The Small Posterior Cranial Fossa Syndrome and Chiari Malformation Type 0
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Symptomatic Conditions with Low-Lying Cerebellar Tonsils Found by the Article Search and Selection Process
3.2. Characteristics of Studies Differentiating CM0 from CM1 and Other Conditions
3.3. Risk of Bias in Studies: Epidemiology, Clinical Presentation, and Diagnosis of CM0
3.4. Synthesis: Using Automated Radiographic Measurements May Reduce the Risk of Bias in Studies of CM0
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morgenstern, P.F.; Tosi, U.; Uribe-Cardenas, R.; Greenfield, J.P. Ventrolateral Tonsillar Position Defines Novel Chiari 0.5 Classification. World Neurosurg. 2020, 136, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Turgut, M. Defining the Chiari Malformations: Past and Newer Classifications. In The Chiari Malformations, 2nd ed.; Tubbs, R.S., Turgut, M., Oakes, W.J., Eds.; Springer Nature Publishing: Cham, Switzerland, 2020; pp. 21–40. [Google Scholar]
- Haddad, F.A.; Qaisi, I.; Joudeh, N.; Dajani, H.; Jumah, F.; Elmashala, A.; Adeeb, N.; Chern, J.J.; Tubbs, R.S. The newer classifications of the chiari malformations with clarifications: An anatomical review. Clin. Anat. 2017, 31, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, P.A.; Brodbelt, A.; Bloom, A.B.; Kula, R.W. Chiari I Malformation: Opinions on Diagnostic Trends and Controversies from a Panel of 63 International Experts. World Neurosurg. 2019, 130, e9–e16. [Google Scholar] [CrossRef] [PubMed]
- Bordes, S.; Jenkins, S.; Tubbs, R.S. Defining, diagnosing, clarifying, and classifying the Chiari I malformations. Child’s Nerv. Syst. 2019, 35, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, C. Should we stop using the term “malformation” for Chiari type I? Child’s Nerv. Syst. 2019, 35, 1649–1650. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi, P.; Morana, G.; Anania, P.; Rossi, A.; Consales, A.; Piatelli, G.; Cama, A.; Pavanello, M. Tonsillar herniation spectrum: More than just Chiari I. Update and controversies on classification and management. Neurosurg. Rev. 2020, 43, 1473–1492. [Google Scholar] [CrossRef]
- Frič, R.; Eide, P.K. Chiari type 1—A malformation or a syndrome? A critical review. Acta Neurochir. 2019, 162, 1513–1525. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.N.P. Chiari I—A ‘not so’ congenital malformation? Child’s Nerv. Syst. 2019, 35, 1653–1664. [Google Scholar] [CrossRef]
- van Dellen, J.R. Chiari Malformation: An Unhelpful Eponym. World Neurosurg. 2021, 156, 1–3. [Google Scholar] [CrossRef]
- Iskandar, B.J.; Hedlund, G.L.; Grabb, P.A.; Oakes, W.J. The resolution of syringohydromyelia without hindbrain herniation after posterior fossa decompression. J. Neurosurg. 1998, 89, 212–216. [Google Scholar] [CrossRef]
- Rindler, R.S.; Chern, J.J. Newer Subsets: Chiari 1.5 and Chiari 0 Malformations. In The Chiari Malformations, 2nd ed.; Tubbs, R.S., Turgut, M., Oakes, W.J., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 41–48. [Google Scholar]
