MicroShunt versus Trabeculectomy for Surgical Management of Glaucoma: A Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgery
2.3. Outcome Measures
2.4. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Primary Outcomes
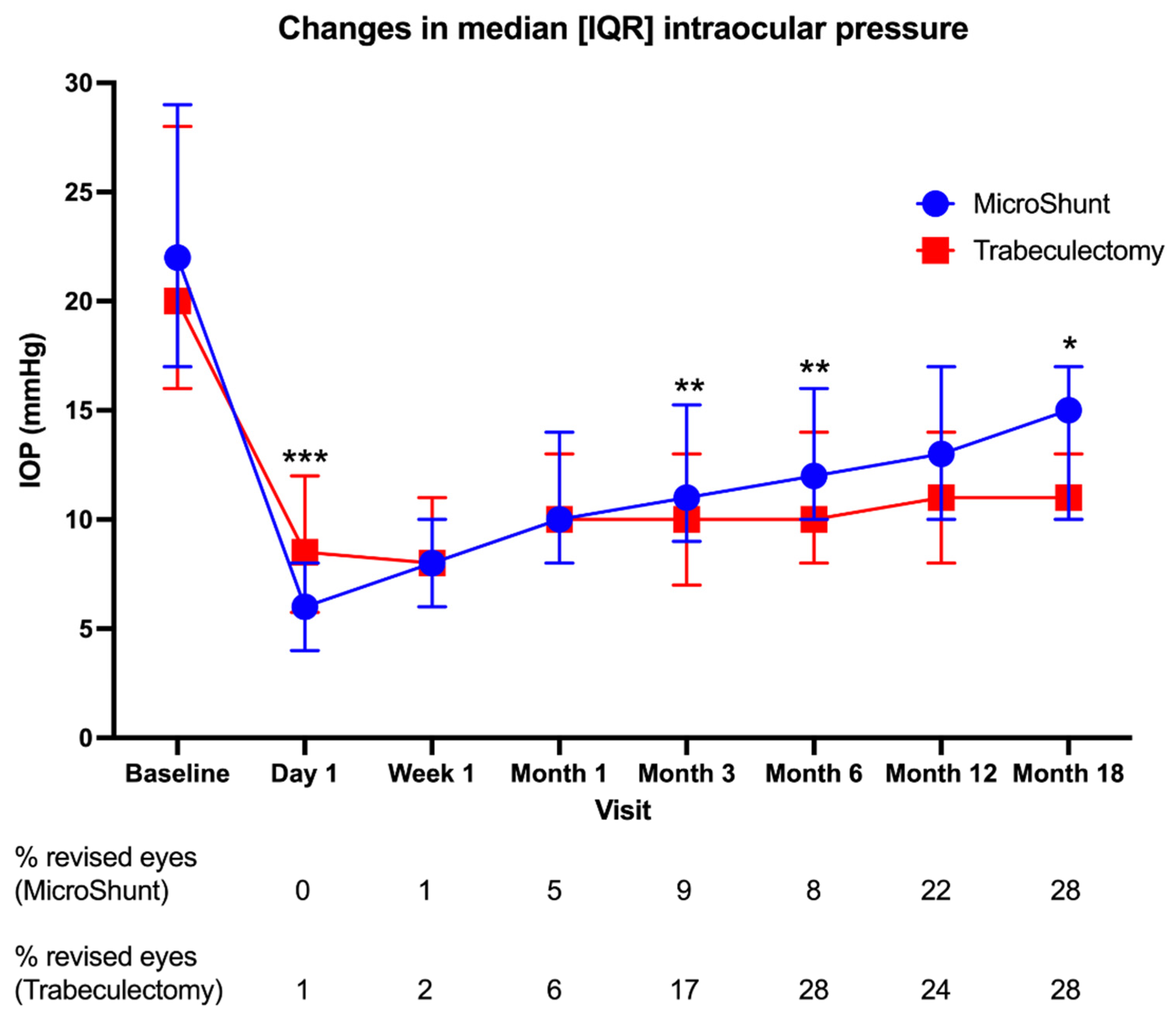
3.2.1. IOP
3.2.2. Medications
3.3. Secondary Outcomes
3.3.1. VF/MD
3.3.2. RNFL Thickness
3.3.3. Success
3.3.4. Complications and Interventions
3.3.5. Operation Time and Number of Visits
4. Discussion
4.1. IOP
4.2. Medications
4.3. VF/MD
4.4. RNFL Thickness
4.5. Success Rates
4.6. Complications and Theatre Interventions
4.7. Postoperative Visits and Operation Times
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quigley, H.; Broman, A.T. The Number of People with Glaucoma Worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Gaasterland, D.E.; Ederer, F.; Beck, A.; Costarides, A.; Leef, D.; Closek, J.; Banks, J.; Jackson, S.; Moore, K.; Vela, A.; et al. The Advanced Glaucoma Intervention Study (AGIS): 7. The Relationship between Control of Intraocular Pressure and Visual Field Deterioration.The AGIS Investigators. Am. J. Ophthalmol. 2000, 130, 429–440. [Google Scholar] [CrossRef]
- Pillunat, K.R.; Herber, R.; Haase, M.A.; Jamke, M.; Jasper, C.S.; Pillunat, L.E. PRESERFLOTM MicroShunt versus Trabeculectomy: First Results on Efficacy and Safety. Acta Ophthalmol. 2021, 100, e779–e790. [Google Scholar] [CrossRef]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L. Treatment Outcomes in the Tube Versus Trabeculectomy (TVT) Study after Five Years of Follow-Up. Am. J. Ophthalmol. 2012, 153, 789–803.e2. [Google Scholar] [CrossRef] [PubMed]
- Landers, J.; Martin, K.; Sarkies, N.; Bourne, R.; Watson, P. A Twenty-Year Follow-up Study of Trabeculectomy: Risk Factors and Outcomes. Ophthalmology 2012, 119, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Richter, G.M.; Coleman, A.L. Minimally Invasive Glaucoma Surgery: Current Status and Future Prospects. Clin. Ophthalmol. 2016, 10, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Batlle, J.F.; Fantes, F.; Riss, I.; Pinchuk, L.; Alburquerque, R.; Kato, Y.P.; Arrieta, E.; Peralta, A.C.; Palmberg, P.; Parrish, R.K.; et al. Three-Year Follow-up of a Novel Aqueous Humor MicroShunt. J. Glaucoma 2016, 25, e58–e65. [Google Scholar] [CrossRef]
- Holland, L.J.; Mercieca, K.J.; Kirwan, J.F. Effect of COVID-19 Pandemic on Glaucoma Surgical Practices in the UK. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Buffault, J.; Graber, M.; Bensmail, D.; Bluwol, É.; Jeanteur, M.N.; Abitbol, O.; Benhatchi, N.; Sauvan, L.; Lachkar, Y. Efficacy and Safety at 6 Months of the XEN Implant for the Management of Open Angle Glaucoma. Sci. Rep. 2020, 10, 4527. [Google Scholar] [CrossRef]
