The Role of Silicone Oil in the Surgical Management of Endophthalmitis: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria: Participants, Interventions, Comparators, and Outcomes (PICO)
2.2. Search Strategy
2.3. Risk of Bias and Quality of Evidence Assessment
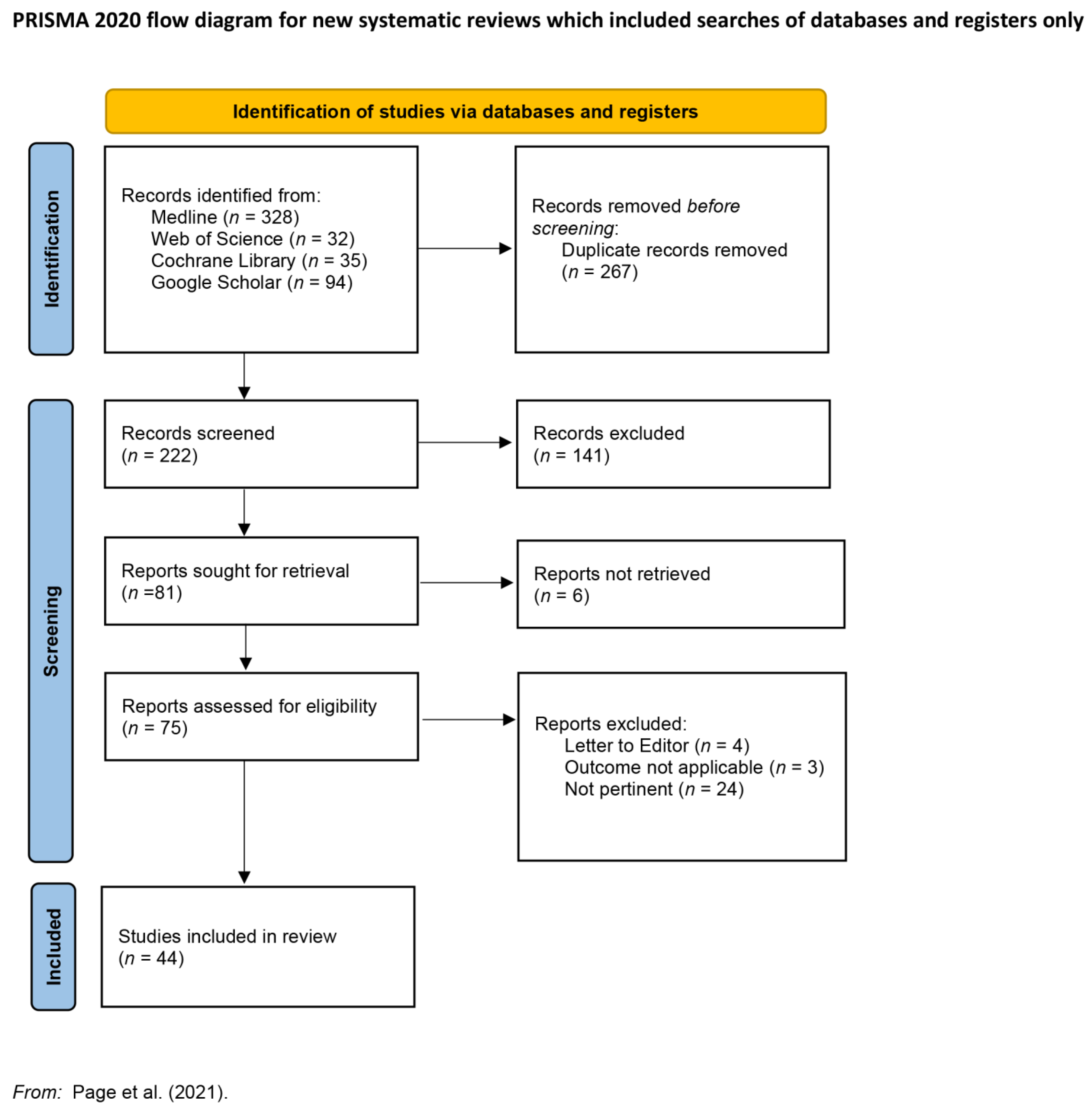
3. Results of Searches
4. Definition
Clinical Features
5. Epidemiology and Causative Agents
6. Therapy
6.1. Medical Therapy
6.1.1. Systemic Antibiotics
6.1.2. Topical Antibiotics
6.1.3. Intravitreal Antibiotics
6.2. Surgical Therapy
7. Antimicrobial Activity of Silicon Oil
8. Anatomical and Visual Outcomes
9. Retinal Detachment and Endophthalmitis
10. Traumatic Endophthalmitis
11. Endogenous Endophthalmitis
12. Endophthalmitis in Silicone Oil Filled Eyes
13. Limitation of Silicone Oil Use
14. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forster, R.K. The Endophthalmitis Vitrectomy Study. Arch. Ophthalmol. 1995, 113, 1555–1557. [Google Scholar] [CrossRef]
- Kuhn, F.; Gini, G. Ten years after… are findings of the Endophthalmitis Vitrectomy Study still relevant today? Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 243, 1197–1199. [Google Scholar] [CrossRef]
- Barry, P.; Cordovés, L.; Gardner, S. ESCRS Guidelines for Prevention and Treatment of Endophthalmitis Following Cataract Surgery: Data, Dilemmas and Conclusions. Available online: http://bmec.swbh.nhs.uk/wp-content/uploads/2013/03/ESCRS-Guidelines-for-use-of-prophylactic-Antibiotics-post-cataract-sugery.pdf (accessed on 14 April 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp (accessed on 15 August 2022).
