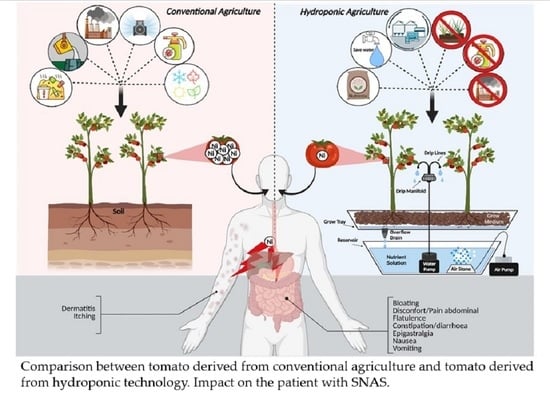
Datterino Trial: A Double Blind, Randomized, Controlled, Crossover, Clinical Trial on the Use of Hydroponic Cultivated Tomato Sauce in Systemic Nickel Allergy Syndrome
Abstract
1. Introduction
2. Materials and Methods
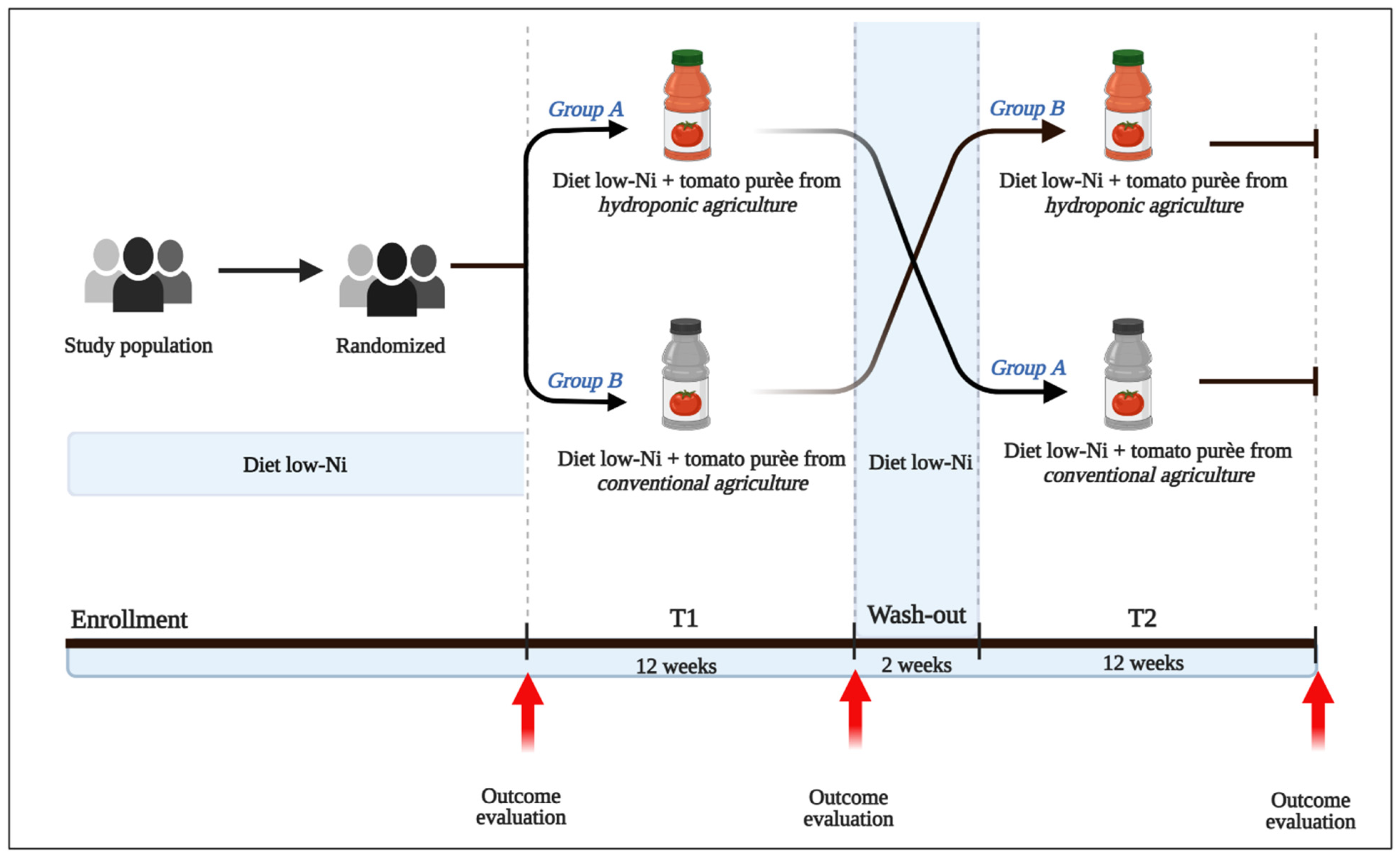
2.1. Design and Trial Objectives
2.2. Trial Location, Enrollment Strategy, and Study Sample
2.3. Randomization, Treatment Assignment, and Masking
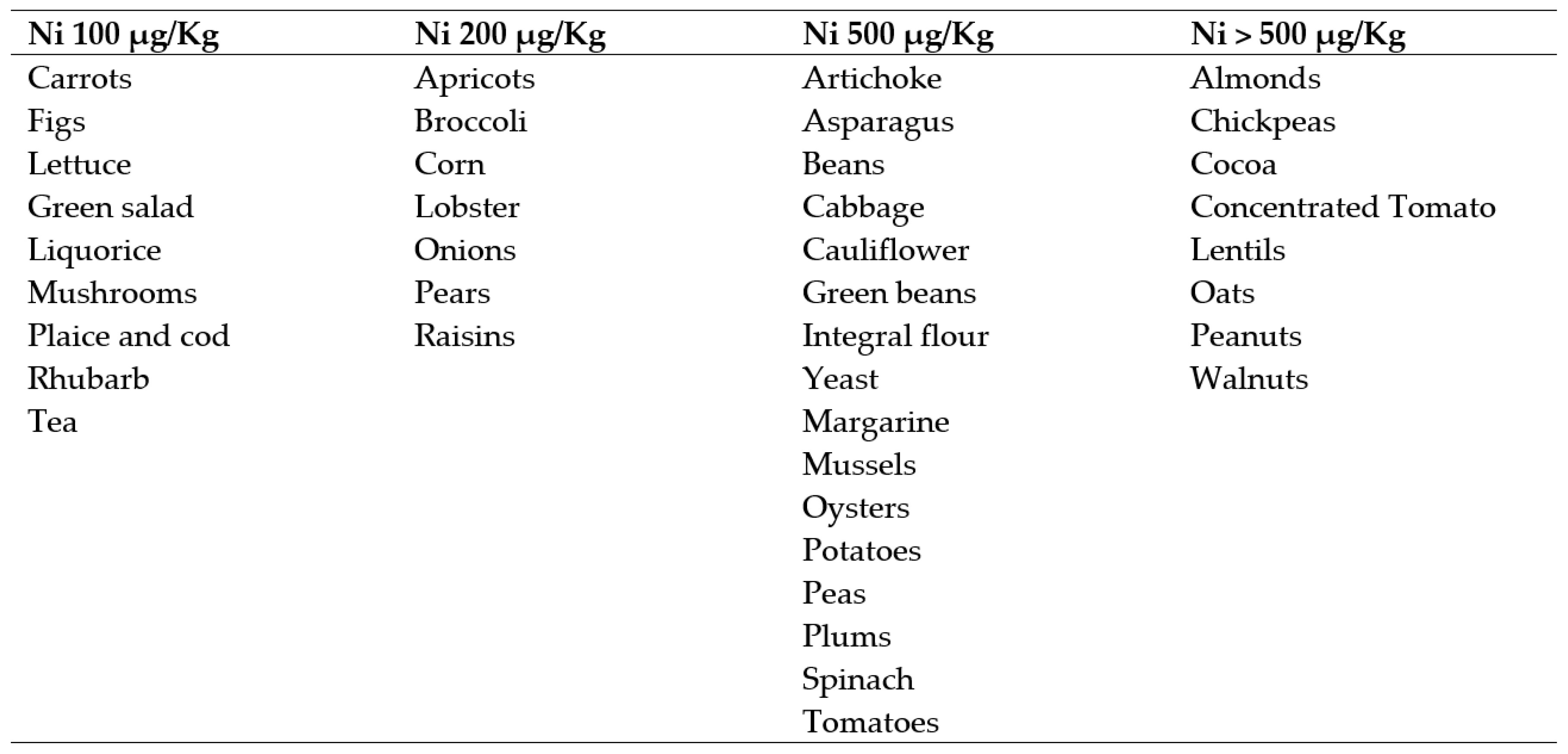
2.4. Trial Interventions
2.5. Outcome Measure
2.5.1. Symptoms
2.5.2. Adherence
2.5.3. Quality of Life
2.6. Sample Size
2.7. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Symptoms
3.3. Adherence
3.4. Quality of Life
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devries, J. Hydroponics. Ball Redb. Greenh. Equip. 2003, 1, 103–114. [Google Scholar]
- Steiner, A.A. The History of Mineral Plant Nutrition till about 1860 as Source of the Origin of Soilless Culture Methods. Soil. Cult. 1985, 1, 7–24. [Google Scholar]
- Gericke, W.F. Aquaculture, a Means of Crop Production. Am. J. Bot. 1929, 16, 862. [Google Scholar]
- Polycarpou, P.; Neokleous, D.; Chimonidou, D.; Papadopoulos, I. A Closed System for Soil Less Culture Adapted to the Cyprus Conditions. In Non-Conv. Water Use; El Gamal, F., Lamaddalen, A.N., Bogliotti, C., Guelloubi, R., Eds.; CIHEAM/EU DG Research: Bari, Italy, 2005; pp. 237–241. [Google Scholar]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an Advanced Technique for Vegetable Production: An Overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Blando, F.; Berland, H.; Maiorano, G.; Durante, M.; Mazzucato, A.; Picarella, M.E.; Nicoletti, I.; Gerardi, C.; Mita, G.; Andersen, Ø.M. Nutraceutical Characterization of Anthocyanin-Rich Fruits Produced by “Sun Black” Tomato Line. Front. Nutr. 2019, 6, 133. [Google Scholar] [CrossRef]