- Schlenker, M.B.; Durr, G.M.; Michaelov, E.; Ahmed, I.I.K. Intermediate Outcomes of a Novel Standalone Ab Externo SIBS Microshunt with Mitomycin C. Am. J. Ophthalmol. 2020, 215, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Nakashima, K.I.; Watanabe-Kitamura, F.; Watanabe, T.; Nakamura, K.; Maki, K.; Shimazaki, A.; Kato, M.; Tanihara, H.; Inoue, T. Intraocular Pressure-Lowering Effects of Trabeculectomy Versus MicroShunt Insertion in Rabbit Eyes. Transl. Vis. Sci. Technol. 2021, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.D.; Barnebey, H.S.; Moster, M.R.; Stiles, M.C.; Vold, S.D.; Khatana, A.K.; Flowers, B.E.; Grover, D.S.; Strouthidis, N.G.; Panarelli, J.F. Ab-Externo MicroShunt versus Trabeculectomy in Primary Open-Angle Glaucoma: One-Year Results from a 2-Year Randomized, Multicenter Study. Ophthalmology 2021, 128, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.M.; Schuster, A.K.; Munder, A.; Muehl, M.; Pfeiffer, N.; Hoffmann, E.M. Comparison of Subconjunctival Microinvasive Glaucoma Surgery and Trabeculectomy. Acta Ophthalmol. 2021, 100, e1120–e1126. [Google Scholar] [CrossRef] [PubMed]
- Lichter, P.R.; Musch, D.C.; Gillespie, B.W.; Guire, K.E.; Janz, N.K.; Wren, P.A.; Mills, M.P.H.R.P. Interim Clinical Outcomes in the Collaborative Initial Glaucoma Treatment Study Comparing Initial Treatment Randomized to Medications or Surgery. Ophthalmology 2001, 108, 1943–1953. [Google Scholar] [CrossRef]
- Shaarawy, T.M.; Sherwood, M.B.; Grehn, F. Guidelines on Design and Reporting of Glaucoma Surgical Trials; Kugler Publications: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Batlle, J.F.; Corona, A.; Albuquerque, R. Long-Term Results of the PRESERFLO MicroShunt in Patients with Primary Open-Angle Glaucoma from a Single-Center Nonrandomized Study. J. Glaucoma 2021, 30, 281–286. [Google Scholar] [CrossRef]
- Tanner, A.; Haddad, F.; Fajardo-Sanchez, J.; Nguyen, E.; Xin Thong, K.; Ah-Moye, S.; Perl, N.; Abu-Bakra, M.; Kulkarni, A.; Trikha, S.; et al. One-Year Surgical Outcomes of the PreserFlo MicroShunt in Glaucoma: A Multicentre Analysis. Br. J. Ophthalmol. 2022, 1–8. [Google Scholar] [CrossRef]
- Gedde, S.J.; Feuer, W.J.; Lim, K.S.; Barton, K.; Goyal, S.; Ahmed, I.I.K.; Brandt, J.D. Treatment Outcomes in the Primary Tube Versus Trabeculectomy Study after 3 Years of Follow-Up. Ophthalmology 2020, 127, 333–345. [Google Scholar] [CrossRef]
- Wanichwecharungruang, B.; Ratprasatporn, N. 24-Month Outcomes of XEN45 Gel Implant versus Trabeculectomy in Primary Glaucoma. PLoS ONE 2021, 16, e0256362. [Google Scholar] [CrossRef]