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Piggott, T.; Morgan, R.L.; Cuello-Garcia, C.A.; Santesso, N.; Mustafa, R.A.; Meerpohl, J.J.; Schünemann, H.J. Grading of Recommendations Assessment, Development, and Evaluations (GRADE) notes: Extremely serious, GRADE’s terminology for rating down by three levels. J. Clin. Epidemiol. 2020, 120, 116–120. [Google Scholar] [CrossRef]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers ‘to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Ozdamar, A.; Aras, C.; Ozturk, R.; Akin, E.; Karacorlu, M.; Ercikan, C. In vitro antimicrobial activity of silicone oil against endophthalmitis-causing agents. Retina 1999, 19, 122–126. [Google Scholar] [CrossRef]
- Arici, C.; Aras, C.; Tokman, H.B.; Torun, M.M. An in Vitro Experimental Study on the Antimicrobial Activity of Silicone Oil against Anaerobic Bacteria. Ocul. Immunol. Inflamm. 2016, 24, 173–177. [Google Scholar] [CrossRef]
- Economou-Stamatelopoulou, C.; Roussopoulos, G.P.; Prouskas, J.C.; Apostolopoulos, M. Antifungal activity of intraocularly used liquids against Aspergillus. Ophthalmologica 2004, 218, 323–327. [Google Scholar] [CrossRef]
- Adams, F.; Romero, I.L.; Silva, C.B.; Manzano, R.P. Evaluation of silicon oil on bacterial growth. Arq. Bras. Oftalmol. 2012, 75, 89–91. [Google Scholar] [CrossRef]
- Ornek, N.; Apan, T.; Oğurel, R.; Ornek, K. Comparison of the antimicrobial effect of heavy silicone oil and conventional silicone oil against endophthalmitis-causing agents. Indian J. Ophthalmol. 2014, 62, 388–391. [Google Scholar] [CrossRef]
- Chrapek, O.; Vecerova, R.; Koukalova, D.; Maresova, K.; Jirkova, B.; Sin, M.; Rehak, J. The in vitro antimicrobial activity of silicone oils used in ophthalmic surgery. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2012, 156, 7–13. [Google Scholar] [CrossRef]
- Dave, V.P.; Joseph, J.; Jayabhasker, P.; Pappuru, R.R.; Pathengay, A.; Das, T. Does ophthalmic-grade silicone oil possess antimicrobial properties? J. Ophthalmic. Inflamm. Infect. 2019, 9, 20. [Google Scholar] [CrossRef]
- Olgun, A.; Imamoğlu, S.; Karapapak, M.; Düzgün, E.; Kaçar, H. Endophthalmitis After XEN Gel Stent Implantation: 2 Cases. J. Glaucoma 2018, 27, e191–e194. [Google Scholar] [CrossRef]
- Baxter, K.R.; Robinson, J.E.; Ruby, A.J. Occlusive vasculitis due to hyperacute Streptococcus mitis endophthalmitis after intravitreal ranibizumab. Retin. Cases Brief Rep. 2015, 9, 201–204. [Google Scholar] [CrossRef]
- Mohd-Ilham, I.; Zulkifli, M.; Yaakub, M.; Muda, R.; Shatriah, I. A Case of a Large Sub-retinal Abscess Secondary to Klebsiella pneumoniae Endophthalmitis in a Pyelonephritis Patient. Cureus 2019, 11, e4656. [Google Scholar] [CrossRef]
- Krėpštė, L.; Žemaitienė, R.; Barzdžiukas, V.; Miliauskas, A. Bilateral endogenous bacterial panophthalmitis. Medicina 2013, 49, 143–147. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Lee, S.U.; Sohn, J.H.; Lee, S.E. Result of early vitrectomy for endogenous Klebsiella pneumoniae endophthalmitis. Retina 2003, 23, 366–370. [Google Scholar] [CrossRef]
- Goel, N.; Bhambhwani, V.; Ghosh, B. Multidrug-resistant Pseudomonas aeruginosa endophthalmitis in a silicone oil-filled eye treated with piperacillin/tazobactam: Report of a case and review of literature. Int. Ophthalmol. 2015, 35, 599–602. [Google Scholar] [CrossRef]
- Siu, G.D.; Lo, E.C.; Young, A. Endogenous endophthalmitis with a visual acuity of 6/6. BMJ Case Rep. 2015, 2015, bcr2014205048. [Google Scholar] [CrossRef]
- Chon, J.; Kim, M. Successful management of late-onset Streptococcus mitis endophthalmitis. Ther. Clin. Risk Manag. 2017, 13, 1439–1442. [Google Scholar] [CrossRef]
- Mackiewicz, J.; Haszcz, D.; Zagórski, Z. Exogenous Candida endophthalmitis in a hop grower—A case report. Ann. Agric. Environ. Med. 2000, 7, 131–132. [Google Scholar]
- Won, J.Y.; Kim, M. Vancomycin-resistant Staphylococcus hominis endophthalmitis following cataract surgery. Clin. Ophthalmol. 2013, 7, 1193–1195. [Google Scholar] [CrossRef]
- Okonkwo, O.N.; Hassan, A.O.; Oderinlo, O.; Gyasi, M.E. Burkholderia cepacia, a cause of post pars plana vitrectomy silicone oil related endophthalmitis: Clinico-pathological presentation and outcome of management. Int. J. Retina Vitr. 2018, 4, 35. [Google Scholar] [CrossRef]
- Steinmetz, R.L.; Vyas, S.; Ashmore, E.; Brooks, H.L. Acute-Onset Postoperative Endophthalmitis in Silicone Oil–Filled Eyes Managed With Intravitreal Antibiotics Alone. J. Vitr. Dis. 2018, 2, 107–110. [Google Scholar] [CrossRef]
- Tayyib, M. Endophthalmitis After Vitrectomy An Silicone Oil Implant. Ann. King Edw. Med. Univ. 1997, 3, 60–61. [Google Scholar]
- Dave, V.P.; Pathengay, A.; Relhan, N.; Sharma, P.; Jalali, S.; Pappuru, R.R.; Tyagi, M.; Narayanan, R.; Chhablani, J.; Das, T.; et al. Endophthalmitis and Concurrent or Delayed-Onset Rhegmatogenous Retinal Detachment Managed With Pars Plana Vitrectomy, Intravitreal Antibiotics, and Silicone Oil. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 546–551. [Google Scholar] [CrossRef]
- Hudieb, A. Early pars plana vitrectomy and silicon oil Endotamponade in the treatment of Acute infectious Endophthalmitis. Azhar Assyot Med. J. 2012, 10, 3. [Google Scholar]
- Aras, C.; Ozdamar, A.; Karacorlu, M.; Ozkan, S. Silicone oil in the surgical treatment of endophthalmitis associated with retinal detachment. Int. Ophthalmol. 2001, 24, 147–150. [Google Scholar] [CrossRef]
- Bali, E.; Huyghe, P.; Caspers, L.; Libert, J. Vitrectomy and silicone oil in the treatment of acute endophthalmitis. Preliminary results. Bull. Soc. Belge Ophtalmol. 2003, 288, 9–14. [Google Scholar]
- Pinarci, E.Y.; Yesilirmak, N.; Bayar, S.A.; Sizmaz, S.; Akkoyun, I.; Yilmaz, G. The results of pars plana vitrectomy and silicone oil tamponade for endophthalmitis after intravitreal injections. Int. Ophthalmol. 2013, 33, 361–365. [Google Scholar] [CrossRef]
- Farouk, M.M.; Mounir, A.; Elagouz, M. The Role of Silicone Oil in Management of Postoperative Endophthalmitis. Sohag Med. J. 2017, 21, 575–581. [Google Scholar] [CrossRef]
- Yan, H.; Lu, Y.; Yu, J.; Han, J.; Zhang, J.; Chen, S.; Xu, Y. Silicone oil in the surgical treatment of traumatic endophthalmitis. Eur. J. Ophthalmol. 2008, 18, 680–684. [Google Scholar] [CrossRef]
- Verma, L.; Chakravarti, A. Prevention and management of postoperative endophthalmitis: A case-based approach. Indian J. Ophthalmol. 2017, 65, 1396–1402. [Google Scholar] [CrossRef]
