Therapeutic Advances in Diabetic Nephropathy
Abstract
:1. Introduction
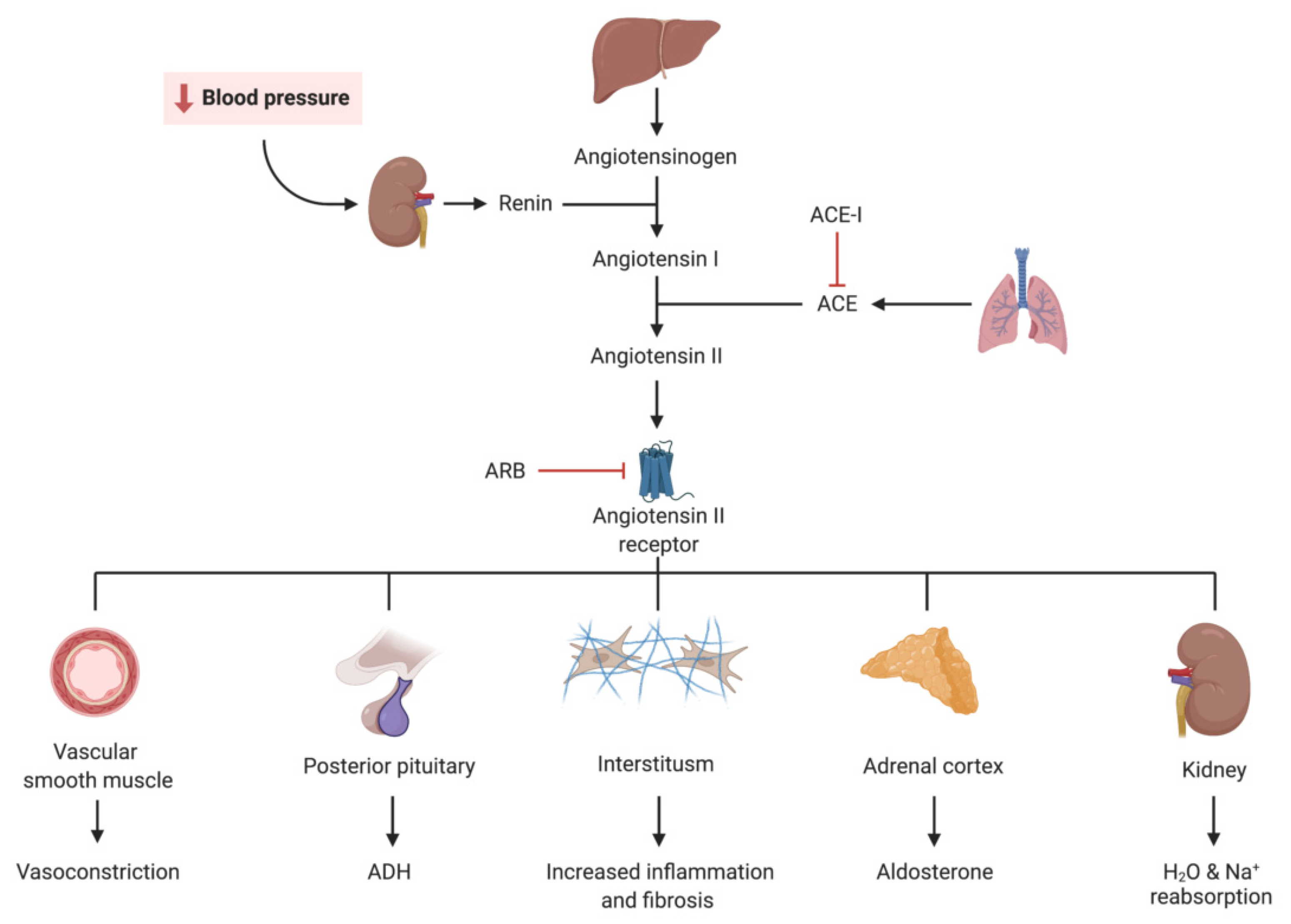
2. Renin Angiotensin Aldosterone System (RAAS) Blockade (ACE-I and ARB)
2.1. Introduction
2.2. Landmark Trials
2.3. Practical Considerations
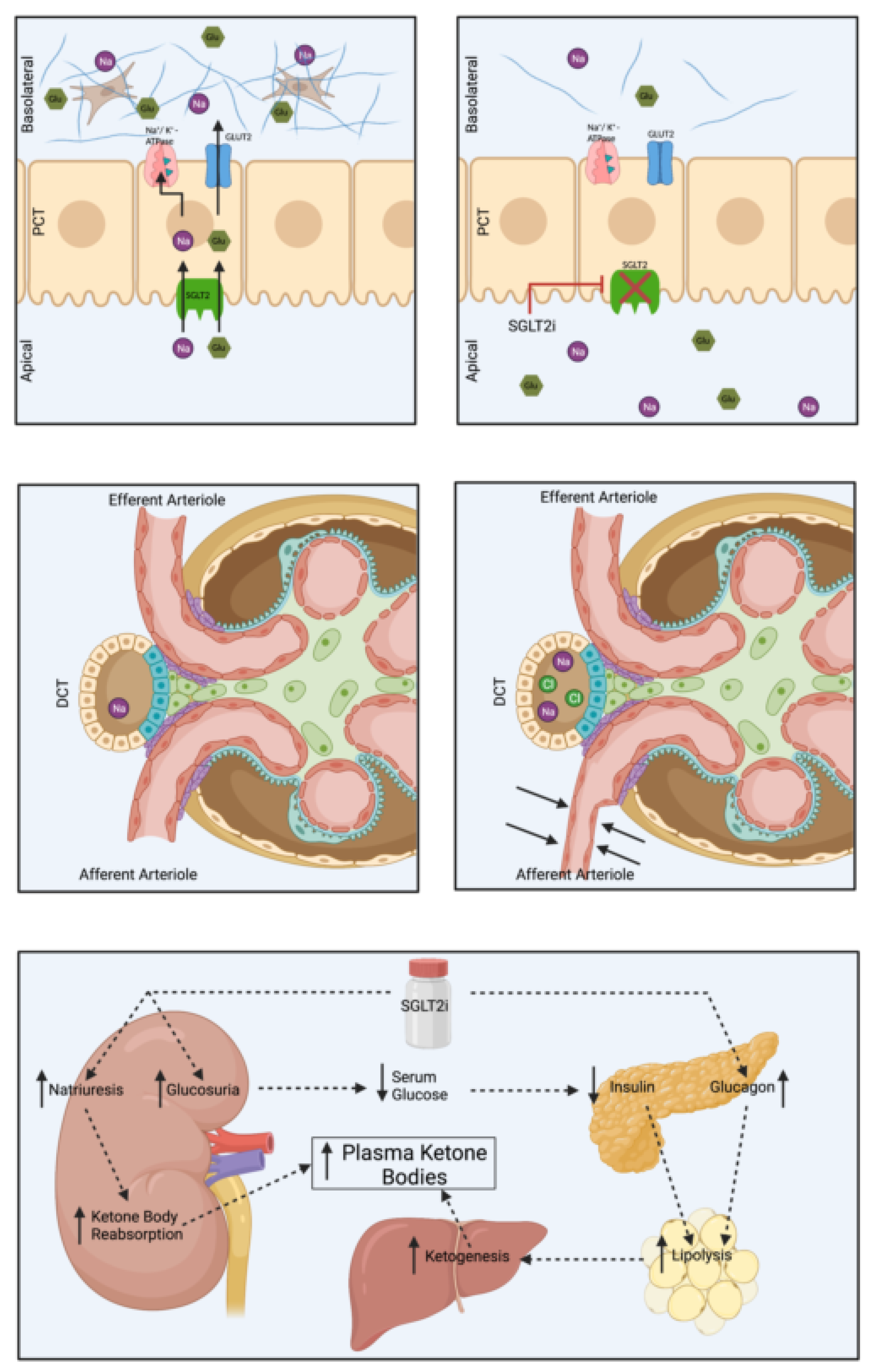
3. SGLT2 Inhibitors
3.1. Introduction
3.2. Landmark Trials
3.3. Practical Considerations
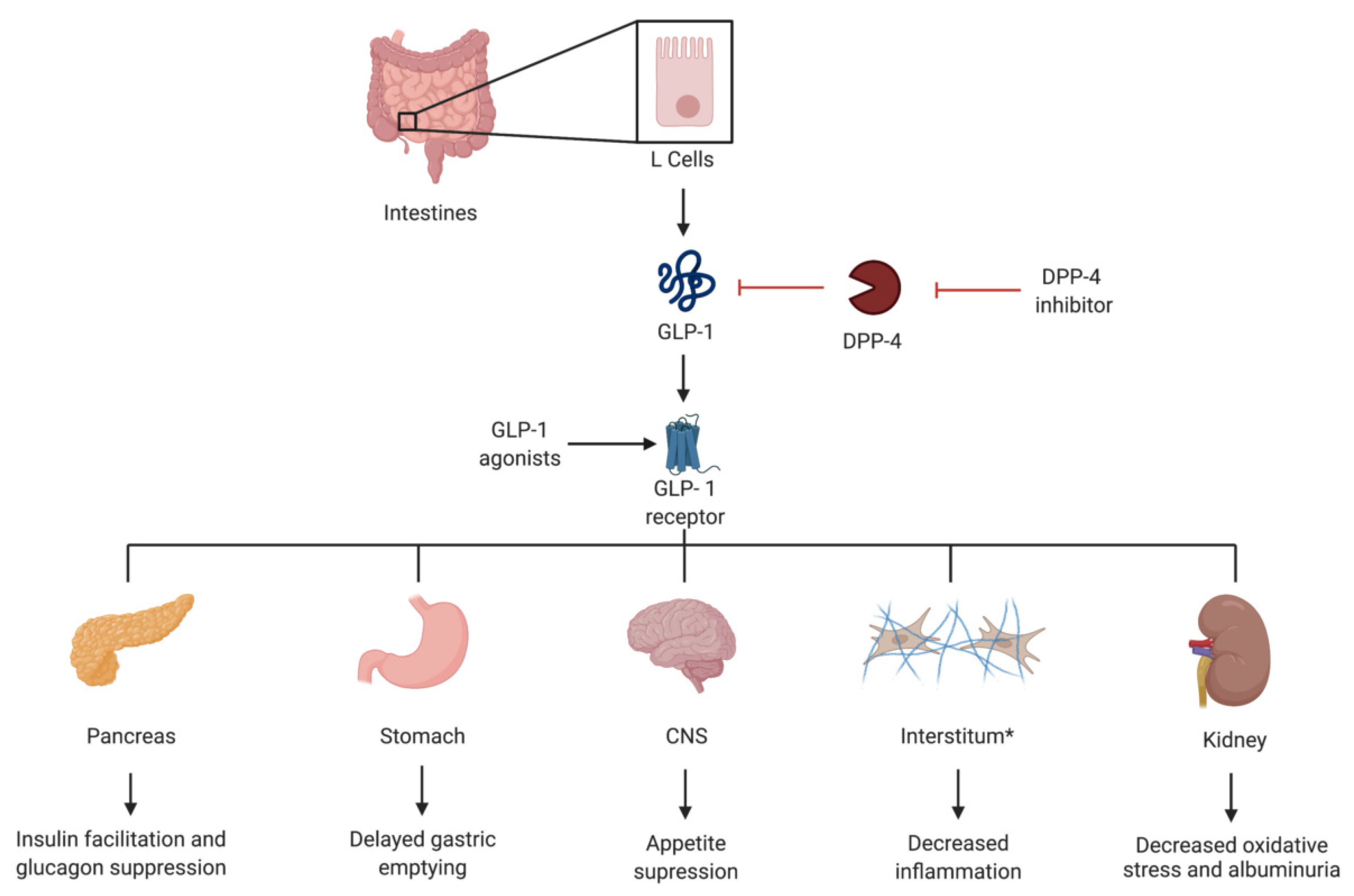
4. GLP-1 Agonists
4.1. Introduction
4.2. Landmark Trials
4.3. Practical Considerations
5. Mineralocorticoid Receptor Antagonists
5.1. Introduction
5.2. Landmark Trials
5.3. Practical Considerations
6. Endothelin Antagonists
6.1. Introduction
6.2. Landmark Trials
6.3. Practical Considerations
7. Potential Future Therapeutic Options
8. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE-I | angiotensin converting enzyme inhibitor |
| ARB | Angiotensin receptor blocker |
| CHF | Congestive heart failure |
| CKD | Chronic kidney disease |
| CKRT | continuous kidney replacement therapy |
| CI | confidence interval |
| CV | cardiovascular |
| DKA | diabetic keto acidosis |
| DKD | diabetic kidney disease |
| ESKD | end stage kidney disease |
| eGFR | estimated glomerular filtration rate |
| ENaC | Epithelial sodium channel |
| GLP-1 | glucagon like peptide-1 |
| HR | Hazard ratio |
| HTN | Hypertension |
| KRT | Kidney replacement therapy |
| MI | myocardial infarction |
| MR | mineralocorticoid receptor |
| MRA | mineralocorticoid receptor antagonist |
| RAAS | renin–angiotensin–aldosterone system |
| SGLT2 | sodium-glucose transport protein 2 |
| T2DM | type II diabetes mellitus |
| UACR | urine albumin to creatinine ratio |
References
- United States Renal Data System. USRDS 2018 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2017. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [Green Version]
- Montinaro, V.; Cicardi, M. ACE inhibitor-mediated angioedema. Int. Immunopharmacol. 2020, 78, 106081. [Google Scholar] [CrossRef]
- Blythe, W.B. Captopril and renal autoregulation. N. Engl. J. Med. 1983, 308, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Hommel, E.; Parving, H.H.; Mathiesen, E.; Edsberg, B.; Damkjaer Nielsen, M.; Giese, J. Effect of captopril on kidney function in insulin-dependent diabetic patients with nephropathy. Br. Med. J. 1986, 293, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Björck, S.; Nyberg, G.; Mulec, H.; Granerus, G.; Herlitz, H.; Aurell, M. Beneficial