- Schargus, M.; Busch, C.; Rehak, M.; Meng, J.; Schmidt, M.; Bormann, C.; Unterlauft, J.D. Functional Monitoring after Trabeculectomy or XEN Microstent Implantation Using Spectral Domain Optical Coherence Tomography and Visual Field Indices—A Retrospective Comparative Cohort Study. Biology 2021, 10, 273. [Google Scholar] [CrossRef]
- Raghu, N.; Pandav, S.S.; Kaushik, S.; Ichhpujani, P.; Gupta, A. Effect of Trabeculectomy on RNFL Thickness and Optic Disc Parameters Using Optical Coherence Tomography. Eye 2012, 26, 1131. [Google Scholar] [CrossRef] [Green Version]
- Quigley, H.A.; Addicks, E.M.; Green, W.R.; Maumenee, A.E. Optic Nerve Damage in Human Glaucoma. II. The Site of Injury and Susceptibility to Damage. Arch. Ophthalmol. 1981, 99, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Ch’ng, T.W.; Gillmann, K.; Hoskens, K.; Rao, H.L.; Mermoud, A.; Mansouri, K. Effect of Surgical Intraocular Pressure Lowering on Retinal Structures—Nerve Fibre Layer, Foveal Avascular Zone, Peripapillary and Macular Vessel Density: 1 Year Results. Eye 2020, 34, 562. [Google Scholar] [CrossRef] [PubMed]
- Mwanza, J.C.; Budenz, D.L.; Warren, J.L.; Webel, A.D.; Reynolds, C.E.; Barbosa, D.T.; Lin, S. Retinal Nerve Fibre Layer Thickness Floor and Corresponding Functional Loss in Glaucoma. Br. J. Ophthalmol. 2015, 99, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.F.; Lockwood, A.J.; Shah, P.; Macleod, A.; Broadway, D.C.; King, A.J.; McNaught, A.I.; Agrawal, P. Trabeculectomy in the 21st Century: A Multicenter Analysis. Ophthalmology 2013, 120, 2532–2539. [Google Scholar] [CrossRef]
- Iwasaki, K.; Takamura, Y.; Nishida, T.; Sawada, A.; Iwao, K.; Shinmura, A.; Kunimatsu-Sanuki, S.; Yamamoto, T.; Tanihara, H.; Sugiyama, K.; et al. Comparing Trabeculectomy Outcomes between First and Second Operated Eyes: A Multicenter Study. PLoS ONE 2016, 11, e0162569. [Google Scholar] [CrossRef]
- Karakosta, A.; Vassilaki, M.; Plainis, S.; Elfadl, N.H.; Tsilimbaris, M.; Moschandreas, J. Choice of Analytic Approach for Eye-Specific Outcomes: One Eye or Two? Am. J. Ophthalmol 2012, 153, 571–579.e1. [Google Scholar] [CrossRef]
- Atik, A.; Fahy, E.; Rhodes, L.A.; Samuels, B.C.; Mennemeyer, S.T.; Girkin, C.A. Comparative Cost-Effectiveness of Trabeculectomy versus MicroShunt in the United States Medicare System. Ophthalmology, 2022, in press. [CrossRef]


| Patient Characteristics | MicroShunt | Trabeculectomy | p-Value |
|---|---|---|---|
| Age (years) | 69 [57–78] | 66 [57–76] | 0.250 * |
| IOP (mmHg) | 22 [17–29] | 20 [16–28] | 0.182 * |
| Number of medications | 4 [3–4] | 4 [3–4] | 0.273 * |
| 94 (93.1%) | 96 (95.0%) | 0.767 # |
| 76 (75.2%) | 85 (84.2%) | 0.161 # |
| 53 (52.5%) | 63 (62.4%) | 0.200 # |
| 91 (90.1%) | 92 (91.1%) | >0.999 # |
| 0 (0.0%) | 1 (1.0%) | >0.999 # |