- Cakir, M.; Imamoğlu, S.; Cekiç, O.; Bozkurt, E.; Alagöz, N.; Oksüz, L.; Yilmaz, O.F. An outbreak of early-onset endophthalmitis caused by Fusarium species following cataract surgery. Curr. Eye Res. 2009, 34, 988–995. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, J.; Wang, R.; Lei, J.; Zhou, Y. Visual Outcomes and Prognostic Factors after Pars Plana Vitrectomy for Traumatic Endophthalmitis. Biomed. Res. Int. 2017, 2017, 5851318. [Google Scholar] [CrossRef]
- Jin, W.; Xu, Y.; Wang, W.; Xing, Y.; Yang, A. Efficacy and Safety of 23-Gauge Pars Plana Vitrectomy/Silicone Oil Tamponade Combination for Treatment of Pediatric Post-Traumatic Endophthalmitis. Curr. Eye Res. 2017, 42, 1143–1148. [Google Scholar] [CrossRef]
- Zhang, J.; Han, F.; Zhai, X. Clinical analysis of 23-gauge vitrectomy for the treatment of acute endophthalmitis after cataract surgery. Eur. J. Ophthalmol. 2015, 25, 503–506. [Google Scholar] [CrossRef]
- Yospaiboon, Y.; Intarapanich, A.; Laovirojjanakul, W.; Ratanapakorn, T.; Sinawat, S.; Sanguansak, T.; Bhoomibunchoo, C. Factors affecting visual outcomes after treatment of infectious endophthalmitis in northeastern Thailand. Clin. Ophthalmol. 2018, 12, 765–772. [Google Scholar] [CrossRef]
- Yospaiboon, Y.; Meethongkam, K.; Sinawat, S.; Laovirojjanakul, W.; Ratanapakorn, T.; Sanguansak, T.; Bhoomibunchoo, C. Predictive factors in the treatment of streptococcal endophthalmitis. Clin. Ophthalmol. 2018, 12, 859–864. [Google Scholar] [CrossRef]
- Nagpal, M.; Jain, P.; Nagpal, K. Pars Plana Vitrectomy With or Without Silicone Oil Endotamponade in Surgical Management of Endophthalmitis. Asia Pac. J. Ophthalmol. 2012, 1, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Azad, R.; Ravi, K.; Talwar, D.; Rajpal; Kumar, N. Pars plana vitrectomy with or without silicone oil endotamponade in post-traumatic endophthalmitis. Graefes Arch. Clin. Exp. Ophthalmol. 2003, 241, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Hon, D.N.; Aung, T.; Hien, N.D.; Cowan, C.L., Jr. Bacterial endogenous endophthalmitis in Vietnam: A randomized controlled trial comparing vitrectomy with silicone oil versus vitrectomy alone. Clin. Ophthalmol. 2014, 8, 1633–1640. [Google Scholar] [CrossRef]
- Khaqan, H.A.; Imtiaz, U.; Buksh, H.M.; REHMAN, H.A.; Raheela, N.J.C.; Trauma, E.O. Outcomes of Early Pars Plana Vitrectomy for Acute Post Operative Endophthalmitis with or without Silicone Oil. Clin. Exp. Ocul. Trauma Infect. 2017, 2, 56–60. [Google Scholar]
- Kapoor, K.G.; Khurshid, G.S.J.I.O.; Science, V. Silicone Oil As An Adjunct In The Surgical Management Of Endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2619. [Google Scholar]
- Kaynak, S.; Oner, F.H.; Koçak, N.; Cingil, G. Surgical management of postoperative endophthalmitis: Comparison of 2 techniques. J. Cataract Refract. Surg. 2003, 29, 966–969. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Gil, A.D.; Canamary, F.; Minari, M.; Jorge, R. Pars plana vitrectomy and silicone oil tamponade for acute endophthalmitis treatment. Arq. Bras. Oftalmol. 2009, 72, 28–32. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.L.; Wang, Y.; Chen, S.J.; Huang, X.Y.; Wu, N.; Ying, X. Endotamponades in pars plana vitrectomy for metallic intraocular foreign body associated with endophthalmitis. Int. J. Ophthalmol. 2011, 4, 95–99. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Wang, Y.X.; Yao, T.T.; Yang, Y.; Wang, Z.Y. Traumatic endophthalmitis and the outcome after vitrectomy in oung children. Int. J. Ophthalmol. 2020, 13, 406–411. [Google Scholar] [CrossRef]
- Lin, H.; Ling, S.; Liu, Z.; Zhong, X.; Chen, W. Preventive scleral buckling and silicone oil tamponade are important for posttraumatic endophthalmitis successfully managed with vitrectomy. Ophthalmologica 2011, 226, 214–219. [Google Scholar] [CrossRef]
- Patel, A.; Gentile, R. Pars Plana Vitrectomy With or Without Silicone Oil Endotamponade in Surgical Management of Endophthalmitis. Asia Pac. J. Ophthalmol. 2012, 1, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.L. Bacterial and Fungal Endophthalmitis. Clin. Microbiol. Rev. 2017, 30, 597–613. [Google Scholar] [CrossRef]
- Kresloff, M.S.; Castellarin, A.A.; Zarbin, M.A. Endophthalmitis. Surv. Ophthalmol. 1998, 43, 193–224. [Google Scholar] [CrossRef]
- Du, D.T.; Wagoner, A.; Barone, S.B.; Zinderman, C.E.; Kelman, J.; MaCurdy, T.; Forshee, R.A.; Worrall, C.; Izurieta, H.S.J.O. Incidence of endophthalmitis after corneal transplant or cataract surgery in a medicare population. Ophthalmology 2014, 121, 290–298. [Google Scholar] [CrossRef]
- Nam, K.Y.; Lee, J.E.; Lee, J.E.; Jeung, W.J.; Park, J.M.; Park, J.M.; Chung, I.Y.; Han, Y.S.; Yun, I.H.; Kim, H.W.; et al. Clinical features of infectious endophthalmitis in South Korea: A five-year multicenter study. BMC Infect. Dis. 2015, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.U.; Flynn, H.W., Jr.; Acar, N.; Dev, S.; Shaikh, S.; Mittra, R.A.; Arevalo, J.F.; Kychenthal, A.; Kunselman, A. Incidence of endophthalmitis after 20-gauge vs 23-gauge vs 25-gauge pars plana vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 377–380. [Google Scholar] [CrossRef]
- Rasul, A.; Subhi, Y.; Sørensen, T.L.; Munch, I.C. Non-physician delivered intravitreal injection service is feasible and safe–a systematic review. Dan. Med. J. 2016, 63, A5229. [Google Scholar]
- Shah, R.E.; Gupta, O. The microsurgical safety task force: Guidelines for minimizing endophthalmitis with vitrectomy surgery. Curr. Opin. Ophthalmol. 2012, 23, 189–194. [Google Scholar] [CrossRef]
- McCannel, C.A. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: Causative organisms and possible prevention strategies. Retina 2011, 31, 654–661. [Google Scholar] [CrossRef]
- Chaudhary, K.M.; Romero, J.M.; Ezon, I.; Fastenberg, D.M.; Deramo, V.A. Pars plana vitrectomy in the management of patients diagnosed with endophthalmitis following intravitreal anti-vascular endothelial growth factor injection. Retina 2013, 33, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Rayess, N.; Rahimy, E.; Shah, C.P.; Wolfe, J.D.; Chen, E.; DeCroos, F.C.; Storey, P.; Garg, S.J.; Hsu, J. Incidence and clinical features of post-injection endophthalmitis according to diagnosis. Br. J. Ophthalmol. 2016, 100, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Storey, P.; Dollin, M.; Pitcher, J.; Reddy, S.; Vojtko, J.; Vander, J.; Hsu, J.; Garg, S.J. The role of topical antibiotic prophylaxis to prevent endophthalmitis after intravitreal injection. Ophthalmology 2014, 121, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.J.; Dollin, M.; Storey, P.; Pitcher, J.D., 3rd; Fang-Yen, N.H.; Vander, J.; Hsu, J. Microbial spectrum and outcomes of endophthalmitis after intravitreal injection versus pars plana vitrectomy. Retina 2016, 36, 351–359. [Google Scholar] [CrossRef]
