Functional Investigation of the Tumoural Heterogeneity of Intrahepatic Cholangiocarcinoma by In Vivo PET-CT Navigation: A Proof-of-Concept Study
Abstract
1. Introduction
2. Materials and Methods
2.1. PET-CT Imaging
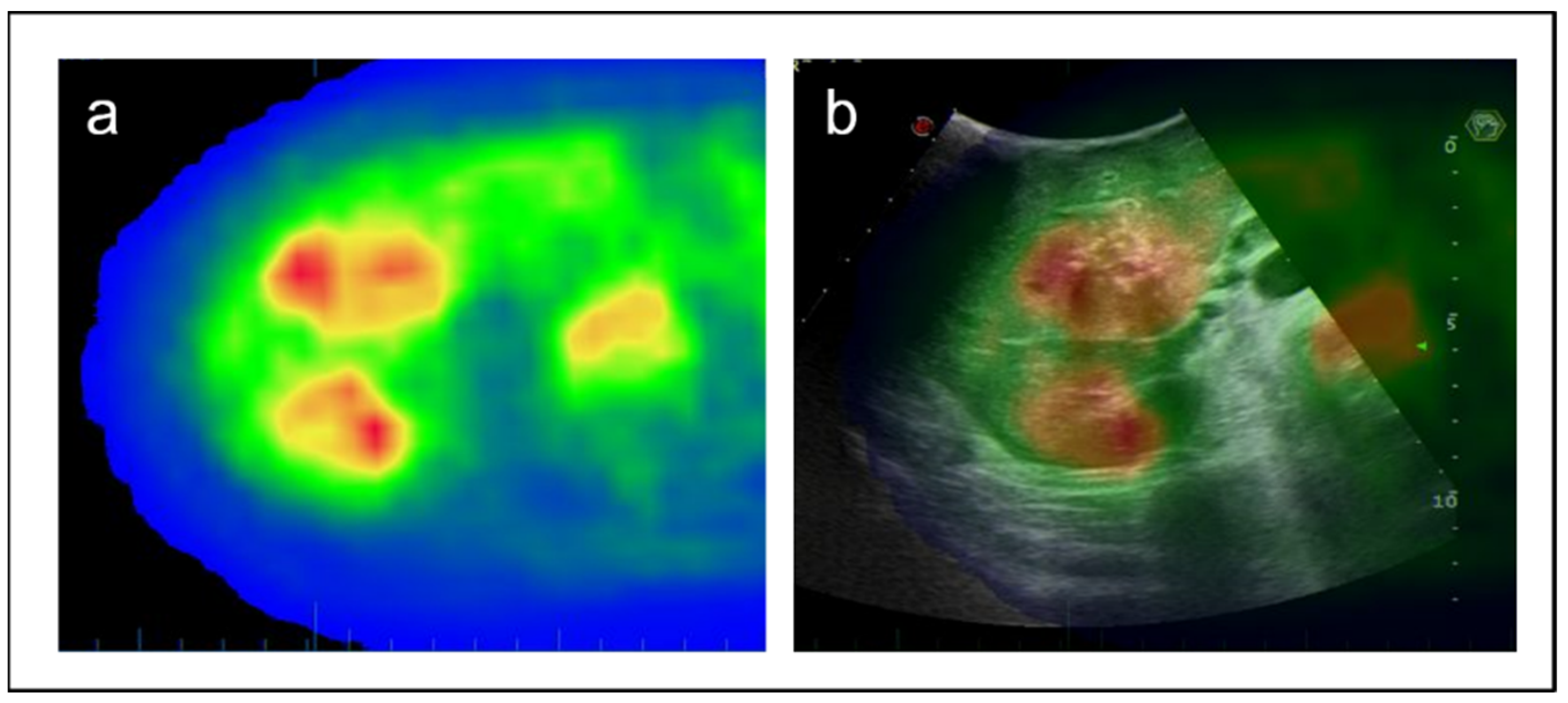
2.2. IOUS and Intraoperative Navigation
2.3. Pathology Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Kreso, A.; O’Brien, C.A.; van Galen, P.; Gan, O.I.; Notta, F.; Brown, A.M.; Ng, K.; Ma, J.; Wienholds, E.; Dunant, C.; et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science 2013, 339, 543–548. [Google Scholar] [CrossRef]
- Laurent-Puig, P.; Pekin, D.; Normand, C.; Kotsopoulos, S.K.; Nizard, P.; Perez-Toralla, K.; Rowell, R.; Olson, J.; Srinivasan, P.; Le Corre, D.; et al. Clinical relevance of KRAS-mutated subclones detected with picodroplet digital PCR in advanced colorectal cancer treated with anti-EGFR therapy. Clin. Cancer Res. 2015, 21, 1087–1097. [Google Scholar] [CrossRef]
- Haberkorn, U.; Ziegler, S.I.; Oberdorfer, F.; Trojan, H.; Haag, D.; Peschke, P.; Berger, M.R.; Altmann, A.; van Kaick, G. FDG uptake, tumor proliferation and expression of glycolysis associated genes in animal tumor models. Nucl. Med. Biol. 1994, 21, 827–834. [Google Scholar] [CrossRef]
- Paudyal, B.; Oriuchi, N.; Paudyal, P.; Higuchi, T.; Nakajima, T.; Endo, K. Expression of glucose transporters and hexokinase II in cholangiocellular carcinoma compared using [18F]-2-fluro-2-deoxy-D-glucose positron emission tomography. Cancer Sci. 2008, 99, 260–266. [Google Scholar] [CrossRef]
- Seo, S.; Hatano, E.; Higashi, T.; Nakajima, A.; Nakamoto, Y.; Tada, M.; Tamaki, N.; Iwaisako, K.; Mori, A.; Doi, R.; et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts lymph node metastasis, P-glycoprotein expression, and recurrence after resection in mass-forming intrahepatic cholangiocarcinoma. Surgery 2008, 143, 769–777. [Google Scholar] [CrossRef]