- Tubbs, R.S.; Elton, S.; Grabb, P.; Dockery, S.E.; Bartolucci, A.A.; Oakes, W.J. Analysis of the Posterior Fossa in Children with the Chiari 0 Malformation. Neurosurgery 2001, 48, 1050–1055. [Google Scholar] [CrossRef]
- Ciaramitaro, P.; Massimi, L.; Bertuccio, A.; Solari, A.; Farinotti, M.; Peretta, P.; Saletti, V.; Chiapparini, L.; Barbanera, A.; Garbossa, D.; et al. Diagnosis and treatment of Chiari malformation and syringomyelia in adults: International consensus document. Neurol. Sci. 2022, 43, 1327–1342. [Google Scholar] [CrossRef]
- Bogdanov, E.I.; Faizutdinova, A.T.; Heiss, J.D. Posterior cranial fossa and cervical spine morphometric abnormalities in symptomatic Chiari type 0 and Chiari type 1 malformation patients with and without syringomyelia. Acta Neurochir. 2021, 163, 3051–3064. [Google Scholar] [CrossRef]
- Heffez, D.S.; Broderick, J.; Connor, M.; Mitchell, M.; Galezowska, J.; Golchini, R.; Ghorai, J. Is there a relationship between the extent of tonsillar ectopia and the severity of the clinical Chiari syndrome? Acta Neurochir. 2019, 162, 1531–1538. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Nishikawa, M.; Kula, R.W.; Dlugacz, Y.D. Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir. 2010, 152, 1117–1127. [Google Scholar] [CrossRef]
- Bogdanov, E.I.; Faizutdinova, A.T.; Mendelevich, E.G.; Sozinov, A.S.; Heiss, J.D. Epidemiology of Symptomatic Chiari Malformation in Tatarstan: Regional and Ethnic Differences in Prevalence. Neurosurgery 2018, 84, 1090–1097. [Google Scholar] [CrossRef]
- Faizutdinova, A.T.; Bogdanov, E.I. Clinical and radiological rationale for distinguishing subtypes of primary Chiari I malformation. Zh Nevrol Psikhiatr Im S S Korsakova 2020, 120, 64–69. [Google Scholar] [CrossRef]
- Nishikawa, M.; Bolognese, P.A.; Kula, R.W.; Ikuno, H.; Ohata, K. Pathogenesis and Classification of Chiari Malformation Type I Based on the Mechanism of Ptosis of the Brain Stem and Cerebellum: A Morphometric Study of the Posterior Cranial Fossa and Craniovertebral Junction. J. Neurol. Surg. Part B Skull Base 2019, 82, 277–284. [Google Scholar] [CrossRef]
- Nwotchouang, B.S.T.; Eppelheimer, M.S.; Ibrahimy, A.; Houston, J.R.; Biswas, D.; Labuda, R.; Bapuraj, J.R.; Allen, P.A.; Frim, D.; Loth, F. Clivus length distinguishes between asymptomatic healthy controls and symptomatic adult women with Chiari malformation type I. Neuroradiology 2020, 62, 1389–1400. [Google Scholar] [CrossRef]
- Sekula, R.F., Jr.; Jannetta, P.J.; Casey, K.F.; Marchan, E.M.; Sekula, L.K.; McCrady, C.S. Dimensions of the posterior fossa in patients symptomatic for Chiari I malformation but without cerebellar tonsillar descent. Cereb. Fluid Res. 2005, 2, 11. [Google Scholar] [CrossRef] [Green Version]
- Buell, T.J.; Heiss, J.D.; Oldfield, E.H. Pathogenesis and Cerebrospinal Fluid Hydrodynamics of the Chiari I Malformation. Neurosurg. Clin. N. Am. 2015, 26, 495–499. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Chou, M.W.; Trinidad, E.M.; Kula, R.W.; Mandell, M.; Wolpert, C.; Speer, M.C. Chiari I Malformation Redefined: Clinical and Radiographic Findings for 364 Symptomatic Patients. Neurosurgery 1999, 44, 1005–1017. [Google Scholar] [CrossRef]