- Ahmed, Y.; Schimel, A.M.; Pathengay, A.; Colyer, M.H.; Flynn, H.W., Jr. Endophthalmitis following open-globe injuries. Eye 2012, 26, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Simakurthy, S.; Tripathy, K. Endophthalmitis. In StatPearls; StatPearls Publishing Copyright© 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gentile, R.C.; Shukla, S.; Shah, M.; Ritterband, D.C.; Engelbert, M.; Davis, A.; Hu, D.N. Microbiological spectrum and antibiotic sensitivity in endophthalmitis: A 25-year review. Ophthalmology 2014, 121, 1634–1642. [Google Scholar] [CrossRef]
- Clarke, B.; Williamson, T.H.; Gini, G.; Gupta, B. Management of bacterial postoperative endophthalmitis and the role of vitrectomy. Surv. Ophthalmol. 2018, 63, 677–693. [Google Scholar] [CrossRef]
- Lott, M.N.; Fuller, J.J.; Hancock, H.A.; Singh, J.; Singh, H.; McGwin, G., Jr.; Marcus, D.M. Vitreal penetration of oral moxifloxacin in humans. Retina 2008, 28, 473–476. [Google Scholar] [CrossRef]
- Brockhaus, L.; Goldblum, D.; Eggenschwiler, L.; Zimmerli, S.; Marzolini, C. Revisiting systemic treatment of bacterial endophthalmitis: A review of intravitreal penetration of systemic antibiotics. Clin. Microbiol. Infect. 2019, 25, 1364–1369. [Google Scholar] [CrossRef]
- Jackson, T.L.; Eykyn, S.J.; Graham, E.M.; Stanford, M.R. Endogenous bacterial endophthalmitis: A 17-year prospective series and review of 267 reported cases. Surv. Ophthalmol. 2003, 48, 403–423. [Google Scholar] [CrossRef]
- Radhika, M.; Mithal, K.; Bawdekar, A.; Dave, V.; Jindal, A.; Relhan, N.; Albini, T.; Pathengay, A.; Flynn, H.W. Pharmacokinetics of intravitreal antibiotics in endophthalmitis. J. Ophthalmic Inflamm. Infect. 2014, 4, 22. [Google Scholar] [CrossRef]
- Reddy, A.K.; Reddy, R.R.; Paruvelli, M.R.; Ambatipudi, S.; Rani, A.; Lodhi, S.A.; Reddy, J.M.; Reddy, K.R.; Pandey, N.; Videkar, R.; et al. Susceptibility of bacterial isolates to vancomycin and ceftazidime from patients with endophthalmitis: Is there a need to change the empirical therapy in suspected bacterial endophthalmitis? Int. Ophthalmol. 2015, 35, 37–42. [Google Scholar] [CrossRef]
- Endophthalmitis Vitrectomy Study Group. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch. Ophthalmol. 1995, 113, 1479–1496. [Google Scholar] [CrossRef]
- Giordano, G.G.; Refojo, M.F. Silicone oils as vitreous substitutes. Prog. Polym. Sci. 1998, 23, 509–532. [Google Scholar] [CrossRef]
- Thomas, B.J.; Yonekawa, Y.; Ruby, A.J.; Capone, A., Jr. Aggressive Surgical Therapy With Early Vitrectomy, Panretinal Photocoagulation, and Silicone Oil Tamponade for Streptococcus mitis Endophthalmitis. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 893–895. [Google Scholar] [CrossRef]
- Suganeswari, G.; Shah, D.; Anand, A.R. Intravitreal piperacillin-tazobactam in endophthalmitis caused by Mycobacterium abscessus in silico ne-filled eye: A case report. Indian J. Ophthalmol. 2020, 68, 1471–1473. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, K.D.; Flynn, H.W., Jr.; Alfonso, E.C.; Miller, D. Fusarium endophthalmitis following keratitis associated with contact lenses. Ophthalmic Surg. Lasers Imaging 2006, 37, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, N.; Dong, X.G.; Yuan, G.Q.; Yu, B.; Xie, L.X. Surgical management of fungal endophthalmitis resulting from fungal keratitis. Int. J. Ophthalmol. 2016, 9, 848–853. [Google Scholar] [CrossRef]
- Abou Shousha, M.; Eleiwa, T.; Gibbons, A.; Smith, C.; Edelstein, S.; Kontadakis, G.; Schmitz, Z.; Abernathy, J.; Chod, R.; Bodnar, Z.; et al. Risk of Endophthalmitis in Boston Type 1 Keratoprosthesis Combined with Vitrectomy and Silicone Oil Insertion. J. Ophthalmol. 2019, 2019, 9648614. [Google Scholar] [CrossRef]
- Nelsen, P.T.; Marcus, D.A.; Bovino, J.A. Retinal detachment following endophthalmitis. Ophthalmology 1985, 92, 1112–1117. [Google Scholar] [CrossRef]
- Doft, B.M.; Kelsey, S.F.; Wisniewski, S.R. Retinal detachment in the endophthalmitis vitrectomy study. Arch. Ophthalmol. 2000, 118, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.E.; Rubsamen, P.E.; Joondeph, B.C.; Flynn, H.W., Jr.; Smiddy, W.S. Concurrent endophthalmitis and retinal detachment. Ophthalmology 1994, 101, 490–498. [Google Scholar] [CrossRef]
- Olson, J.C.; Flynn, H.W., Jr.; Forster, R.K.; Culbertson, W.W. Results in the treatment of postoperative endophthalmitis. Ophthalmology 1983, 90, 692–699. [Google Scholar] [CrossRef]
- Lu, X.; Xia, H.; Jin, C.; Chen, W.; Siu-Chun Ng, D.; Yan, H.; Chen, H. Prognostic factors associated with visual outcome of salvageable eyes with posttraumatic endophthalmitis. Sci. Rep. 2019, 9, 12678. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.P.; de Juan, E., Jr.; McCuen, B.W., 2nd; Landers, M.B., 3rd. Endophthalmitis in a silicone oil-filled eye. Am. J. Ophthalmol. 1986, 102, 660–661. [Google Scholar] [CrossRef]
- Al Taisan, A.; Semidey, V.A. Culture-Positive Acute Postvitrectomy Endophthalmitis in a Silicone Oil-Filled Eye. Retin Cases Brief Rep. 2022, 16, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Zimmer-Galler, I.E.; Santos, A.; Haller, J.A.; Campochiaro, P.A. Management of endophthalmitis in a silicone oil-filled eye. Retina 1997, 17, 507–509. [Google Scholar] [CrossRef]
- Dogra, M.; Bhutani, G.; Gupta, V. Mucormycosis Endophthalmitis in a Silicone Oil-Filled Eye of an Immunocompetent Patient. Ocul. Immunol. Inflamm. 2019, 27, 1293–1295. [Google Scholar] [CrossRef]
- Nakamura, K.; Refojo, M.F.; Crabtree, D.V.; Pastor, J.; Leong, F.L. Ocular toxicity of low-molecular-weight components of silicone and fluorosilicone oils. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3007–3020. [Google Scholar]
- Conway, M.D.; Jermak, C.M.; Peyman, G.A.; Swanson, H.T.; Blake, D.A. Buffering capacity of bovine vitreous. Retina 2008, 28, 150–153. [Google Scholar] [CrossRef]