effects of angiotensin converting enzyme inhibition on renal function in patients with diabetic nephropathy. Br. Med. J. 1986, 293, 471–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagrue, G.; Robeva, R.; Laurent, J. Antiproteinuric effect of captopril in primary glomerular disease. Nephron 1987, 46, 99–100. [Google Scholar] [CrossRef]
- Ikeda, T.; Nakayama, D.; Gomi, T.; Sakurai, J.; Yamazaki, T.; Yuhara, M. Captopril, an angiotensin I-converting enzyme inhibitor, decreases proteinuria in hypertensive patients with renal diseases. Nephron 1989, 52, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Heeg, J.E.; de Jong, P.E.; van der Hem, G.K.; de Zeeuw, D. Reduction of proteinuria by angiotensin converting enzyme inhibition. Kidney Int. 1987, 32, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The Effect of Angiotensin-Converting-Enzyme Inhibition on Diabetic Nephropathy. N. Engl. J. Med. 1993, 329, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S.; et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I.; et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, S.; Teo, K.K.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P.; Anderson, C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 2008, 358, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: Evidence-based advances in monitoring and treatment. Kidney Int. 2020, 98, 839–848. [Google Scholar] [CrossRef]
- Sha, S.; Devineni, D.; Ghosh, A.; Polidori, D.; Chien, S.; Wexler, D.; Shalayda, K.; Demarest, K.; Rothenberg, P. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes. Metab. 2011, 13, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Devineni, D.; Morrow, L.; Hompesch, M.; Skee, D.; Vandebosch, A.; Murphy, J.; Ways, K.; Schwartz, S. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes. Metab. 2012, 14, 539–545. [Google Scholar] [CrossRef]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2016, 39, S165–S171. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, R.E. Sodium-glucose linked transporter-2 inhibitors: Potential for renoprotection beyond blood glucose lowering? Kidney Int. 2014, 86, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Hasan, R.; Lasker, S.; Hasan, A.; Zerin, F.; Zamila, M.; Parvez, F.; Rahman, M.M.; Khan, F.; Subhan, N.; Alam, M.A. Canagliflozin ameliorates renal oxidative stress and inflammation by stimulating AMPK-Akt-eNOS pathway in the isoprenaline-induced oxidative stress model. Sci. Rep. 2020, 10, 14659. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Desai, M.; Jardine, M.; Balis, D.; Meininger, G.; Perkovic, V. Canagliflozin Slows Progression of Renal Function Decline Independently of Glycemic Effects. J. Am. Soc. Nephrol. 2017, 28, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrannini, E.; Mark, M.; Mayoux, E. CV Protection in the EMPA-REG OUTCOME Trial: A “Thrifty Substrate” Hypothesis. Diabetes Care 2016, 39, 1108–1114. [Google Scholar] [CrossRef] [Green Version]
- Mudaliar, S.; Alloju, S.; Henry, R.R. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care 2016, 39, 1115–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Wanner, C. EMPA-REG OUTCOME: The Nephrologist’s Point of View. Am. J. Med. 2017, 130, S63–S72. [Google Scholar] [CrossRef] [Green Version]
- Rhee, J.J.; Jardine, M.J.; Chertow, G.M.; Mahaffey, K.W. Dedicated kidney disease-focused outcome trials with sodium-glucose cotransporter-2 inhibitors: Lessons from CREDENCE and expectations from DAPA-HF, DAPA-CKD, and EMPA-KIDNEY. Diabetes Obes. Metab. 2020, 22, 46–54. [Google Scholar] [CrossRef]
- Tanaka, T.; Higashijima, Y.; Wada, T.; Nangaku, M. The potential for renoprotection with incretin-based drugs. Kidney Int. 2014, 86, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Turton, M.D.; O’Shea, D.; Gunn, I.; Beak, S.A.; Edwards, C.M.; Meeran, K.; Choi, S.J.; Taylor, G.M.; Heath, M.M.; Lambert, P.D.; et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996, 379, 69–72. [Google Scholar] [CrossRef]
- Bullock, B.P.; Heller, R.S.; Habener, J.F. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 1996, 137, 2968–2978. [Google Scholar] [CrossRef] [Green Version]
- Campos, R.V.; Lee, Y.C.; Drucker, D.J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 1994, 134, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Mima, A.; Hiraoka-Yamomoto, J.; Li, Q.; Kitada, M.; Li, C.; Geraldes, P.; Matsumoto, M.; Mizutani, K.; Park, K.; Cahill, C.; et al. Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCβ activation in diabetes. Diabetes 2012, 61, 2967–2979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendarto, H.; Inoguchi, T.; Maeda, Y.; Ikeda, N.; Zheng, J.; Takei, R.; Yokomizo, H.; Hirata, E.; Sonoda, N.; Takayanagi, R. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism 2012, 61, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Kodera, R.; Shikata, K.; Kataoka, H.U.; Takatsuka, T.; Miyamoto, S.; Sasaki, M.; Kajitani, N.; Nishishita, S.; Sarai, K.; Hirota, D.; et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011, 54, 965–978. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, K.R.; Lakshmanan, M.C.; Rayner, B.; Busch, R.S.; Zimmermann, A.G.; Woodward, D.B.; Botros, F.T. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 605–617. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Sattar, N.; Rosenstock, J.; Ramasundarahettige, C.; Pratley, R.; Lopes, R.D.; Lam, C.S.P.; Khurmi, N.S.; Heenan, L.; Del Prato, S.; et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 896–907. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef] [Green Version]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.M.; Evans, M. Semaglutide: Charting New Horizons in GLP-1 Analogue Outcome Studies. Diabetes Ther. Res. Treat. Educ Diabetes Relat Disord 2020, 11, 2221–2235. [Google Scholar] [CrossRef]
- Buonafine, M.; Bonnard, B.; Jaisser, F. Mineralocorticoid Receptor and Cardiovascular Disease. Am. J. Hypertens 2018, 31, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, E.; Gomez-Sanchez, C.E. The multifaceted mineralocorticoid receptor. Compr. Physiol. 2014, 4, 965–994. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Sanchez, E.P.; Gomez-Sanchez, C.E. Central regulation of blood pressure by the mineralocorticoid receptor. Mol. Cell Endocrinol. 2012, 350, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Menuet, D.; Isnard, R.; Bichara, M.; Viengchareun, S.; Muffat-Joly, M.; Walker, F.; Zennaro, M.C.; Lombès, M. Alteration of cardiac and renal functions in transgenic mice overexpressing human mineralocorticoid receptor. J. Biol. Chem. 2001, 276, 38911–38920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrera-Chimal, J.; Pérez-Villalva, R.; Rodríguez-Romo, R.; Reyna, J.; Uribe, N.; Gamba, G.; Bobadilla, N.A. Spironolactone prevents chronic kidney disease caused by ischemic acute kidney injury. Kidney Int. 2013, 83, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejía-Vilet, J.M.; Ramírez, V.; Cruz, C.; Uribe, N.; Gamba, G.; Bobadilla, N.A. Renal ischemia-reperfusion injury is prevented by the mineralocorticoid receptor blocker spironolactone. Am. J. Physiol. Ren. Physiol. 2007, 293, F78–F86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zannad, F.; McMurray, J.J.V.