- Nishikawa, M.; Sakamoto, H.; Hakuba, A.; Nakanishi, N.; Inoue, Y. Pathogenesis of Chiari malformation: A morphometric study of the posterior cranial fossa. J. Neurosurg. 1997, 86, 40–47. [Google Scholar] [CrossRef]
- Noudel, R.; Jovenin, N.; Eap, C.; Scherpereel, B.; Pierot, L.; Rousseaux, P. Incidence of basioccipital hypoplasia in Chiari malformation type I: Comparative morphometric study of the posterior cranial fossa. Clinical article. J. Neurosurg. 2009, 111, 1046–1052. [Google Scholar] [CrossRef]
- Bogdanov, E.I.; Heiss, J.D.; Mendelevich, E.G.; Mikhaylov, I.M.; Haass, A. Clinical and neuroimaging features of “idiopathic” syringomyelia. Neurology 2004, 62, 791–794. [Google Scholar] [CrossRef]
- Barkovich, A.J.; Wippold, F.J.; Sherman, J.L.; Citrin, C.M. Significance of cerebellar tonsillar position on MR. AJNR Am. J. Neuroradiol. 1986, 7, 795–799. [Google Scholar]
- Smith, B.W.; Strahle, J.; Bapuraj, J.R.; Muraszko, K.M.; Garton, H.J.; Maher, C.O. Distribution of cerebellar tonsil position: Implications for understanding Chiari malformation. J. Neurosurg. 2013, 119, 812–819. [Google Scholar] [CrossRef]
- Ciaramitaro, P.; Ferraris, M.; Massaro, F.; Garbossa, D. Clinical diagnosis—Part I: What is really caused by Chiari I. Child’s Nerv. Syst. 2019, 35, 1673–1679. [Google Scholar] [CrossRef]
- Elster, A.D.; Chen, M.Y. Chiari I malformations: Clinical and radiologic reappraisal. Radiology 1992, 183, 347–353. [Google Scholar] [CrossRef]
- Meadows, J.; Kraut, M.; Guarnieri, M.; Haroun, R.I.; Carson, B.S. Asymptomatic Chiari Type I malformations identified on magnetic resonance imaging. J. Neurosurg. 2000, 92, 920–926. [Google Scholar] [CrossRef]
- Godzik, J.; Kelly, M.P.; Radmanesh, A.; Kim, D.; Holekamp, T.F.; Smyth, M.D.; Lenke, L.G.; Shimony, J.S.; Park, T.S.; Leonard, J.; et al. Relationship of syrinx size and tonsillar descent to spinal deformity in Chiari malformation Type I with associated syringomyelia. J. Neurosurg. Pediatr. 2014, 13, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Singhal, A.; Cheong, A.; Steinbok, P. International survey on the management of Chiari 1 malformation and syringomyelia: Evolving worldwide opinions. Child’s Nerv. Syst. 2018, 34, 1177–1182. [Google Scholar] [CrossRef]
- McClugage, S.G.; Oakes, W.J. The Chiari I malformation. J. Neurosurg. Pediatr. 2019, 24, 217–226. [Google Scholar] [CrossRef]
- Aboulezz, A.O.; Sartor, K.; Geyer, C.A.; Gado, M.H. Position of Cerebellar Tonsils in the Normal Population and in Patients with Chiari Malformation: A quantitative approach with MR imaging. J. Comput. Assist. Tomogr. 1985, 9, 1033–1036. [Google Scholar] [CrossRef]
- Chern, J.J.; Gordon, A.J.; Mortazavi, M.M.; Tubbs, R.S.; Oakes, W.J. Pediatric Chiari malformation Type 0: A 12-year institutional experience. J. Neurosurg. Pediatr. 2011, 8, 1–5. [Google Scholar] [CrossRef]
- Markunas, C.A.; Lock, E.; Soldano, K.; Cope, H.; Ding, C.K.; Enterline, D.S.; Grant, G.; Fuchs, H.; Ashley-Koch, A.E.; Gregory, S.G. Identification of Chiari Type I Malformation subtypes using whole genome expression profiles and cranial base morphometrics. BMC Med. Genom. 2014, 7, 39. [Google Scholar] [CrossRef]