- Nowroozzadeh, M. The equivocal role of silicone oil in the treatment of traumatic endophthalmitis. Eur. J. Ophthalmol. 2009, 19, 496–497, author reply 497–499. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, H.M.; Kivilcim, M.; Peyman, G.A.; Unal, M.H.; Liang, C.; Molinari, L.C.; Kazi, A.A. Evaluation of toxicity of intravitreal ceftazidime, vancomycin, and ganciclovir in a silicone oil-filled eye. Retina 1999, 19, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Aras, C.; Yolar, M.; Sevim, O. Surgical management of postoperative endophthalmitis. J. Cataract Refract. Surg. 2004, 30, 1612. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Study Design | Silicone Oil | Purpose | Pathogens Studied | Outcomes | Antimicrobial Activity |
|---|---|---|---|---|---|---|---|
| Ozdamar et al. [9] | 1999 | In vitro experimental study | 1300 cSt | Compared Microorganism growth in saline, growth medium and silicone oil medium to analyze SO antimicrobial activity | S. aureus, S. epidermidids, P. aeruginosa, C. albicans and Asperigillus spp. | CFU number | YES (Silicone oil showed antimicrobial activity against S. aureus, S. epidermidids, P. aeruginosa, C. albicans and Asperigillus spp.) |
| Arici et al. [10] | 2016 | In vitro experimental study | 1300 cSt | To investigate the in vitro antimicrobial activity of silicone oil against anaerobic agents | Propionibacterium acnes, Peptostreptococcus spp., Peptostreptococcus anaerobius, Bacteroides fragilis, Fusobacterium spp., and Clostridium tertium | CFU number | NO (Propionibacterium acnes showed resistance against the antimicrobial effect of silicone oil) |
| Economou-Stamatelopoulou et al. [11] | 2004 | In vitro experimental study | 1000 cS and 5000 cSt | To investigate the in vitro antifungal activity of silicone oil | Aspergillus spp. | CFU number | YES (SO and PFCL conform to standards, effective against Asperigillus spp.) |
| Adams et al. [12] | 2012 | in vitro experimental study | 1000 cSt | To verify the effect of the silicon oil on in vitro bacterial growth of selected microorganisms. | Pseudomonas aeruginosa; Escherichia coli; Staphylococcus aureus; Staphylococcus epidermidis; Candida albicans; Klebsiella pneumoniae; Streptococcus pneumoniae | Inhibition halos, CFU | NO (The silicon oil 1000 cps does not have any effect on the bacterial growth of any of the studied microorganisms. Pseudomonas aeruginosa; Escherichia coli; Staphylococcus aureus; Staphylococcus epidermidis; Candida albicans; Klebsiella pneumoniae; Streptococcus pneumoniae) |
| Ornek et al. [13] | 2014 | In vitro experimental study | conventional silicone oil (RS OIL 5000) and heavy silicone oil (heavy Sil 1500) | To compare the effectiveness of conventional silicone oil and heavy silicone oil against endophthalmitis-causing agents | S. aureus, S.epidermidis, E. coli, P. aeruginosa, and C. albican | CFU number | YES (Conventional silicone oil decreased the colony numbers of all bacteria except for C. albicans, but heavy silicone oil demonstrated a superior antimicrobial effect on all pathogens including C. albicans) |
| Chrapek et al. [14] | 2012 | In vitro experimental study | Arciolane 1300 centistokes, Arciolane 5500 centistokes and Oxane Hd, heavy silicone oil | To investigate and compare the antimicrobial activity of three types of silicone oils used in ophthalmic surgery (Arciolane 1300 centistokes, Arciolane 5500 centistokes and Oxane Hd, heavy silicone oil) | Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Bacillus sp., Pseudomonas aeruginosa, Candida albicans and Aspergillus fumigatus | CFU number | YES (The Oxane Hd silicone oil exhibited the highest antimicrobial activity, both antibacterial and antifungal. It inhibited the growth activity of all inoculated bacteria, albeit after various times; after 14 days, it acted upon candida and aspergilli as well) |
| Dave et al. [15] | 2019 | In vitro experimental study | Aurosil 1000 cSt, Aurosil Plus 5000 cSt | To test the antimicrobial properties of silicon oil (Aurosil 1000 cst, Aurosil Plus 5000 cst) on in vitro growth of common microorganisms causing endophthalmitis | Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, MDR strain of Klebsiella pneumoniae, Escherichia coli, Candida albicans, and Aspergillus flavus | CFU number | YES (For 1000 cSt SO, complete bactericidal action was noted by day 21 of exposure, and for the 5000 cSt SO, it was noted by day 30. Both the oils show a similar fungistatic action) |
| Okonkwo et al. [26] | 2018 | Case series | 5000 cSt | To report the long-term outcome of the management of a series of culture-proven post pars plana vitrectomy endophthalmitis in which the infective agent was in the silicone oil used as an endotamponade | Burkholderia cepacia | BCVA e and Anatomical outcomes | NO (Gram-negative bacilli can colonize silicone oil resulting in post pars plana vitrectomy endophthalmitis) |
| Steinmetz et al. [27] | 2018 | Case series | 5000 cSt | to describe 2 cases of endophthalmitis successfully treated with an office injection of intravitreal antibiotics. | Not Applicable | BCVA, IOP, Anatomical outcomes | NO Infectious endophthalmitis in silicone oil-filled eyes. |
| Tayyib et al. [28] | 1997 | Case series | Not reported | To assess the onset of sterile endophthalmitis in silicone oil-filled eyes | Not Applicable | Postoperative complication rate, incidence of endophthalmitis after vitrectomy and silicone oil tamponade | YES (Silicone oil showed antimicrobial activity against S. Aureus, S. Epidermidids, P. aeruginosa, C. albicans and Asperigillus spp.) |
| Goel et al. [21] | 2015 | Case report | 1000 cSt | to report the first case of multidrug-resistant endophthalmitis following pars plana vitrectomy in a silicone oil-filled eye | Pseudomonas aeruginosa | At 1 month there was an attached retina and resolved periphlebitis | NO endophthalmitis in silicone filled eyes (Pseudopmonas). Silicone oil may be an impediment to intravitreal delivery of antibiotics, as it may result in dangerously high concentrations. |
| Author | Year | Study Design | Sample | Groups | Purpose | Anatomical Outcomes | Visual Outcomes |
|---|---|---|---|---|---|---|---|
| Dave et al. [29] | 2017 | Case Series | 93 | Group 1 (concurrent RD), Group 2 (Delayed RD). | To analyze the treatment outcomes in patients with endophthalmitis and concurrent or delayed-onset retinal detachment managed with pars plana vitrectomy, intravitreal antibiotics, and silicone oil. | The complete retinal reattachment rate was 73.7% in Group 1 and 98.5% in Group 2. | Visual acuity of 20/400 or better rate was 30.0% in Group 1 and 39.7% in Group 2 |