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B.; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Alexandrou, M.-E.; Papagianni, A.; Tsapas, A.; Loutradis, C.; Boutou, A.; Piperidou, A.; Papadopoulou, D.; Ruilope, L.; Bakris, G.; Sarafidis, P. Effects of mineralocorticoid receptor antagonists in proteinuric kidney disease: A systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2019, 37, 2307–2324. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Nigwekar, S.U.; Sehgal, A.R.; Strippoli, G.F.M. Aldosterone antagonists for preventing the progression of chronic kidney disease: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 542–551. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.V.; O’Meara, E. Treatment of heart failure with spironolactone—Trial and tribulations. N. Engl. J. Med. 2004, 351, 526–528. [Google Scholar] [CrossRef]
- Kolkhof, P.; Nowack, C.; Eitner, F. Nonsteroidal antagonists of the mineralocorticoid receptor. Curr. Opin. Nephrol. Hypertens 2015, 24, 417–424. [Google Scholar] [CrossRef]
- Pitt, B.; Kober, L.; Ponikowski, P.; Gheorghiade, M.; Filippatos, G.; Krum, H.; Nowack, C.; Kolkhof, P.; Kim, S.-Y.; Zannad, F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur. Heart J. 2013, 34, 2453–2463. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Chan, J.C.; Cooper, M.E.; Gansevoort, R.T.; Haller, H.; Remuzzi, G.; Rossing, P.; Schmieder, R.E.; Nowack, C.; et al. Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial. JAMA 2015, 314, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharm. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benigni, A. Defining the role of endothelins in renal pathophysiology on the basis of selective and unselective endothelin receptor antagonist studies. Curr. Opin. Nephrol. Hypertens 1995, 4, 349–353. [Google Scholar] [CrossRef]
- Neuhofer, W.; Pittrow, D. Endothelin receptor selectivity in chronic kidney disease: Rationale and review of recent evidence. Eur. J. Clin. Investig. 2009, 39, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Fenhammar, J.; Andersson, A.; Frithiof, R.; Forestier, J.; Weitzberg, E.; Sollevi, A.; Hjelmqvist, H. The endothelin receptor antagonist tezosentan improves renal microcirculation in a porcine model of endotoxemic shock. Acta Anaesthesiol. Scand. 2008, 52, 1385–1393. [Google Scholar] [CrossRef]