- Moncho, D.; Poca, M.A.; Minoves, T.; Ferré, A.; Cañas, V.; Sahuquillo, J. Are evoked potentials clinically useful in the study of patients with Chiari malformation Type 1? J. Neurosurg. 2017, 126, 606–619. [Google Scholar] [CrossRef]
- Urbizu, A.; Martin, B.A.; Moncho, D.; Rovira, A.; Poca, M.A.; Sahuquillo, J.; Macaya, A.; Español, M.I. Machine learning applied to neuroimaging for diagnosis of adult classic Chiari malformation: Role of the basion as a key morphometric indicator. J. Neurosurg. 2018, 129, 779–791. [Google Scholar] [CrossRef]
- Basaran, R.; Efendioglu, M.; Senol, M.; Ozdogan, S.; Isik, N. Morphometric analysis of posterior fossa and craniovertebral junction in subtypes of Chiari malformation. Clin. Neurol. Neurosurg. 2018, 169, 1–11. [Google Scholar] [CrossRef]
- Markunas, C.A.; Tubbs, R.S.; Moftakhar, R.; Ashley-Koch, A.E.; Gregory, S.G.; Oakes, W.J.; Speer, M.C.; Iskandar, B.J. Clinical, radiological, and genetic similarities between patients with Chiari Type I and Type 0 malformations. J. Neurosurg. Pediatr. 2012, 9, 372–378. [Google Scholar] [CrossRef]
- Cavender, R.K.; Schmidt, J.H., 3rd. Tonsillar ectopia and Chiari malformations: Monozygotic triplets. Case report. J. Neurosurg. 1995, 82, 497–500. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Wellons, J.C., 3rd; Oakes, W.J. Asymmetry of tonsillar ectopia in Chiari I malformation. Pediatr. Neurosurg. 2002, 37, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Boyles, A.L.; Enterline, D.S.; Hammock, P.H.; Siegel, D.G.; Slifer, S.H.; Mehltretter, L.; Gilbert, J.R.; Hu-Lince, D.; Stephan, D.; Batzdorf, U.; et al. Phenotypic definition of Chiari type I malformation coupled with high-density SNP genome screen shows significant evidence for linkage to regions on chromosomes 9 and 15. Am. J. Med Genet. Part A 2006, 140, 2776–2785. [Google Scholar] [CrossRef]
- Markunas, C.A.; Enterline, D.S.; Dunlap, K.; Soldano, K.; Cope, H.; Stajich, J.; Grant, G.; Fuchs, H.; Gregory, S.G.; Ashley-Koch, A.E. Genetic evaluation and application of posterior cranial fossa traits as endophenotypes for Chiari type I malformation. Ann. Hum. Genet. 2014, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Musolf, A.M.; Ho, W.S.C.; Long, K.A.; Zhuang, Z.; Argersinger, D.P.; Sun, H.; Moiz, B.A.; Simpson, C.L.; Mendelevich, E.G.; Bogdanov, E.I.; et al. Small posterior fossa in Chiari I malformation affected families is significantly linked to 1q43-44 and 12q23-24.11 using whole exome sequencing. Eur. J. Hum. Genet. 2019, 27, 1599–1610. [Google Scholar] [CrossRef]
- Hammersley, J.; Haughton, V.; Wang, Y.; del Rio, A.M. Tapering of the cervical spinal canal in patients with scoliosis with and without the Chiari I malformation. AJNR Am. J. Neuroradiol. 2012, 33, 1752–1755. [Google Scholar] [CrossRef]
- Hirano, M.; Haughton, V.; del Rio, A.M. Tapering of the Cervical Spinal Canal in Patients with Chiari I Malformations. AJNR Am. J. Neuroradiol. 2012, 33, 1326–1330. [Google Scholar] [CrossRef]