| Hudieb et al. [30] | 2012 | Case series | 34 | Groups 1, 2 and 3 were treated initially without silicone oil, Group 4 was treated initially with silicone oil. | To evaluate the role and outcomes of PPV and oil injection in the treatment of infectious endophthalmitis. | In 22 patients (groups 1, 2 and 3) (55%) who needed further surgery, either for persistent infection or retinal detachment 12 patients (group 4) treated at first with silicone oil had a rapid control of the infectious process and better anatomical results with this procedure only. | Final visual acuity was better in the silicone oil groups (groups 3 and 4) than in the non-silicone groups (groups 1 and 2). |
| Olgun et al. [16] | 2018 | Case Report | 2 | none | To report cases of Endophthalmitis after Xen Gel stent implantation treated with PPV and SOI | Infection control and attached retina were obtained in both cases. | Final visual acuity was hand motion in Case 1 and 20/400 in Case 2 |
| Baxter et al. [17] | 2015 | Case Report | 1 | none | To report a case of S. Mitis endophthalmitis treated first with PPV and IOAB, then SOI. | At postoperative Month 1 attached retina with improved retinal perfusion on fluorescein angiography was observed. | At postoperative Month 1 BCVA was 20/200 |
| Aras et al. [31] | 2001 | Case Series | 6 | none | To investigate the use of silicone oil in patients who had undergone vitrectomy for the treatment of endophthalmitis associated with retinal detachment | Final retinal reattachment and treatment of endophthalmitis were achieved in 5 eyes at the end of follow-up | Final visual acuity was 20/40 in 1 case, counting fingers in 4 cases and no light perception in 1 case. |
| Bali et al. [32] | 2003 | Case Series | 34 | (group 1), PPV + IOAB. (group 2) PPV + IOAB; PPV (group 3) PPV + IOAB; PPV + IOAB + SOI. (group 4), PPV + IOAB + SOI | To evaluate the role of PPV and silicone oil injection in the treatment strategy of severe endophthalmitis. | In 22 patients (groups 1, 2 and 3) (55%) who needed further surgery, either for persistent infection or retinal detachment 12 patients (group 4) treated at first with silicone oil had a rapid control of the infectious process and better anatomical results with this procedure only. | Final visual acuity was better in the silicone oil groups (groups 3 and 4) than in the non-silicone groups (groups 1 and 2). |
| Pinarci et al. [33] | 2013 | Case Series | 8 | none | To report the role of early vitrectomy and silicone oil tamponade in acute endophthalmitis following intravitreal injection. | There was no retinal detachment or phthisis bulbi during the follow-up period (1–4 years). | BCVA at final follow-up was 0.05 in two patients (25%), 0.1 in three patients (37.5%), 0.3 in two patients (25%), and 0.8 in one patient (12%). |
| Farouk et al. [34] | 2017 | Case Series | 26 | Group 1 in which vitrectomy was done without silicon oil and group 2 in which vitrectomy was done with silicon oil. All cases were followed for 6 months. | To evaluate the outcomes of vitrectomy with and without silicone oil injection for the treatment of infectious endophthalmitis after cataract surgery when the retina is severely affected. | Four cases (19%) suffered from the persistence of infection after vitrectomy and 3 cases (14.2%) had a postoperative retinal detachment in group 1. These complications were not reported in any case of group 2. | In Group 1 the mean logMAR visual acuity was 2.09 ± 0.82, in Group 2 the mean logMAR visual acuity was 2.04 ± 0.75. |
| Yan et al. [35] | 2008 | Case Series | 18 | none | To explore the effects of vitrectomy combined with silicone oil injection in the treatment of traumatic endophthalmitis without retinal detachment and analyze the relative factors. | There was no retinal detachment or ocular atrophy. | The postoperative visual acuity ranged from light perception to 0.8. The visual acuity increased in 15 eyes (83%) and was stable in 3 eyes (17%). |
| Lin et al. [52] | 2011 | Case Series | 62 | Patients were divided into groups according to the VA at presentation. Group A (12 cases; VA = LP) and group B (50 cases; VA > LP). | The authors investigated initial ocular conditions, surgical management and outcomes of PTE patients and analyzed their relationship to find the necessary management for different patients’ conditions. | Initial VA, preventive scleral buckling and silicone oil tamponade may be good predictors of anatomic outcome. | In conclusion, for PTE, 69.4% (43/62) of the eyes with a good final visual outcome (VA >/= HM) were successfully managed with PPV, and six of them underwent silicone oil injection. |
| Khaqan et al. [46] | 2017 | Prospective Study | 112 | In group 1 patients undergoing PPV with endotamponade (silicon oil) Group 2 patients undergoing PPV without endotamponade. | To evaluate the anatomical and functional outcomes of pars plana vitrectomy (PPV) in acute postoperative endophthalmitis with or without endotamponade. | 23 (76.66%) patients out of 30 who underwent PPV only (Group 2) showed retinal detachment within the first four weeks of follow-up, while among 30 patients of Group 1 who underwent PPV with endotamponade, no patient showed retinal detachment in the first four weeks post-operatively. | Seventy-six (92.68%) participants showed improved vision (6/36-6/60) in Group 1 and in Group 2, 07 (23.33%) participants showed improved vision (6/36-6/60). |
| Mohd-Ilham et al. [18] | 2019 | Case Report | 1 | none | To report a case of a large subretinal abscess secondary to Klebsiella pneumoniae endophthalmitis in a pyelonephritis patient. | The patient showed complete regression of the intraocular inflammation and subretinal abscess. | The patient regained her vision on 6/36. |
| Mackiewicz et al. [24] | 2000 | Case Report | 1 | none | the patient was treated with three vitrectomies. During the third vitrectomy, the retinal detachment was repaired with a circumferential buckle and silicone oil tamponade. | If inflammatory changes of the retina are found during surgery, it seems advisable to administer silicone oil as a protection against a detachment of the retina. | During the 2-year observation period, the visual acuity in the present case was 0.1. |