- Verdoliva, S.G.; Gwyn-Jones, D.; Detheridge, A.; Robson, P. Controlled Comparisons between Soil and Hydroponic Systems Reveal Increased Water Use Efficiency and Higher Lycopene and β-Carotene Contents in Hydroponically Grown Tomatoes. Sci. Hortic. 2021, 279, 109896. [Google Scholar] [CrossRef]
- Künsch, U.; Schärer, H.; Hurter, J. Do Differences Exist in the Quality between Soilless and Conventionally Produced Tomatoes and Head Lettuce? Mitt. Geb. Lebensm. Hyg. 1994, 85, 18. [Google Scholar]
- Benoit, F.; Ceustermans, N. Some Qualitative Aspects of Tomatoes Grown on NFT. Soil. Cult. 1987, 3, 3–7. [Google Scholar]
- Fanasca, S.; Colla, G.; Maiani, G.; Venneria, E.; Rouphael, Y.; Azzini, E.; Saccardo, F. Changes in Antioxidant Content of Tomato Fruits in Response to Cultivar and Nutrient Solution Composition. J. Agric. Food Chem. 2006, 54, 4319–4325. [Google Scholar] [CrossRef]
- Erba, D.; Casiraghi, M.C.; Ribas-Agustí, A.; Cáceres, R.; Marfà, O.; Castellari, M. Nutritional Value of Tomatoes (Solanum lycopersicum L.) Grown in Greenhouse by Different Agronomic Techniques. J. Food Compos. Anal. 2013, 31, 245–251. [Google Scholar] [CrossRef]
- Braga, M.; Quecchia, C.; Perotta, C.; Timpini, A.; Maccarinelli, K.; Di Tommaso, L.; Di Gioacchino, M. Systemic Nickel Allergy Syndrome: Nosologic Framework and Usefulness of Diet Regimen for Diagnosis. Int. J. Immunopathol. Pharm. 2013, 26, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Di Gioacchino, M.; Ricciardi, L.; De Pità, O.; Minelli, M.; Patella, V.; Voltolini, S.; Di Rienzo, V.; Braga, M.; Ballone, E.; Mangifesta, R.; et al. Nickel Oral Hyposensitization in Patients with Systemic Nickel Allergy Syndrome. Ann. Med. 2014, 46, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, A.; Nucera, E.; Schiavino, D. Metal Allergy and Tolerance Development. In Metal Allergy; Springer: Berlin/Heidelberg, Germany, 2018; pp. 97–105. [Google Scholar]