- Rutan, E.; Madan, N.; Zea, R.; Haughton, V. Spinal subarachnoid space tapering in patients with syringomyelia. Neuroradiol. J. 2019, 32, 382–385. [Google Scholar] [CrossRef]
- Thakar, S.; Sivaraju, L.; Jacob, K.S.; Arun, A.A.; Aryan, S.; Mohan, D.; Kiran, N.A.S.; Hegde, A.S. A points-based algorithm for prognosticating clinical outcome of Chiari malformation Type I with syringomyelia: Results from a predictive model analysis of 82 surgically managed adult patients. J. Neurosurg. Spine 2018, 28, 23–32. [Google Scholar] [CrossRef]
- Zhu, Z.; Sha, S.; Sun, X.; Liu, Z.; Yan, H.; Zhu, W.; Wang, Z.; Qiu, Y. Tapering of the cervical spinal canal in patients with distended or nondistended syringes secondary to Chiari type I malformation. AJNR Am. J. Neuroradiol. 2014, 35, 2021–2026. [Google Scholar] [CrossRef]
- Thakar, S.; Siddappa, A.K.; Aryan, S.; Mohan, D.; Kiran, N.A.S.; Hegde, A.S. Does the mesodermal derangement in Chiari Type I malformation extend to the cervical spine? Evidence from an analytical morphometric study on cervical paraspinal muscles. J. Neurosurg. Spine 2017, 27, 421–427. [Google Scholar] [CrossRef]
- Alperin, N.; Loftus, J.R.; Oliu, C.J.; Bagci, A.M.; Lee, S.H.; Ertl-Wagner, B.; Green, B.; Sekula, R. Magnetic Resonance Imaging Measures of Posterior Cranial Fossa Morphology and Cerebrospinal Fluid Physiology in Chiari Malformation Type I. Neurosurgery 2014, 75, 515–522. [Google Scholar] [CrossRef]
- Badie, B.; Mendoza, D.; Batzdorf, U. Posterior Fossa Volume and Response to Suboccipital Decompression in Patients with Chiari I Malformation. Neurosurgery 1995, 37, 214–218. [Google Scholar] [CrossRef]
- Biswas, D.; Eppelheimer, M.S.; Houston, J.R.; Ibrahimy, A.; Bapuraj, J.R.; Labuda, R.; Allen, P.A.; Frim, D.; Loth, F. Quantification of Cerebellar Crowding in Type I Chiari Malformation. Ann. Biomed. Eng. 2019, 47, 731–743. [Google Scholar] [CrossRef]
- Botelho, R.V.; Heringer, L.C.; Botelho, P.B.; Lopes, R.A.; Waisberg, J. Posterior fossa dimensions of Chiari malformation patients compared with normal subjects: Systematic review and meta-analysis. World Neurosurg. 2020, 138, 521–529.e522. [Google Scholar] [CrossRef]
- Dagtekin, A.; Avci, E.; Kara, E.; Uzmansel, D.; Dagtekin, O.; Koseoglu, A.; Talas, D.; Bagdatoglu, C. Posterior cranial fossa morphometry in symptomatic adult Chiari I malformation patients: Comparative clinical and anatomical study. Clin. Neurol. Neurosurg. 2011, 113, 399–403. [Google Scholar] [CrossRef]
- Houston, J.R.; Eppelheimer, M.S.; Pahlavian, S.H.; Biswas, D.; Urbizu, A.; Martin, B.A.; Bapuraj, J.R.; Luciano, M.; Allen, P.A.; Loth, F. A morphometric assessment of type I Chiari malformation above the McRae line: A retrospective case-control study in 302 adult female subjects. J. Neuroradiol. 2018, 45, 23–31. [Google Scholar] [CrossRef]
- Nyland, H.; Krogness, K.G. Size of posterior fossa in Chiari type 1 malformation in adults. Acta Neurochir. 1978, 40, 233–242. [Google Scholar] [CrossRef]