- Mauri, G.; Gennaro, N.; De Beni, S.; Ierace, T.; Goldberg, S.N.; Rodari, M.; Solbiati, L.A. Real-Time US-(18)FDG-PET/CT Image Fusion for Guidance of Thermal Ablation of (18)FDG-PET-Positive Liver Metastases: The Added Value of Contrast Enhancement. Cardiovasc. Interv. Radiol. 2019, 42, 60–68. [Google Scholar] [CrossRef]
- Hallet, J.; Gayet, B.; Tsung, A.; Wakabayashi, G.; Pessaux, P.; 2nd International Consensus Conference on Laparoscopic Liver Resection group. Systematic review of the use of pre-operative simulation and navigation for hepatectomy: Current status and future perspectives. J. Hepatobiliary Pancreat Sci. 2015, 22, 353–362. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Brandi, G.; Farioli, A.; Astolfi, A.; Biasco, G.; Tavolari, S. Genetic heterogeneity in cholangiocarcinoma: A major challenge for targeted therapies. Oncotarget 2015, 6, 14744–14753. [Google Scholar] [CrossRef]
- Putra, J.; de Abreu, F.B.; Peterson, J.D.; Pipas, J.M.; Mody, K.; Amos, C.I.; Tsongalis, G.J.; Suriawinata, A.A. Molecular profiling of intrahepatic and extrahepatic cholangiocarcinoma using next generation sequencing. Exp. Mol. Pathol. 2015, 99, 240–244. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Limpaiboon, T.; Tapdara, S.; Jearanaikoon, P.; Sripa, B.; Bhudhisawasdi, V. Prognostic significance of microsatellite alterations at 1p36 in cholangiocarcinoma. World J. Gastroenterol. 2006, 12, 4377–4382. [Google Scholar] [CrossRef]
- Kam, A.E.; Masood, A.; Shroff, R.T. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol. Hepatol. 2021, 6, 956–969. [Google Scholar] [CrossRef]
- Search Results for “Cholangiocarcinoma” on the FDA Search Tool. Available online: ; https://www.fda.gov/search?s=cholangiocarcinoma (accessed on 27 August 2022).