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel Allergy and Allergic Contact Dermatitis: A Clinical Review of Immunology, Epidemiology, Exposure, and Treatment. Contact Dermat. 2019, 81, 227–241. [Google Scholar] [CrossRef]
- Falagiani, P.; Gioacchino, M.D.; Ricciardi, L.; Minciullo, P.L.; Saitta, S.; Carní, A.; Santoro, G.; Gangemi, S.; Minelli, M.; Bozzetti, M.P.; et al. Systemic Nickel Allergy Syndrome (SNAS): A Review. Rev. Port. Imunoalergologia 2008, 16, 135–147. [Google Scholar]
- Panzani, R.C.; Schiavino, D.; Nucera, E.; Pellegrino, S.; Fais, G.; Schinco, G.; Patriarca, G. Oral Hyposensitization to Nickel Allergy: Preliminary Clinical Results. Int. Arch. Allergy Immunol. 1995, 107, 251–254. [Google Scholar] [CrossRef]
- Nielsen, F.H. Possible Future Implications of Nickel, Arsenic, Silicon, Vanadium, and Other Ultratrace Elements in Human Nutrition; AR Liss: New York, NY, USA, 1982. [Google Scholar]
- Rizzi, A.; Nucera, E.; Laterza, L.; Gaetani, E.; Valenza, V.; Corbo, G.M.; Inchingolo, R.; Buonomo, A.; Schiavino, D.; Gasbarrini, A. Irritable Bowel Syndrome and Nickel Allergy: What Is the Role of the Low Nickel Diet? J. Neurogastroenterol. Motil. 2017, 23, 101–108. [Google Scholar] [CrossRef]
- Lombardi, F.; Fiasca, F.; Minelli, M.; Maio, D.; Mattei, A.; Vergallo, I.; Cifone, M.G.; Cinque, B.; Minelli, M. The Effects of Low-Nickel Diet Combined with Oral Administration of Selected Probiotics on Patients with Systemic Nickel Allergy Syndrome (SNAS) and Gut Dysbiosis. Nutrients 2020, 12, 1040. [Google Scholar] [CrossRef]
- Nucera, E.; Rizzi, A.; Chini, R.; Giangrossi, S.; Lohmeyer, F.M.; Parrinello, G.; Musca, T.; Miggiano, G.A.D.; Gasbarrini, A.; Inchingolo, R. Diet Intervention Study through Telemedicine Assistance for Systemic Nickel Allergy Syndrome Patients during the COVID-19 Pandemic. Nutrients 2021, 13, 2897. [Google Scholar] [CrossRef]