- Shuman, W.H.; DiRisio, A.; Carrasquilla, A.; Lamb, C.D.; Quinones, A.; Pionteck, A.; Yang, Y.; Kurt, M.; Shrivastava, R.K. Is there a morphometric cause of Chiari malformation type I? Analysis of existing literature. Neurosurg. Rev. 2021, 45, 263–273. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Z.; Xu, R.; Liao, Z.; Yan, Y.; Tang, W.; Xia, Y. Chiari Malformations Type I without Basilar Invagination in Adults: Morphometric and Volumetric Analysis. World Neurosurg. 2020, 143, e640–e647. [Google Scholar] [CrossRef]
- Roller, L.A.; Bruce, B.B.; Saindane, A.M. Demographic confounders in volumetric MRI analysis: Is the posterior fossa really small in the adult Chiari 1 malformation? AJR Am. J. Roentgenol. 2015, 204, 835–841. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Hill, M.; Loukas, M.; Shoja, M.M.; Oakes, W.J. Volumetric analysis of the posterior cranial fossa in a family with four generations of the Chiari malformation Type I. J. Neurosurg. Pediatr. 2008, 1, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Dlouhy, B.J.; Dawson, J.D.; Menezes, A.H. Intradural pathology and pathophysiology associated with Chiari I malformation in children and adults with and without syringomyelia. J. Neurosurg. Pediatr. 2017, 20, 526–541. [Google Scholar] [CrossRef]
- Frič, R.; Eide, P.K. Comparative observational study on the clinical presentation, intracranial volume measurements, and intracranial pressure scores in patients with either Chiari malformation Type I or idiopathic intracranial hypertension. J. Neurosurg. 2017, 126, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Heffez, D.S.; Golchini, R.; Ghorai, J.; Cohen, B. Operative findings and surgical outcomes in patients undergoing Chiari 1 malformation decompression: Relationship to the extent of tonsillar ectopia. Acta Neurochir. 2019, 162, 1539–1547. [Google Scholar] [CrossRef]
- Chae, J.K.; Haghdel, A.; Kelly, A.; Cruz, A.; Wood, M.; Luhmann, G.; Greenfield, J.P. Ventral Tonsillar Herniation Predicts Headaches in Adults with Chiari Malformation. World Neurosurg. 2021, 155, e453–e459. [Google Scholar] [CrossRef]
- Ozsoy, K.M.; Oktay, K.; Cetinalp, N.E.; Gezercan, Y.; Erman, T. The Role of Cine Flow Magnetic Resonance Imaging in Patients with Chiari 0 Malformation. Turk. Neurosurg. 2018, 28, 251–256. [Google Scholar] [CrossRef]
- Kyoshima, K.; Kuroyanagi, T.; Oya, F.; Kamijo, Y.; El-Noamany, H.; Kobayashi, S. Syringomyelia without hindbrain herniation: Tight cisterna magna. Report of four cases and a review of the literature. J. Neurosurg. 2002, 96, 239–249. [Google Scholar] [CrossRef]
- Taylor, D.G.; Chatrath, A.; Mastorakos, P.; Paisan, G.; Chen, C.J.; Buell, T.J.; Jane, J.A. Cerebrospinal fluid area and syringogenesis in Chiari malformation type I. J. Neurosurg. 2020, 134, 825–830. [Google Scholar] [CrossRef]
- Klekamp, J.; Iaconetta, G.; Batzdorf, U.; Samii, M. Syringomyelia associated with foramen magnum arachnoiditis. J. Neurosurg. Spine 2002, 97, 317–322. [Google Scholar] [CrossRef]
- Newton, E.J. Syringomyelia as a manifestation of defective fourth ventricular drainage. Ann. R. Coll. Surg. Engl. 1969, 44, 194–213. [Google Scholar] [PubMed]
