0.2) | 1.0 (0.07) | 0.290 |
| PTT ratio, mean (SD) | 0.9 (0.2) | 0.9 (0.3) | 0.95 (0.07) | 0.855 |
| Fibrinogen, mg/dL, mean (SD) | 512.6 (130.5) | 516 (134.8) | 492.5 (109.1) | 0.688 |
| Ferritin ng/mL, mean (SD) | 706.9 (648.1) | 735.4 (683.9) | 507.4 (233) | 0.356 |
| All Patients (N = 65) | Survivors (N = 57) | Non-Survivors (N = 8) | p Value | No Oxygen Therapy (N = 8) | Oxygen Therapy and NIV (N = 57) | p Value | |
|---|---|---|---|---|---|---|---|
| SOLDATI total LUS score mean (SD) | 20.2 (8.4) | 19.8 (8.0) | 23.1 (11.6) | 0.335 | 13.6 (6.9) | 21.3 (8.2) | 0.017 |
| VETRUGNO total LUS score mean (SD) | 16.4 (6.4) | 16.2 (6.1) | 17.9 (9.2) | 0.461 | 12.0 (4.3) | 17.0 (6.5) | 0.023 |
| All Patients (N = 65) | Survivors (N = 57) | Non-Survivors (N = 8) | p Value | Reference Values | |
|---|---|---|---|---|---|
| sE-selectin (ng/mL), mean (SD) | 25.0 (15.6) | 24.9 (15.9) | 25.5 (15.2) | 0.931 | 13.0–51.03 |
| Thrombomodulin (pg/mL), mean (SD) | 5143.2 (2317.3) | 4800.7 (1771.0) | 7283.9 (3961.6) | 0.004 | 2353–4541 |
| IL-6 (pg/mL), mean (SD) | 44.8 (102.4) | 40.3 (102.8) | 73.1 (101.9) | 0.404 | <10 |
| FVIII (%), mean (SD) | 160.6 (57.4) | 164.8 (59.7) | 136.1 (35.7) | 0.194 | 54–133 |
| PC (%), mean (SD) | 103.8 (25.7) | 104.5 (25.7) | 99.9 (26.7) | 0.640 | 84–145 |
| VWF:Ag (%), mean (SD) | 251.8 (90.9) | 245.3 (83.4) | 288.9 (126.6) | 0.215 | 70–194 |
| D-dimer standardized (ng/mL), mean (SD) | 893.9 (567.0) | 846.6 (564.9) | 1165.6 (532.5) | 0.144 | <500 |
| FVIII/Protein C, mean (SD) | 1.578 (0.504) | 1.610 (0.527) | 1.394 (0.313) | 0.268 | 1 |
| sEPCR (ng/mL), mean (SD) | 80.13 (49.0) | 76.5 (47.3) | 100.9 (56) | 0.197 | 12–64 |
| VEGF (pg/mL), mean (SD) | 455.6 (318.5) | 435.9 (316.9) | 579.1 (320.7) | 0.241 | 62–707 |
| sC5b9 (ng/mL), mean (SD) | 472.5 (389.5) | 483.2 (410.5) | 405.1 (223.3) | 0.603 | 139–463 |
| C5a (ng/mL), mean (SD) | 16.2 (5.9) | 16.1 (5.7) | 16.6 (7.5) | 0.819 | 0.37–74 |
| tPA (ng/mL), mean (SD) | 10.9 (6.2) | 10.6 (5.8) | 12.9 (8.3) | 0.340 | <10 |
| PAI-1 activity (ng/mL), mean (SD) | 10.2 (28.8) | 11.5 (30.8) | 2.5 (4.3) | 0.420 | <5 |
| C3c (%), mean (SD) | 122 (20.2) | 123 (20.3) | 116 (1990) | 0.403 | 70–130 |
| C4 (%), mean (SD) | 180 (52.7) | 180 (51.7) | 179 (62.6) | 0.961 | 60–140 |
| ICAM-1 (ng/mL), mean (SD) | 324 (110.6) | 325 (116.7) | 318 (65.9) | 0.864 | 99–320 |
| VCAM-1 (ng/mL), mean (SD) | 1568 (575.8) | 1451 (456.2) | 2299 (730.5) | <0.001 | 349–991 |
| Length of Stay °° | Time to Oxygen Support Weaning °° | Death ° | |
|---|---|---|---|
| Age | 0.02 (0.01, 0.03) ** | 0.03 (0.01, 0.04) ** | 1.07 (1.01, 1.16) * |