- Yousaf, A.; Hagen, R.; Mitchell, M.; Ghareeb, E.; Fang, W.; Correa, R.; Zinn, Z.; Gayam, S. The Effect of a Low-Nickel Diet and Nickel Sensitization on Gastroesophageal Reflux Disease: A Pilot Study. Indian J. Gastroenterol. 2021, 40, 137–143. [Google Scholar] [CrossRef]
- Borghini, R.; De Amicis, N.; Bella, A.; Greco, N.; Donato, G.; Picarelli, A. Beneficial Effects of a Low-Nickel Diet on Relapsing IBS-Like and Extraintestinal Symptoms of Celiac Patients during a Proper Gluten-Free Diet: Nickel Allergic Contact Mucositis in Suspected Non-Responsive Celiac Disease. Nutrients 2020, 12, 2277. [Google Scholar] [CrossRef] [PubMed]
- Pizzutelli, S. Systemic Nickel Hypersensitivity and Diet: Myth or Reality? Eur. Ann. Allergy Clin. Immunol. 2011, 43, 5–18. [Google Scholar] [PubMed]
- Jones, J.B., Jr. Hydroponics: A Practical Guide for the Soilless Grower, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0-429-12230-9. [Google Scholar]
- Lee, S.; Lee, J. Beneficial Bacteria and Fungi in Hydroponic Systems: Types and Characteristics of Hydroponic Food Production Methods. Sci. Hortic. 2015, 195, 206–215. [Google Scholar] [CrossRef]
- Bengtsson, M.; Ohlsson, B. The Brief Visual Analogue Scale for Irritable Bowel Syndrome Questionnaire Can Be Used to Evaluate Psychological Well-Being in Patients with Irritable Bowel Syndrome. Eur. J. Intern. Med. 2013, 24, e82–e83. [Google Scholar] [CrossRef]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, Validation and Norming. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Grossi, E.; Groth, N.; Mosconi, P.; Cerutti, R.; Pace, F.; Compare, A.; Apolone, G. Development and Validation of the Short Version of the Psychological General Well-Being Index (PGWB-S). Health Qual. Life Outcomes 2006, 4, 88. [Google Scholar] [CrossRef]