- Walter, D.; Doring, C.; Feldhahn, M.; Battke, F.; Hartmann, S.; Winkelmann, R.; Schneider, M.; Bankov, K.; Schnitzbauer, A.; Zeuzem, S.; et al. Intratumoral heterogeneity of intrahepatic cholangiocarcinoma. Oncotarget 2017, 8, 14957–14968. [Google Scholar] [CrossRef]
- Goyal, L.; Saha, S.K.; Liu, L.Y.; Siravegna, G.; Leshchiner, I.; Ahronian, L.G.; Lennerz, J.K.; Vu, P.; Deshpande, V.; Kambadakone, A.; et al. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Dis. 2017, 7, 252–263. [Google Scholar] [CrossRef]
- Borad, M.J.; Champion, M.D.; Egan, J.B.; Liang, W.S.; Fonseca, R.; Bryce, A.H.; McCullough, A.E.; Barrett, M.T.; Hunt, K.; Patel, M.D.; et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014, 10, e1004135. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef]
- Vigano, L.; Soldani, C.; Franceschini, B.; Cimino, M.; Lleo, A.; Donadon, M.; Roncalli, M.; Aghemo, A.; Di Tommaso, L.; Torzilli, G. Tumor-Infiltrating Lymphocytes and Macrophages in Intrahepatic Cholangiocellular Carcinoma. Impact on Prognosis after Complete Surgery. J. Gastrointest Surg. 2019, 23, 2216–2224. [Google Scholar] [CrossRef]
- Asukai, K.; Kawamoto, K.; Eguchi, H.; Konno, M.; Nishida, N.; Koseki, J.; Noguchi, K.; Hasegawa, S.; Ogawa, H.; Yamada, D.; et al. Prognostic Impact of Peritumoral IL-17-Positive Cells and IL-17 Axis in Patients with Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S1524–S1531. [Google Scholar] [CrossRef]
- Kowalik, M.A.; Guzzo, G.; Morandi, A.; Perra, A.; Menegon, S.; Masgras, I.; Trevisan, E.; Angioni, M.M.; Fornari, F.; Quagliata, L.; et al. Metabolic reprogramming identifies the most aggressive lesions at early phases of hepatic carcinogenesis. Oncotarget 2016, 7, 32375–32393. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Katoh, M.; Nakagama, H. FGF receptors: Cancer biology and therapeutics. Med. Res. Rev. 2014, 34, 280–300. [Google Scholar] [CrossRef]
- King, M.J.; Hectors, S.; Lee, K.M.; Omidele, O.; Babb, J.S.; Schwartz, M.; Tabrizian, P.; Taouli, B.; Lewis, S. Outcomes assessment in intrahepatic cholangiocarcinoma using qualitative and quantitative imaging features. Cancer Imaging 2020, 20, 43. [Google Scholar] [CrossRef]
- Fiz, F.; Jayakody Arachchige, V.S.; Gionso, M.; Pecorella, I.; Selvam, A.; Wheeler, D.R.; Sollini, M.; Viganò, L. Radiomics of Biliary Tumors: A Systematic Review of Current Evidence. Diagnostics 2022, 12, 826. [Google Scholar] [CrossRef]
- Yugawa, K.; Itoh, S.; Iseda, N.; Kurihara, T.; Kitamura, Y.; Toshima, T.; Harada, N.; Kohashi, K.; Baba, S.; Ishigami, K.; et al. Obesity is a risk factor for intrahepatic cholangiocarcinoma progression associated with alterations of metabolic activity and immune status. Sci. Rep. 2021, 11, 5845. [Google Scholar] [CrossRef]
- Fiz, F.; Masci, C.; Costa, G.; Sollini, M.; Chiti, A.; Ieva, F.; Torzilli, G.; Viganò, L. PET/CT-based radiomics of mass-forming intrahepatic cholangiocarcinoma improves prediction of pathology data and survival. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3387–3400. [Google Scholar] [CrossRef]
- Watanabe, A.; Harimoto, N.; Yokobori, T.; Araki, K.; Kubo, N.; Igarashi, T.; Tsukagoshi, M.; Ishii, N.; Yamanaka, T.; Handa, T.; et al. FDG-PET reflects tumor viability on SUV in colorectal cancer liver metastasis. Int. J. Clin. Oncol. 2020, 25, 322–329. [Google Scholar] [CrossRef]
- Goasguen, N.; de Chaisemartin, C.; Brouquet, A.; Julié, C.; Prevost, G.P.; Laurent-Puig, P.; Penna, C. Evidence of heterogeneity within colorectal liver metastases for allelic losses, mRNA level expression and in vitro response to chemotherapeutic agents. Int. J. Cancer 2010, 127, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Wlochowitz, D.; Wachter, A.; Conradi, L.C.; Wolff, A.; Scheel, A.H.; Korf, U.; Wiemann, S.; Schildhaus, H.U.; Bohnenberger, H.; et al. High-Throughput Profiling of Colorectal Cancer Liver Metastases Reveals Intra- and Inter-Patient Heterogeneity in the EGFR and WNT Pathways Associated with Clinical Outcome. Cancers 2022, 14, 2084. [Google Scholar] [CrossRef]