| Ethnic group | - | ||
| 41 (40.6%) | 41 (40.6%) | |
| 17 (16.8%) | 17 (16.8%) | |
| 14 (13.9%) | 14 (13.9%) | |
| 15 (14.9%) | 15 (14.9%) | |
| 14 (13.9%) | 14 (13.9%) | |
| Identify as female | 37 (36.6%) | 37 (36.6%) | - |
| Identify as male | 64 (63.4%) | 64 (63.4%) | - |
| Best-corrected visual acuity (logMAR) | 0.2 [0.0–0.5] | 0.2 [0.0–0.5] | 0.814 * |
| Visual field mean deviation (dB) | −13.35 ± 8.10 | −14.38 ± 8.13 | 0.546 ** |
| Average RNFL thickness (microns) | 57 [47–71] | 55 [47–68] | 0.670 * |
| Type of glaucoma | - | ||
| 74 (73.3%) | 74 (73.3%) | |
| 12 (11.9%) | 12 (11.9%) | |
| 14 (13.9%) | 14 (13.9%) | |
| 1 (1.0%) | 1 (1.0%) | |
| Previous laser treatment | 37 (36.6%) | 29 (28.7%) | 0.294 # |
| 13 (12.9%) | 7 (6.9%) | 0.238 # |
| 7 (6.9%) | 8 (7.9%) | >0.999 # |
| 14 (13.9%) | 10 (9.9%) | 0.515 # |
| 8 (7.9%) | 6 (5.9%) | 0.783 # |
| 1 (1.0%) | 0 (0.0%) | >0.999 # |
| Previous MIGS | 12 (11.9%) | 10 (9.9%) | 0.822 # |
| 8 (7.9%) | 5 (5.0%) | 0.568 # |
| 4 (4.0%) | 6 (5.0%) | 0.748 # |
| First or second eye undergoing glaucoma surgery | - | ||
| 77 (76.2%) | 77 (76.2%) | |
| 24 (23.8%) | 24 (23.8%) | |
| Co-morbidities | |||
| 20 (19.8%) | 22 (21.8%) | 0.863 # |
| 48 (47.5%) | 42 (41.6%) | 0.479 # |
| 3 (3.0%) | 2 (2.0%) | >0.999 # |
| ASA grade | 0.030 + | ||
| 18 (17.8%) | 34 (33.7%) | |
| 69 (68.3%) | 53 (52.5%) | |
| 13 (12.9%) | 13 (12.9%) | |
| Pseudophakic lens status | 51 (50.5%) | 41 (40.6%) | 0.220 # |
| MicroShunt | p-Value (Comparison with Baseline) | Trabeculectomy | p-Value (Comparison with Baseline) | p-Value (Comparison between Groups) | |
|---|---|---|---|---|---|
| Baseline | |||||
| VF MD (dB) | −13.35 ± 8.10 | - | −14.38 ± 8.13 | - | 0.546 ** |
| RNFL (μm) | 57 [47–71] | - | 55 [47–68] | - | 0.670 * |
| Day-1 | |||||
| RNFL (μm) | 59 [46–78] | <0.001 # | 59 [49–75] | 0.002 # | 0.795 * |
| Week-1 | |||||
| RNFL (μm) | 64 [52–75] | <0.001 # | 56 [46–78] | 0.011 # | 0.151 * |
| Month-1 | |||||
| RNFL (μm) | 56 [49–71] | 0.732 # | 56 [48–68] | 0.181 # | 0.947 * |
| Month-3 | |||||
| RNFL (μm) | 56 [46–66] | 0.012 # | 54 [47–65] | <0.001 # | 0.663 * |
| Month-6 | |||||
| VF MD (dB) | −13.80 ± 7.53 | 0.668 ## | −14.47 ± 8.56 | 0.147 ## | 0.735 ** |
| RNFL (μm) | 57 [48–66] | 0.072 # | 50 [44–55] | <0.001 # | 0.005 * |
| Month-12 | |||||
| VF MD (dB) | −14.52 ± 8.18 | 0.348 ## | −15.73 ± 7.39 | 0.192 ## | 0.638 ** |
| RNFL (μm) | 55 [47–69] | 0.114 # | 50 [43–55] | 0.003 # | 0.046 * |
| Month-18 | |||||
| VF MD (dB) | −15.97 ± 8.26 | 0.009 ## | −16.45 ± 8.08 | 0.026 ## | 0.882 ** |
| RNFL (μm) | 52 [46–63] | 0.101 # | 51 [46–61] | 0.016 # | 0.674 * |
| Complication/Intervention | MicroShunt n (%) | Trabeculectomy n (%) | p-Value |