- Veien, N.K.; Hattel, T.; Laurberg, G. Low Nickel Diet: An Open, Prospective Trial. J. Am. Acad. Derm. 1993, 29, 1002–1007. [Google Scholar] [CrossRef]
- Rizzi, A.; Di Rienzo, A.; Buonomo, A.; Aruanno, A.; Carusi, V.; Ricci, A.G.; Centrone, M.; Mezzacappa, S.; Romeo, L.; Schiavino, D.; et al. Impact of Nickel Oral Hyposensitization on Quality of Life in Systemic Nickel Allergy Syndrome. Int. J. Immunopathol. Pharm. 2020, 34, 2058738420934629. [Google Scholar] [CrossRef]
- Di Gioacchino, M.; Gatta, A.; Valle, L.D.; Farinelli, A.; Caruso, R.; Pini, C.; Malandra, A.; Mangifesta, R.; Cavallucci, E.; Petrarca, C. Systemic Nickel Allergy Syndrome. In Metal Allergy: From Dermatitis to Implant and Device Failure; Chen, J.K., Thyssen, J.P., Eds.; Springer International Publishing: Cham, Switerland, 2018; pp. 551–561. ISBN 978-3-319-58503-1. [Google Scholar]
- Production of Fruit and Vegetables. Apples and Tomatoes Were the Top Fruit and Vegetable Produced in the EU in 2015. Spain, Italy and Poland: Main Producers; Eurostat: Luxembourg, 2016.
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 31 May 2022).
- Roccotiello, E.; Nicosia, E.; Pierdonà, L.; Marescotti, P.; Ciardiello, M.A.; Giangrieco, I.; Mari, A.; Zennaro, D.; Dozza, D.; Brancucci, M.; et al. Tomato (Solanum lycopersicum L.) Accumulation and Allergenicity in Response to Nickel Stress. Sci. Rep. 2022, 12, 5432. [Google Scholar] [CrossRef]
- Commission Directive 98/82/EC of 27 October 1998 Amending the Annexes to Council Directives 86/362/EEC, 86/363/EEC and 90/642/EEC on the Fixing of Maximum Levels for Pesticide Residues in and on Cereals, Foodstuffs of Animal Origin and Certain Products of Plant Origin, Including Fruit and Vegetables Respectively (Text with EEA Relevance); European Union: Luxembourg, 1998; Volume 290.