- Graf, J.; Pape, U.F.; Jann, H.; Denecke, T.; Arsenic, R.; Brenner, W.; Pavel, M.; Prasad, V. Prognostic Significance of Somatostatin Receptor Heterogeneity in Progressive Neuroendocrine Tumor Treated with Lu-177 DOTATOC or Lu-177 DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Damjanovic, J.; Janssen, J.C.; Prasad, V.; Diederichs, G.; Walter, T.; Brenner, W.; Makowski, M.R. (68)Ga-PSMA-PET/CT for the evaluation of liver metastases in patients with prostate cancer. Cancer Imaging 2019, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lee, Z.; Awadallah, A.; Zhou, L.; Xin, W. Peritumoral/vascular expression of PSMA as a diagnostic marker in hepatic lesions. Diagn. Pathol. 2020, 15, 92. [Google Scholar] [CrossRef] [PubMed]

| Patient #1 | Patient #2 | Patient #3 | |
|---|---|---|---|
| Age | 76 | 60 | 71 |
| Sex | Male | Male | Male |
| Tumour size, mm | 120 | 60 | 97 |
| Number of tumours | 1 | 1 | 1 |
| Grading | G2 | G2 | G3 |
| Surgical margin, mm | 3 | 10 | 1 |
| Microscopic vascular invasion | Y | N | Y |
| Perineural infiltration | N | N | Y |
| Patient #1 | Patient #2 | Patient #3 | ||||
|---|---|---|---|---|---|---|
| Area SUV Min | Area SUV Max | Area SUV Min | Area SUV Max | Area SUV Min | Area SUV Max | |
| SUV | 5.1 | 14.7 | 5.5 | 9.9 | 5.4 | 8.9 |
| Morphology | stroma < cells | cells > stroma | cells = stroma | cells = stroma | cells = stroma | cells = stroma |
| Phenotype | CK7+ CK19−/+ | CK7+ CK19−/+ | CK7+ CK19/+ | CK7+ CK19−/+ | CK7+ CK19−/+ | CK7+ CK19− |
| Grading | G1 | G2 | G2 | G2 | G3 | G3 |
| Proliferation index (KI67) | 15% | 15% | 10% | 70% | 20% | 70% |
| P53 | 20% | 10% | 30% | 30% | 60% | 60% |
| PDL-1 | Neg | Neg | Neg | Neg | Neg | Neg |
| PD1 | 5% | Neg | 5% | 10% | 5% | Neg |
| FGFR2 | WT | WT | WT | Translocated | WT | WT |
| 1p36 | LOH | LOH | Conserved | Conserved | Conserved | Conserved |
| Immune infiltrate | ||||||
| CD3 | 10% | 10% | 10% | 10% | 10% | 20% |
| CD4 | 10% | 20% | 10% | 40% | 20% | 20% |
| CD8 | 5% | 10% | 10% | 20% | 5% | 15% |
| CD68 | 10% | 20% | 10% | 30% | 20% | 20% |
| CD163 | 20% | 20% | 20% | 50% | 5% | 10% |
| Metabolic indexes | ||||||
| G6PD | 80% | 80% | 40% | 100% | 50% | 50% |
| CS | 100% | 60% | 80% | 100% | 20% | 20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viganò, L.; Lopci, E.; Di Tommaso, L.; Destro, A.; Aghemo, A.; Rimassa, L.; Solbiati, L.; Chiti, A.; Torzilli, G.; Fiz, F. Functional Investigation of the Tumoural Heterogeneity of Intrahepatic Cholangiocarcinoma by In Vivo PET-CT Navigation: A Proof-of-Concept Study. J. Clin. Med. 2022, 11, 5451. https://doi.org/10.3390/jcm11185451
Viganò L, Lopci E, Di Tommaso L, Destro A, Aghemo A, Rimassa L, Solbiati L, Chiti A, Torzilli G, Fiz F. Functional Investigation of the Tumoural Heterogeneity of Intrahepatic Cholangiocarcinoma by In Vivo PET-CT Navigation: A Proof-of-Concept Study. Journal of Clinical Medicine. 2022; 11(18):5451. https://doi.org/10.3390/jcm11185451
Chicago/Turabian StyleViganò, Luca, Egesta Lopci, Luca Di Tommaso, Annarita Destro, Alessio Aghemo, Lorenza Rimassa, Luigi Solbiati, Arturo Chiti, Guido Torzilli, and Francesco Fiz. 2022. "Functional Investigation of the Tumoural Heterogeneity of Intrahepatic Cholangiocarcinoma by In Vivo PET-CT Navigation: A Proof-of-Concept Study" Journal of Clinical Medicine 11, no. 18: 5451. https://doi.org/10.3390/jcm11185451
APA StyleViganò, L., Lopci, E., Di Tommaso, L., Destro, A., Aghemo, A., Rimassa, L., Solbiati, L., Chiti, A., Torzilli, G., & Fiz, F. (2022). Functional Investigation of the Tumoural Heterogeneity of Intrahepatic Cholangiocarcinoma by In Vivo PET-CT Navigation: A Proof-of-Concept Study. Journal of Clinical Medicine, 11(18), 5451. https://doi.org/10.3390/jcm11185451