| Kapoor et al. [55] | 2012 | Interventional consecutive retrospective study | 30 | Group 1 (n = 14) PPV + 1000 centistoke silicone oil tamponade for 12 weeks; Group 2 (n = 16) PPV + 1000 centistoke silicone oil tamponade for 24 weeks. | to evaluate the efficacy of early vitrectomy with adjunctive silicone oil to treat endophthalmitis. | Additional surgery was required in 3% (1/30) of the study group. | At 9 months, 73% of all patients (22/30) achieved the best corrected visual acuity (BCVA) of 20/40 or better. |
| Kaynak et al. [48] | 2003 | Retrospective Study | 56 | Group 1 (n = 24) eyes core vitrectomy; Group 2 (n = 28) eyes total PPV, encircling band, silicone tamponade, and endolaser. | To evaluate the results of 2 surgical techniques in eyes with postoperative endophthalmitis. | The number of additional procedures was significantly less, and the rate of surgical success was significantly higher in Group 2 than in Group 1 (p < 0.01). | There was no statistically significant difference between the 2 groups in final visual acuity (p > 0.05). |
| Verma et al. [36] | 2017 | Case Series | 9 | none | Describes nine different real-world scenarios of endophthalmitis responding to intravitreal antibiotics alone and cases requiring intraocular lens removal, radical vitrectomy with hyaloid peeling, base dissection, and silicone oil | Not reported for all patients | Not reported for all patients |
| Krėpštė et al. [19] | 2013 | Case Report | 1 | none | To present a case of meningitis with bilateral endogenous bacterial panophthalmitis in a previously healthy individual. | Right eye was enucleated, two weeks after the removal of silicone oil the left eye suffered hypotony and subsequent phthisis bulbi. | At 13 weeks (BCVA) of the left eye was 0.07, Two weeks after the removal of silicone oil, visual acuity decreased to light perception. |
| Nagpal et al. [43] | 2012 | Prospective Randomized Study | 129 | Group 1 (n = 65) eyes, which underwent vitrectomy alone, were compared with group 2 (n = 64) eyes, in whom complete PPV with SOI was done. | To compare outcomes of pars plana vitrectomy (PPV) with and without silicone oil injection (SOI) in the surgical management of endophthalmitis. | Rate of retinal detachment was 6.2% in group 2 as compared with 25.5% in group 1. Groups 1 and 2 required additional subsequent procedures in 27 eyes (41.54%) and 5 eyes (7.8%), respectively (p < 0.0001). | Mean best corrected visual acuity improvement was 0.867 ± 1.13 and 1.140 ± 0.88 in groups 1 and 2, respectively (p < 0.005). In the posttraumatic subgroup, the difference between groups 1 and 2 in mean change in best corrected visual acuity was statistically significant (0.580 ± 1.10 and 1.132 ± 0.8 respectively, p < 0.05). |
| Cakir et al. [37] | 2009 | Case Series | 8 | none | to review microbiologic and medical records of eight cases of endophthalmitis caused by Fusarium species after cataract surgery. | All patients underwent multiple vitrectomies with silicone oil injections. One patient with corneal involvement underwent evisceration despite a variety of treatments. One patient with unregulated diabetes was pre phthisical without recurrence of infection. The final visual acuity of patients was between light perception and 20/100. | The final visual acuity of patients was between light perception and 20/100. |
| Azad et al. [44] | 2003 | Prospective Randomized Controlled Study | 24 | Group 1: core vitrectomy Group 2: complete vitrectomy with silicone oil endotamponade | to compare core vitrectomy with complete vitrectomy and silicone oil in posttraumatic endophthalmitis. | Four (33.33%) of the 12 patients in group 1 developed retinal detachment following vitrectomy. In group 2 Complete vitrectomy ensures the complete removal of vitreous membranes and prevents fibrous proliferation and tractional detachment. | In group 1, 41.66% of patients (5/12) achieved a useful visual outcome (≥20/400) and only one patient achieved a final visual acuity ≥20/200. In group 2, 75% of patients (9/12) had a visual outcome better than 20/400 (p = 0.07) and 58.3% (7/12) achieved a visual acuity greater than 20/200 (p = 0.02). |
| Siqueira et al. [49] | 2009 | Retrospective Study | 35 | Group 1: intravitreal antibiotic injection, associated with topical and oral antibiotics Group 2: vitrectomy with intravitreal antibiotic injection and silicone oil injection | To evaluate the outcomes of pars plana vitrectomy and silicone oil injection for the treatment of infectious endophthalmitis | Group 1: Six patients (25%) had retinal detachment during the first month of follow-up and also required PPV and SOI. Group 2: 2 patients (n = 11), all of them had controlled infection on the first procedure. In one case (9.09%), a severe proliferative vitreoretinopathy (PVR) induced loss of vision (NLP). | Group 1: Nine patients (37.5%) had worsening visual acuity, 10 patients (41.6%) improved and 5 patients (20.83%) did not change. Group 2: One patient (9.09%) showed worsening visual acuity, 5 patients (45.45%) improved and 5 patients (45.45%) did not change. |
| Wang et al. [50] | 2011 | Retrospective Study | 36 | Group 1: 4 eyes without obvious retinal damage with BSS. Group 2: Sixteen eyes that had mild retinal damage filled with C3F8 gas. Group 3: 16 eyes with serious retinal damage were treated with silicone oil. | To study the criterion-reference of endotamponade in pars plana vitrectomy for the metallic intraocular foreign body (MIOFD) associated with endophthalmitis | Group 1: There was no postoperative complication. Group 2: Only 2 cases occurred with postoperative retinal detachment. Group 3: higher incidence of postoperative complications (18.8% retinal detachment, 25% ocular Hypertension, 31.3% needed secondary surgical treatment). | Group1: The visual acuity (VA) was improved Group 2: postoperative VA improved in 10 eyes (62.5%), 4 eyes (25.0%) remained unchanged and 2 eyes (12.5%) decreased. Group 3: Postoperative VA of 9 eyes (56.3%) improved, 3 eyes (18.8%) remained unchanged and 4 eyes (25.0%) decreased. |
| Do et al. [45] | 2014 | Randomized Controlled Trial | 108 | Group 1 (53) standard PPV + IVT antibiotics. Group 2 (55) standard PPV + IVT antibiotics + silicone oil. | to compare treatment outcomes with and without silicone oil tamponade in patients undergoing (PPV for severe BEE. | The anatomical result in Group 2 also had a trend of being better (Group 1, 64.2% versus Group 2, 80%; p = 0.07). | The rate of VA improvement ≥ over baseline tended to be better in Group 2, 40% versus 22.6% in Group 1 (p = 0.0521). |