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; et al. Update of the Risk Assessment of Nickel in Food and Drinking Water. EFSA J. 2020, 18, e06268. [Google Scholar] [CrossRef]
- Palacios, G.; Gómez, I.; Carbonell-Barrachina, A.; Pedreño, J.N.; Mataix, J. Effect of Nickel Concentration on Tomato Plant Nutrition and Dry Matter Yield. J. Plant Nutr. 1998, 21, 2179–2191. [Google Scholar] [CrossRef]
- Karagiannidis, N.; Stavropoulos, N.; Tsakelidou, K. Yield Increase in Tomato, Eggplant, and Pepper Using Thermanox Manganese Soil Amendment. Commun. Soil Sci. Plant Anal. 2002, 33, 2247–2258. [Google Scholar] [CrossRef]
- Gad, N.; El-Sherif, M.H.; El-Gereedly, N.H.M. Influence of Nickel on Some Physiological Aspects of Tomato Plants. Aust. J. Basic Appl. Sci. 2007, 1, 286–293. [Google Scholar]
- Correia, L.; Marrocos, P.; Montalván Olivares, D.M.; Velasco, F.G.; Luzardo, F.H.M.; Mota de Jesus, R. Bioaccumulation of Nickel in Tomato Plants: Risks to Human Health and Agro-Environmental Impacts. Environ. Monit. Assess. 2018, 190, 317. [Google Scholar] [CrossRef]
- Swain, A.; Chatterjee, S.; Vishwanath, M. Hydroponics in vegetable crops: A review. Pharma Innov. J. 2021, 10, 629–634. [Google Scholar]
- Maucieri, C.; Nicoletto, C.; van Os, E.; Anseeuw, D.; Havermaet, R.V.; Junge, R. Hydroponic Technologies. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switerland, 2019; pp. 77–110. ISBN 978-3-030-15943-6. [Google Scholar]
- Bussell, W.T.; Mckennie, S. Rockwool in Horticulture, and Its Importance and Sustainable Use in New Zealand. N. Z. J. Crop Hortic. Sci. 2004, 32, 29–37. [Google Scholar] [CrossRef]

| Patients (n = 30) | ||||||
|---|---|---|---|---|---|---|
| Variables | N | % | Group A (13 pts) | % | Group B (9 pts) | % |
| Female gender | 29 | 97 | 12 | 92 | 9 | 100 |
| Age (mean ± SD 1 (range)) | 39 (±12; 22–61) | 42 (±12, 22–61) | 38 (±13, 22–56) | |||
| Social class | ||||||
| Employed | 19 | 63 | 9 | 69 | 6 | 67 |
| Unemployed | 2 | 7 | 1 | 8 | 0 | 0 |
| Householder chores | 4 | 13 | 1 | 8 | 1 | 11 |
| Student | 5 | 17 | 2 | 15 | 2 | 22 |
| Education | ||||||
| Less than high school | 2 | 7 | 1 | 8 | 1 | 11 |
| Short or medium high educ. | 9 | 30 | 3 | 23 | 1 | 11 |
| University degree | 19 | 63 | 9 | 69 | 7 | 78 |
| Marital status | ||||||
| Married | 15 | 50 | 6 | 46 | 4 | 44 |
| Single | 15 | 50 | 7 | 54 | 5 | 56 |
| Lactose intolerance | 12 | 40 | 8 | 62 | 2 | 22 |
| Parental history of allergies | 22 | 73 | 7 | 54 | 9 | 100 |
| Concomitant other allergies | ||||||
| known allergies (all) | 17 | 9 | 5 | |||
| Respiratory allergies | 13 | 7 | 4 | |||
| Food allergies | 3 | 2 | 0 | |||
| Adverse drug reaction | 7 | 4 | 1 | |||
| Concomitant positivity to other haptens (palladium chloride, cobalt chloride, potassium dichromate) | 17 | 57 | 8 | 62 | 4 | 44 |