- Heiss, J.D.; Patronas, N.; DeVroom, H.L.; Shawker, T.; Ennis, R.; Kammerer, W.; Eidsath, A.; Talbot, T.; Morris, J.; Eskioglu, E.; et al. Elucidating the pathophysiology of syringomyelia. J. Neurosurg. 1999, 91, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E.H. Pathogenesis of Chiari I—Pathophysiology of syringomyelia: Implications for therapy: A summary of 3 decades of clinical research. Neurosurgery 2017, 64, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E.H.; Muraszko, K.; Shawker, T.H.; Patronas, N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J. Neurosurg. 1994, 80, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Heiss, J.D.; Argersinger, D.P. Epidemiology of Chiari I Malformation. In The Chiari Malformations; Tubbs, R.S., Turgut, M., Oakes, W.J., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 263–274. [Google Scholar]
- Strahle, J.; Muraszko, K.M.; Kapurch, J.; Bapuraj, J.R.; Garton, H.J.; Maher, C.O. Chiari malformation Type I and syrinx in children undergoing magnetic resonance imaging. J. Neurosurg. Pediatr. 2011, 8, 205–213. [Google Scholar] [CrossRef]
- Vernooij, M.W.; Ikram, M.A.; Tanghe, H.L.; Vincent, A.J.; Hofman, A.; Krestin, G.P.; Niessen, W.J.; Breteler, M.M.; van der Lugt, A. Incidental Findings on Brain MRI in the General Population. N. Engl. J. Med. 2007, 357, 1821–1828. [Google Scholar] [CrossRef]
- Basaran, R.; Bozdogan, C.; Senol, M.; Gundogan, D.; Isik, N. Long-term outcomes of surgical management in subtypes of Chiari malformation. Neurol. Res. 2021, 43, 760–766. [Google Scholar] [CrossRef]
- Whitney, N.; Sun, H.; Pollock, J.M.; Ross, D.A. The human foramen magnum—Normal anatomy of the cisterna magna in adults. Neuroradiology 2013, 55, 1333–1339. [Google Scholar] [CrossRef]
- Haller, G.; Sadler, B.; Kuensting, T.; Lakshman, N.; Greenberg, J.K.; Strahle, J.M.; Park, T.S.; Dobbs, M.B.; Gurnett, C.A.; Limbrick, D.D. Obex position is associated with syringomyelia and use of posterior fossa decompression among patients with Chiari I malformation. J. Neurosurg. Pediatr. 2020, 26, 45–52. [Google Scholar] [CrossRef]
- Seaman, S.C.; Li, L.; Menezes, A.H.; Dlouhy, B.J. Fourth ventricle roof angle as a measure of fourth ventricle bowing and a radiographic predictor of brainstem dysfunction in Chiari malformation type I. J. Neurosurg. Pediatr. 2021, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fischbein, R.; Saling, J.R.; Marty, P.; Kropp, D.; Meeker, J.; Amerine, J.; Chyatte, M.R. Patient-reported Chiari malformation type I symptoms and diagnostic experiences: A report from the national Conquer Chiari Patient Registry database. Neurol. Sci. 2015, 36, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.M.; Oro, J.J. Prospective analysis of presenting symptoms among 265 patients with radiographic evidence of Chiari malformation type I with or without syringomyelia. J. Am. Acad. Nurse Pract. 2004, 16, 134–138. [Google Scholar] [CrossRef]
- Batzdorf, U. Clinical Presentation of Adult Chiari I. In The Chiari Malformations, 2nd ed.; Tubbs, R.S., Turgut, M., Oakes, W.J., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 391–397. [Google Scholar]