| Jiang et al. [38] | 2017 | Case Series | 121 | none | To evaluate visual outcomes and identify prognostic factors after PPV surgery for traumatic endophthalmitis. | none | The use of silicone oil tamponade was not a significant factor resulting in better BCVA. |
| Jin et al. [39] | 2017 | Case Series | 107 | none | to determine visual and anatomical outcomes of pediatric patients with posttraumatic endophthalmitis following 23 gauge PPV combined with silicone oil endotamponade | Anatomical recovery 91.59% Hypotony 0% Silicone oil sustained eyes 1.87% Silicone oil, low IOP 2.8% Atrophy 0.93% Uncontrolled inflammation 1.87% Evisceration in subsequent procedure 2.8% Evisceration in primary procedure 0%. | BCVAs were not only favourable, but also often better than those predicted by OTS. |
| Lu et al. [56] | 2019 | Retrospective Study | 98 | Group 1: only IV antibiotics (38). Group 2: IV antibiotics + PPV (30). Group 3: IV antibiotics + PPV + SO (27). Group 4: intracameral antibiotics (3). | to evaluate the prognostic factors associated with visual outcomes in the salvageable eyes with post-traumatic endophthalmitis between 2008 and 2015. | The silicone oil group had fewer repeated intravitreal injections than the group without oil tamponade. | The number of intravitreal injections was independently associated with poor visual outcomes. |
| Won et al. [25] | 2013 | Case Report | 1 | none | to report a case of acute postoperative endophthalmitis caused by vancomycin-resistant Staphylococcus hominis, treated with intraocular lens removal, and silicone oil tamponade. | At 3 months, the retina was attached. | At 3 months, the visual acuity of the silicone oil-treated eye was 20/400. |
| Siu et al. [22] | 2015 | Case Report | 1 | none | to report a case of endogenous endophthalmitis from K. pneumoniae. | At 3 months retinal re-detachment and reoperation. | At three months after the second operation BCVA 6/60. |
| Zhang et al. [40] | 2015 | Case Series | 21 | none | to evaluate the surgical efficacy and timing of 23-G vitrectomy for acute endophthalmitis following cataract surgery, and to determine when silicone oil tamponade and intraocular lens (IOL) removal are indicated during vitrectomy for endophthalmitis. | In all patients, the surgery resolved the endophthalmitis, one patient experienced a recurrence of endophthalmitis 2 months after vitrectomy. Two patients required primary silicone oil tamponade. In 2 other patients, retinal detachment occurred with subsequent vitrectomy and silicone oil tamponade. | Two patients (9.5%) had BCVA > 0.05 before treatment, 14 patients (66.7%) overall had BCVA > 0.05 after treatment. The difference was significant (χ2 = 15.003, p = 0.002). |
| Chon et al. [23] | 2017 | Case Report | 1 | none | To report successful management of late-onset Streptococcus mitis endophthalmitis treated by vitrectomy, panretinal photocoagulation (PRP) and silicone oil tamponade | One month after the surgery, intraocular inflammation was stabilized. | visual acuity was improved from light perception to 20/200. |
| Yospaiboon et al. [41] | 2018 | Case Series | 417 | none | To determine factors affecting visual outcomes after treatment of infectious endophthalmitis. Methods of treatment were medical treatment (18.71%) and surgical treatment (81.29%), including pars plana vitrectomy with or without silicone oil tamponade (62.59%) and destructive surgery (18.71%). | none | After treatment, visual improvement was noted in 44.6%, stable vision in 18.47%, and worse vision in 36.93%. Factors associated with improved visual outcomes were types of endophthalmitis, causative organisms, and initial visual acuity before treatment. |
| Yospaiboon et al. [42] | 2018 | Case Series | 45 | none | To evaluate visual outcomes and possible predictive factors in the treatment of infectious endophthalmitis caused by Streptococcus species. Methods of treatment were medical treatment (18.71%) and surgical treatment (81.29%), including pars plana vitrectomy with or without silicone oil tamponade (62.59%) and destructive surgery (18.71%). | none | Nine patients (20%) had improved vision after treatment. The only predictive factor associated with improved visual outcomes was vitrectomy within 3 days. Medical treatment and PPV with antibiotics demonstrated more improved visual outcomes than PPV with antibiotics and silicone oil tamponade, but the difference was not statistically significant (p = 0.072). |
| Zhou et al. [51] | 2020 | Retrospective Study | 22 | Primary PPV + SO:18 eyes. Primary PPV + C3F8: 4 eyes. | To explore the traumatic endophthalmitis in young children and the outcomes of pars plana vitrectomy | Five patients had retinal detachment (RD) within 3–4 d of initial presentation. Four patients had traction RD after the second PPV, as a complication of surgery. Four patients exhibited band-shaped degeneration of the cornea during follow-up after the third operation. The final IOP was 8.9 ± 1.8 mm Hg. | The final BCVAs were 20/200 or better in five patients, two patients could count fingers, eight patients could detect hand movement, one patient had light perception and one patient had no light perception. Final BCVAs were not available for three patients. Whose (66.7%) had retinal injury exhibited worse BCVA (p = 0.019, Fisher’s exact test). Eyes underwent SO tamponade exhibited worse final BCVA than that with C3F8 in the primary PPV (p = 0.026, Fisher’s exact test). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinisi, F.; Della Santina, M.; Loiudice, P.; Figus, M.; Casini, G. The Role of Silicone Oil in the Surgical Management of Endophthalmitis: A Systematic Review. J. Clin. Med. 2022, 11, 5445. https://doi.org/10.3390/jcm11185445
Sinisi F, Della Santina M, Loiudice P, Figus M, Casini G. The Role of Silicone Oil in the Surgical Management of Endophthalmitis: A Systematic Review. Journal of Clinical Medicine. 2022; 11(18):5445. https://doi.org/10.3390/jcm11185445
Chicago/Turabian StyleSinisi, Fabrizio, Marco Della Santina, Pasquale Loiudice, Michele Figus, and Giamberto Casini. 2022. "The Role of Silicone Oil in the Surgical Management of Endophthalmitis: A Systematic Review" Journal of Clinical Medicine 11, no. 18: 5445. https://doi.org/10.3390/jcm11185445
APA StyleSinisi, F., Della Santina, M., Loiudice, P., Figus, M., & Casini, G. (2022). The Role of Silicone Oil in the Surgical Management of Endophthalmitis: A Systematic Review. Journal of Clinical Medicine, 11(18), 5445. https://doi.org/10.3390/jcm11185445