| Patch grade nickel | ||||||
| Grade A (±) | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade B (+) | 6 | 20 | 2 | 15 | 1 | 11 |
| Grade C (++) | 11 | 37 | 3 | 23 | 3 | 33 |
| Grade D (+++) | 13 | 43 | 8 | 62 | 5 | 56 |
| Group A: 13 patients randomized to Datterino tomato puree deriving from hydroponic technology during phase 1 and Datterino tomato puree deriving from conventional cultivation during phase 2 | |||||
| Symptoms | Baseline M (±SD) | T1 * M (±SD) | T2 ** M (±SD) | p-value † (T1 vs. baseline) | p-value † (T1 vs. T2) |
| Bloating | 6 (±3) | 4 (±3) | 5 (±3) | 0.0111 | NS |
| Discomfort | 4 (±4) | 2 (±4) | 2 (±3) | NS | NS |
| Flatulence | 5 (±3) | 3 (±5) | 5 (±4) | 0.0090 | NS |
| Abdominal cramps | 4 (±3) | 2 (±2) | 1 (±3) | 0.0207 | NS |
| Constipation | 5 (±4) | 3 (±3) | 4 (±3) | 0.0395 | NS |
| Diarrhea | 3 (±3) | 2 (±2) | 2 (±2) | 0.0105 | NS |
| Epigastralgia | 4 (±4) | 2 (±2) | 2 (±3) | NS | NS |
| Nausea | 3 (±3) | 2 (±3) | 2 (±3) | NS | NS |
| Vomiting | 0 | 0 | 0 | - | - |
| Itching | 3 (±4) | 3 (±3) | 2 (±3) | NS | NS |
| Dermatitis | 5 (±4) | 3 (±3) | 2 (±3) | NS | NS |
| Group B: 9 patients randomized to Datterino tomato puree deriving from conventional cultivation during phase 1 and Datterino tomato puree deriving from hydroponic technology during phase 2 | |||||
| Symptoms | Baseline M (±SD) | T1 * M (±SD) | T2 ** M (±SD) | p-value † (T1 vs. baseline) | p-value † (T1 vs. T2) |
| Bloating | 7 (±2) | 6 (±3) | 3 (±3) | NS | 0.0060 |
| Discomfort | 4 (±2) | 3 (±3) | 2 (±3) | NS | NS |
| Flatulence | 4 (±2) | 3 (±3) | 2 (±3) | NS | NS |
| Abdominal cramps | 4 (±3) | 2 (±2) | 1 (±1) | NS | NS |
| Constipation | 5 (±4) | 4 (±4) | 2 (±4) | NS | NS |
| Diarrhea | 3 (±3) | 1 (±1) | 1 (±3) | NS | NS |
| Epigastralgia | 2 (±2) | 2 (±3) | 1 (±1) | NS | NS |
| Nausea | 1 (±1) | 1 (±2) | 0 | NS | NS |
| Vomiting | 0 | 0 | 0 | - | - |
| Itching | 2 (±3) | 1 (±1) | 1 (±3) | NS | NS |
| Dermatitis | 5 (±4) | 1 (±1) | 2 (±4) | 0.0084 | NS |
| QoL Questionnaires | ARM 1 * M (±SD) | ARM 2 * M (±SD) | p-Value † (ARM 1 vs. ARM 2) |
|---|---|---|---|
| SF-36v2 ~ | |||
| Physical functioning | 87 (±20) | 97 (±5) | 0.0004 |
| Role physical | 87 (±19) | 81 (±39) | 0.0148 |
| Bodily pain | 70 (±22) | 78 (±20) | NS |
| General health | 54 (±26) | 68 (±7) | 0.0005 |
| Vitality | 52 (±28) | 57 (±23) | NS |
| Social functioning | 73 (±20) | 82 (±20) | NS |
| Role emotional | 77 (±34) | 78 (±44) | NS |
| Mental health | 72 (±23) | 74 (±22) | NS |
| Physical component summary | 49 (±7) | 53 (±5) | NS |
| Mental component summary | 46 (±12) | 50 (±11) | NS |
| PGWBI ’, total score | 58 (±5) | 56 (±4) | NS |