|---|---|---|---|
| Hypotony | 46 (45.5%) | 51 (50.5%) | 0.573 |
| 44 (43.6%) | 50 (49.5%) | 0.890 |
| 4 (4.0%) | 16 (15.8%) | 0.008 |
| Chronic hypotony | 0 (0.0%) | 10 (9.9%) | 0.002 |
| Choroidal effusion | 13 (12.9%) | 15 (14.9%) | 0.839 |
| Choroidal detachment | 0 (0.0%) | 2 (2.0%) | 0.498 |
| Hypotony maculopathy | 1 (1.0%) | 9 (8.9%) | 0.019 |
| Hyphaema | 17 (16.8%) | 11 (10.9%) | 0.309 |
| Malignant glaucoma | 1 (1.0%) | 0 (0.0%) | >0.999 |
| Laser suture lysis | 0 (0.0%) | 10 (9.9%) * | 0.002 |
| Flat AC | 4 (4.0%) | 6 (5.9%) | 0.748 |
| AC reformation in theatre | 0 (0.0%) | 4 (4.0%) * | 0.121 |
| AC washout in clinic | 1 (1.0%) | 0 (0.0%) | >0.999 |
| AC washout in theatre | 2 (2.0%) | 0 (0.0%) | 0.498 |
| AC Avastin injection in theatre | 0 (0.0%) | 2 (2.0%) | 0.498 |
| Bleb revision in clinic | 3 (3.0%) * ** | 1 (1.0%) | 0.621 |
| Bleb revision in theatre | 11 (10.9%) * *** | 25 (24.8%) * ** *** **** | 0.016 |
| Secondary Cyclodiode treatment | 2 (2.0%) * | 0 (0.0%) | 0.498 |
| Secondary IOP-lowering surgery | 2 (2.0%) | 0 (0.0%) | 0.498 |
| Laser suture lysis | 0 (0.0%) | 4 (4.0%) * | 0.121 |
| Suture removal in theatre | 0 (0.0%) | 3 (3.0%) | 0.246 |
| Total n with complications (excluding hypotony) | 32 (31.7%) | 46 (45.5%) | 0.060 |
| 59 (48.5%) | 66 (65.3%) | 0.385 |
| Total n with theatre interventions | 17 (16.8%) | 31 (30.7%) | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, M.X.; Normando, E.M.; Luk, S.M.H.; Deshmukh, M.; Ahmed, F.; Crawley, L.; Ameen, S.; Vig, N.; Cordeiro, M.F.; Bloom, P.A. MicroShunt versus Trabeculectomy for Surgical Management of Glaucoma: A Retrospective Analysis. J. Clin. Med. 2022, 11, 5481. https://doi.org/10.3390/jcm11185481
Fu MX, Normando EM, Luk SMH, Deshmukh M, Ahmed F, Crawley L, Ameen S, Vig N, Cordeiro MF, Bloom PA. MicroShunt versus Trabeculectomy for Surgical Management of Glaucoma: A Retrospective Analysis. Journal of Clinical Medicine. 2022; 11(18):5481. https://doi.org/10.3390/jcm11185481
Chicago/Turabian StyleFu, Michael X., Eduardo M. Normando, Sheila M. H. Luk, Mira Deshmukh, Faisal Ahmed, Laura Crawley, Sally Ameen, Niten Vig, Maria Francesca Cordeiro, and Philip A. Bloom. 2022. "MicroShunt versus Trabeculectomy for Surgical Management of Glaucoma: A Retrospective Analysis" Journal of Clinical Medicine 11, no. 18: 5481. https://doi.org/10.3390/jcm11185481
APA StyleFu, M. X., Normando, E. M., Luk, S. M. H., Deshmukh, M., Ahmed, F., Crawley, L., Ameen, S., Vig, N., Cordeiro, M. F., & Bloom, P. A. (2022). MicroShunt versus Trabeculectomy for Surgical Management of Glaucoma: A Retrospective Analysis. Journal of Clinical Medicine, 11(18), 5481. https://doi.org/10.3390/jcm11185481