- Bagci, A.M.; Lee, S.H.; Nagornaya, N.; Green, B.A.; Alperin, N. Automated posterior cranial fossa volumetry by MRI: Applications to Chiari malformation type I. AJNR Am. J. Neuroradiol. 2013, 34, 1758–1763. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, E.I.; Heiss, J.D.; Mendelevich, E.G. The post–syrinx syndrome: Stable central myelopathy and collapsed or absent syrinx. J. Neurol. 2006, 253, 707–713. [Google Scholar] [CrossRef]
- Doruk, E.; Ozay, R.; Sekerci, Z.; Durmaz, H.A.; Gunes, S.O.; Hanalioglu, S.; Sorar, M. Cervico-medullary compression ratio: A novel radiological parameter correlating with clinical severity in Chiari type 1 malformation. Clin. Neurol. Neurosurg. 2018, 174, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Lirng, J.-F.; Fuh, J.-L.; Chang, F.-C.; Cheng, H.-C.; Wang, S.-J. Primary Cough Headache is Associated with Posterior Fossa Crowdedness: A Morphometric MRI Study. Cephalalgia 2004, 24, 694–699. [Google Scholar] [CrossRef]
- Kumar, A.; Agrawal, M.; Prakash, S.; Somorendra, S.; Singh, P.K.; Garg, A.; Singh, M.; Sharma, B.S. Acute Foramen Magnum Syndrome Following Single Diagnostic Lumbar Puncture: Consequence of a Small Posterior Fossa? World Neurosurg. 2016, 91, 677.e1–677.e7. [Google Scholar] [CrossRef]
- Liu, J.; Liu, R.; Liu, B.; Zhou, J.; Fan, C.; Jiao, F.; Wang, D.; Li, F.; Hei, B. Small Posterior Cranial Fossa and Cerebellopontine Cistern Volumes Are Associated with Bilateral Trigeminal Neuralgia. Front. Neurol. 2020, 11, 573239. [Google Scholar] [CrossRef]
- Neufeld, E.A.; Menacho, S.T.; Shah, L.M. Craniocervical Junction and Posterior Fossa Dimensions can Affect Need for Decompressive Craniectomy in Posterior Cranial Fossa Hemorrhage. World Neurosurg. 2019, 127, e570–e577. [Google Scholar] [CrossRef]
- Strahle, J.; Muraszko, K.M.; Garton, H.J.; Smith, B.W.; Starr, J.; Kapurch, J.R., II; Maher, C.O. Syrinx location and size according to etiology: Identification of Chiari-associated syrinx. J. Neurosurg. Pediatr. 2015, 16, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]

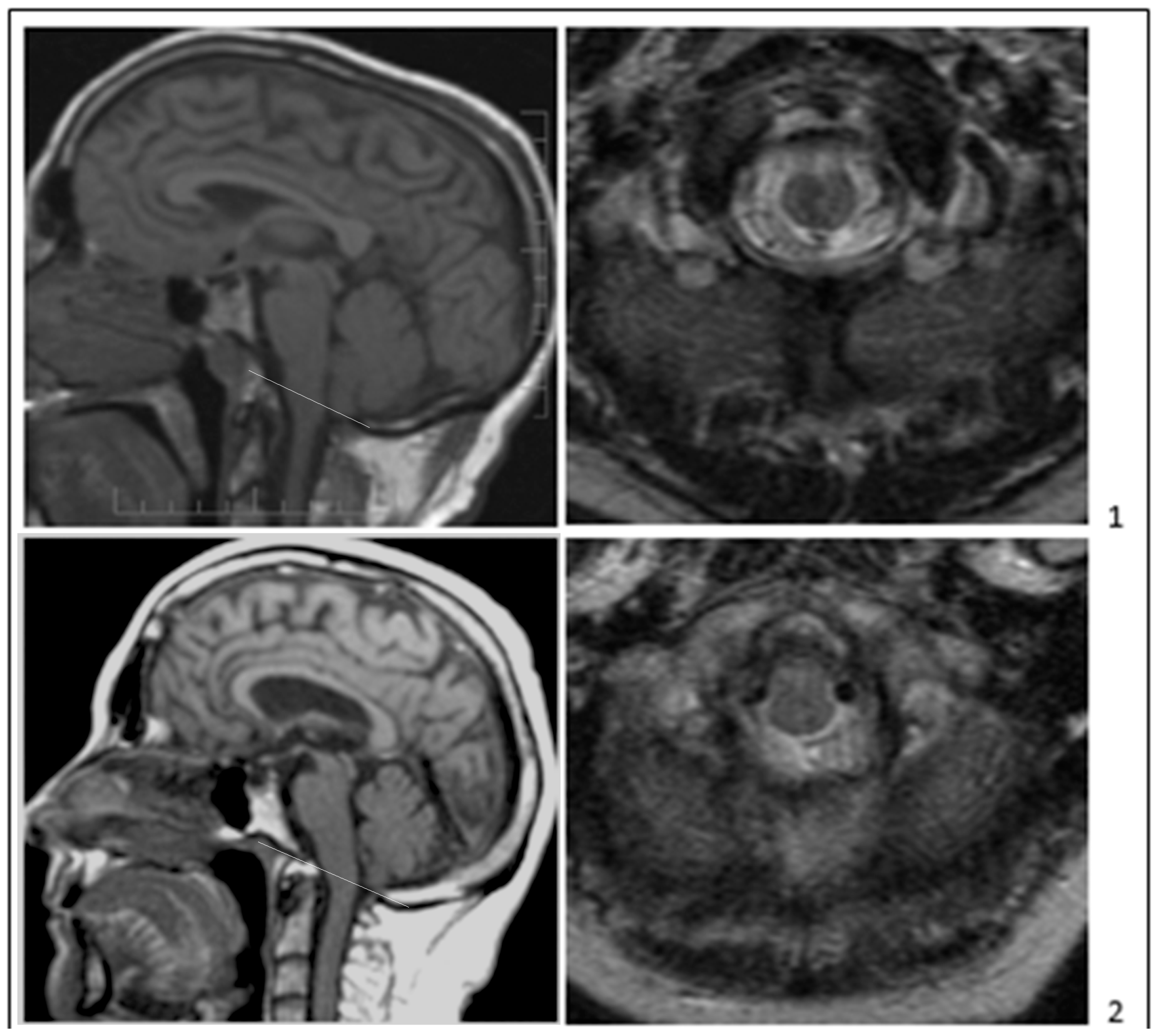
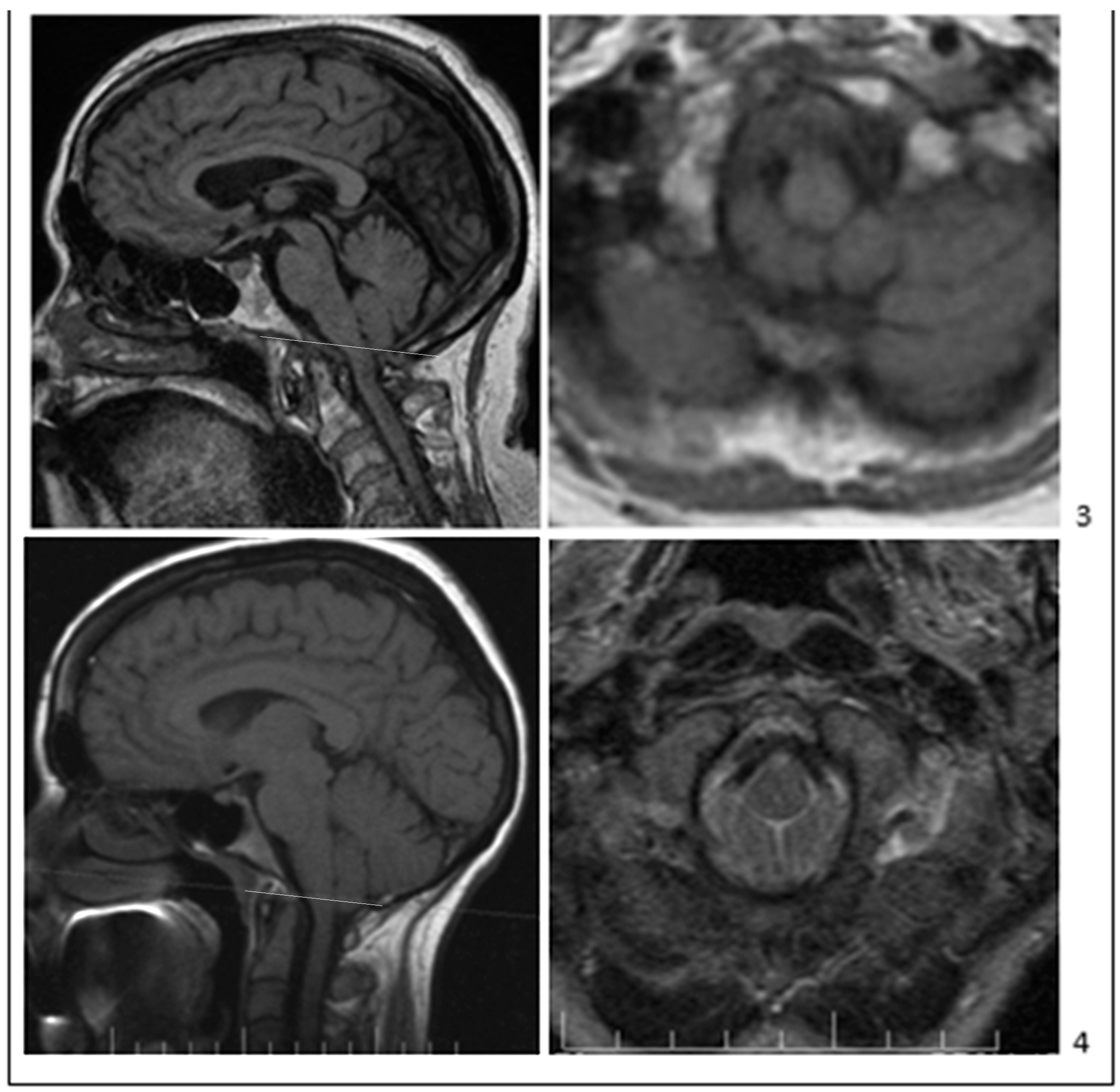
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanov, E.I.; Faizutdinova, A.T.; Heiss, J.D. The Small Posterior Cranial Fossa Syndrome and Chiari Malformation Type 0. J. Clin. Med. 2022, 11, 5472. https://doi.org/10.3390/jcm11185472
Bogdanov EI, Faizutdinova AT, Heiss JD. The Small Posterior Cranial Fossa Syndrome and Chiari Malformation Type 0. Journal of Clinical Medicine. 2022; 11(18):5472. https://doi.org/10.3390/jcm11185472
Chicago/Turabian StyleBogdanov, Enver I., Aisylu T. Faizutdinova, and John D. Heiss. 2022. "The Small Posterior Cranial Fossa Syndrome and Chiari Malformation Type 0" Journal of Clinical Medicine 11, no. 18: 5472. https://doi.org/10.3390/jcm11185472
APA StyleBogdanov, E. I., Faizutdinova, A. T., & Heiss, J. D. (2022). The Small Posterior Cranial Fossa Syndrome and Chiari Malformation Type 0. Journal of Clinical Medicine, 11(18), 5472. https://doi.org/10.3390/jcm11185472