- de Zeeuw, D.; Coll, B.; Andress, D.; Brennan, J.J.; Tang, H.; Houser, M.; Correa-Rotter, R.; Kohan, D.; Lambers Heerspink, H.J.; Makino, H.; et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1083–1093. [Google Scholar] [CrossRef] [Green Version]
- Mann, J.F.E.; Green, D.; Jamerson, K.; Ruilope, L.M.; Kuranoff, S.J.; Littke, T.; Viberti, G.; ASCEND Study Group. Avosentan for overt diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Heerspink, H.J.L.; Parving, H.-H.; Andress, D.L.; Bakris, G.; Correa-Rotter, R.; Hou, F.-F.; Kitzman, D.W.; Kohan, D.; Makino, H.; McMurray, J.J.V.; et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): A double-blind, randomised, placebo-controlled trial. Lancet 2019, 393, 1937–1947. [Google Scholar] [CrossRef]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenstock, J.; Kahn, S.E.; Johansen, O.E.; Zinman, B.; Espeland, M.A.; Woerle, H.J.; Pfarr, E.; Keller, A.; Mattheus, M.; Baanstra, D.; et al. Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA 2019, 322, 1155–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttle, K.R.; Brosius, F.C.; Adler, S.G.; Kretzler, M.; Mehta, R.L.; Tumlin, J.A.; Tanaka, Y.; Haneda, M.; Liu, J.; Silk, M.E.; et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: Results from a Phase 2 randomized controlled clinical trial. Nephrol. Dial. Transpl. 2018, 33, 1950–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Lee, K.; Ni, Z.; He, J.C. Diabetic Kidney Disease: Challenges, Advances, and Opportunities. Kidney Dis. 2020, 6, 215–225. [Google Scholar] [CrossRef]
- Gorin, Y.; Cavaglieri, R.C.; Khazim, K.; Lee, D.-Y.; Bruno, F.; Thakur, S.; Fanti, P.; Szyndralewiez, C.; Barnes, J.L.; Block, K.; et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Ren. Physiol. 2015, 308, F1276–F1287. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.J.; Min, H.S.; Kim, K.T.; Kim, J.E.; Ghee, J.Y.; Kim, H.W.; Lee, J.E.; Han, J.Y.; Lee, G.; Ha, H.J.; et al. APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury. Lab. Investig. 2017, 97, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Boels, M.G.S.; Koudijs, A.; Avramut, M.C.; Sol, W.M.P.J.; Wang, G.; van Oeveren-Rietdijk, A.M.; van Zonneveld, A.J.; de Boer, H.C.; van der Vlag, J.; van Kooten, C.; et al. Systemic Monocyte Chemotactic Protein-1 Inhibition Modifies Renal Macrophages and Restores Glomerular Endothelial Glycocalyx and Barrier Function in Diabetic Nephropathy. Am. J. Pathol. 2017, 187, 2430–2440. [Google Scholar] [CrossRef] [Green Version]