| Charlson score | 0.13 (0.06, 0.20) ** | 0.13 (0.03, 0.22) * | 1.91 (1.32, 3.08) * |
| Creatinine | 0.02 (0.00, 0.04) * | 0.03 (0.00, 0.05) * | 1.15 (1.03, 1.35) * |
| CPR | 0.45 (0.13, 0.78) * | 0.33 (−0.09, 0.76) | 1.05 (0.18, 3.67) |
| LDH | 0.02 (0.00, 0.05) * | 0.03 (0.00, 0.05) | 1.05 (0.96, 1.15) |
| P/F ratio | −0.29 (−0.52, −0.01) * | −0.47 (−0.79, −0.15) * | 0.69 (0.24, 1.84) |
| RR | 0.21 (0.05, 0.36) * | 0.24 (0.03, 0.45) * | 1.33 (0.66, 2.64) |
| Thrombomodulin | 0.09 (0.01, 0.16) * | 0.13 (0.03, 0.22) * | 1.44 (1.08, 2.08) * |
| SC5b9 | 0.06 (0.01, 0.10) * | 0.02 (−0.04, 0.09) | 0.93 (0.66, 1.13) |
| D-dimer | 0.03 (0.00, 0.06) * | 0.05 (0.01, 0.10) * | 1.09 (0.96, 1.23) |
| VCAM-1 | 0.02 (−0.01, 0.05) | 0.05 (0.01, 0.09) * | 1.31 (1.12, 1.63) * |
| VWF:Ag | −0.01 (−0.21, 0.19) | 0.06 (−0.24, 0.36) | 1.53 (0.72, 2.98) |
| ICAM-1 | 0.12 (−0.04, 0.27) | 0.22 (−0.01, 0.46) | 0.94 (0.42, 1.79) |
| PAI-1 | 0.00 (−0.01, 0.00) | 0.00 (−0.01, 0.01) | 0.96 (0.80, 1.01) |
| FVIII | 0.01 (−0.02, 0.04) | 0.01 (−0.03, 0.06) | 0.89 (0.73, 1.04) |
| C5a | 0.02 (−0.01, 0.05) | 0.01 (−0.04, 0.05) | 1.02 (0.90, 1.17) |
| tPA | 0.03 (0.00, 0.05) | 0.03 (0.00, 0.07) | 1.06 (0.94, 1.19) |
| Length of Stay °° | Time to Oxygen Support Weaning °° | Death ° | |
|---|---|---|---|
| Vetrugno LUS score | 0.00 (−0.02, 0.03) | 0.00 (−0.03, 0.04) | 1.19 (0.95, 1.66) |
| P/F ratio | Not included | −0.47 (−0.85, −0.09) * | Not included |
| RR | 0.32 (0.14, 0.51) ** | 0.23 (0.01, 0.45) * | Not included |
| VCAM-1 | Not included | Not included | 1.31 (1.06, 1.81) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gualtierotti, R.; Tafuri, F.; Rossio, R.; Rota, M.; Bucciarelli, P.; Ferrari, B.; Giachi, A.; Suffritti, C.; Cugno, M.; Peyvandi, F.; et al. Lung Ultrasound Findings and Endothelial Perturbation in a COVID-19 Low-Intensity Care Unit. J. Clin. Med. 2022, 11, 5425. https://doi.org/10.3390/jcm11185425
Gualtierotti R, Tafuri F, Rossio R, Rota M, Bucciarelli P, Ferrari B, Giachi A, Suffritti C, Cugno M, Peyvandi F, et al. Lung Ultrasound Findings and Endothelial Perturbation in a COVID-19 Low-Intensity Care Unit. Journal of Clinical Medicine. 2022; 11(18):5425. https://doi.org/10.3390/jcm11185425
Chicago/Turabian StyleGualtierotti, Roberta, Francesco Tafuri, Raffaella Rossio, Matteo Rota, Paolo Bucciarelli, Barbara Ferrari, Andrea Giachi, Chiara Suffritti, Massimo Cugno, Flora Peyvandi, and et al. 2022. "Lung Ultrasound Findings and Endothelial Perturbation in a COVID-19 Low-Intensity Care Unit" Journal of Clinical Medicine 11, no. 18: 5425. https://doi.org/10.3390/jcm11185425