| QoL Questionnaires | Group A * (Phase 1) M (±SD ’) | Group B ** (Phase 1) M (± SD) | p-Value † (A versus B, Phase 1) | Group A * (Phase 2) M (±SD) | Group B ** (Phase 2) M (±SD) | p-Value † (A versus B, Phase 2) |
|---|---|---|---|---|---|---|
| SF-36v2 ~ | ||||||
| Physical functioning | 87 (±15) | 97 (±7) | 0.0198 | 86 (±21) | 99 (±2) | 0.0000 |
| Role physical | 87 (±26) | 92 (±18) | NS | 92 (±19) | 89 (±25) | NS |
| Bodily pain | 77 (±22) | 86 (±11) | 0.0258 | 79 (±25) | 92 (±13) | 0.0401 |
| General health | 59 (±24) | 75 (±15) | NS | 57 (±21) | 68 (±19) | NS |
| Vitality | 56 (±25) | 66 (±19) | NS | 60 (±22) | 81 (±8) | 0.0034 |
| Social functioning | 75 (±19) | 82 (±14) | NS | 81 (±19) | 82 (±26) | NS |
| Role emotional | 82 (±35) | 85 (±34) | NS | 85 (±38) | 89 (±24) | NS |
| Mental health | 71 (±22) | 83 (±7) | 0.0024 | 70 (±22) | 86 (±6) | 0.0003 |
| Physical component summary | 50 (±5) | 52 (±7) | NS | 50 (±6) | 53 (±5) | NS |
| Mental component summary | 48 (±11) | 52 (±7) | NS | 50 (±11) | 54 (±5) | 0.0141 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzi, A.; Chini, R.; Porcari, S.; Settanni, C.R.; Persichetti, E.; Mora, V.; Fanali, C.; Leonetti, A.; Parrinello, G.; Lohmeyer, F.M.; et al. Datterino Trial: A Double Blind, Randomized, Controlled, Crossover, Clinical Trial on the Use of Hydroponic Cultivated Tomato Sauce in Systemic Nickel Allergy Syndrome. J. Clin. Med. 2022, 11, 5459. https://doi.org/10.3390/jcm11185459
Rizzi A, Chini R, Porcari S, Settanni CR, Persichetti E, Mora V, Fanali C, Leonetti A, Parrinello G, Lohmeyer FM, et al. Datterino Trial: A Double Blind, Randomized, Controlled, Crossover, Clinical Trial on the Use of Hydroponic Cultivated Tomato Sauce in Systemic Nickel Allergy Syndrome. Journal of Clinical Medicine. 2022; 11(18):5459. https://doi.org/10.3390/jcm11185459
Chicago/Turabian StyleRizzi, Angela, Raffaella Chini, Serena Porcari, Carlo Romano Settanni, Eleonora Persichetti, Vincenzina Mora, Caterina Fanali, Alessia Leonetti, Giuseppe Parrinello, Franziska Michaela Lohmeyer, and et al. 2022. "Datterino Trial: A Double Blind, Randomized, Controlled, Crossover, Clinical Trial on the Use of Hydroponic Cultivated Tomato Sauce in Systemic Nickel Allergy Syndrome" Journal of Clinical Medicine 11, no. 18: 5459. https://doi.org/10.3390/jcm11185459
APA StyleRizzi, A., Chini, R., Porcari, S., Settanni, C. R., Persichetti, E., Mora, V., Fanali, C., Leonetti, A., Parrinello, G., Lohmeyer, F. M., Inchingolo, R., Mele, M. C., Gasbarrini, A., & Nucera, E. (2022). Datterino Trial: A Double Blind, Randomized, Controlled, Crossover, Clinical Trial on the Use of Hydroponic Cultivated Tomato Sauce in Systemic Nickel Allergy Syndrome. Journal of Clinical Medicine, 11(18), 5459. https://doi.org/10.3390/jcm11185459