- Menne, J.; Eulberg, D.; Beyer, D.; Baumann, M.; Saudek, F.; Valkusz, Z.; Więcek, A.; Haller, H.; Emapticap Study Group. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol. Dial. Transpl. 2017, 32, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Tye, S.C.; Denig, P.; Heerspink, H.J.L. Precision medicine approaches for diabetic kidney disease: Opportunities and challenges. Nephrol. Dial. Transpl. 2021, 36, 3–9. [Google Scholar] [CrossRef]

| Trial | Publication Year | Treatment(s) | Primary Composite Kidney Outcome | Risk Reduction |
|---|---|---|---|---|
| CSG Captopril [11] | 1993 | Captopril vs. placebo | Doubling of the base-line serum creatinine concentration | 48% |
| RENAAL [12] | 2001 | Losartan vs. placebo | Doubling of serum creatinine, ESKD or death | 16% |
| IDNT [13] | 2001 | Irbesartan vs. amlodipine vs. placebo | Doubling of serum creatinine, ESKD or death | 20% vs. placebo 23% vs. amlodipine |
| Trial | Year Published | Treatment (s) | Primary or Secondary End-Point | Composite Kidney Outcome | Hazard Ratio (95% CI) |
|---|---|---|---|---|---|
| EMPA-REG OUTCOME [24] | 2015 | Empagliflozin vs. placebo | Secondary | Doubling of serum creatinine, initiation of kidney replacement therapy or death from renal disease | 0.54 (0.40–0.75) |
| CANVAS [25] | 2017 | Canagliflozin vs. placebo | Secondary | Sustained 40% reduction in eGFR, need for kidney replacement therapy, or death from renal cause | 0.6 (0.47–0.77) |
| CREDENCE [26] | 2019 | Canagliflozin vs. placebo | Primary | End-stage kidney disease, doubling of the serum creatinine level, or death from renal or cardiovascular causes | 0.70 (0.59– 0.82) |
| DECLARE-TIMI [27] | 2019 | Dapagliflozin vs. placebo | Secondary | Sustained ≥40% reduction in eGFR to <60 mL/min/1.73 m2, new end-stage kidney disease or death from renal cause | 0.53 (0.43–0.66) |
| DAPA-CKD [28] | 2020 | Dapagliflozin vs. placebo | Primary | Sustained ≥50% reduction in eGFR, end-stage kidney disease, or death from renal or cardiovascular cause | 0.61 (0.51–0.72) |
| EMPEROR-Reduced [29] | 2020 | Empagliflozin vs. placebo | Secondary | Sustained ≥40% reduction in eGFR, chronic dialysis, renal transplant or sustained eGFR < 10–15 mL/min/1.73 m2 | 0.50 (0.32–0.77) |
| EMPA-KIDNEY | 2022 | Empagliflozin vs. placebo | Primary | End-stage kidney disease, a sustained reduction in eGFR to <10 mL/min/1.73 m2, renal death, or a sustained decline of ≥40% in eGFR | Ongoing |
| Trial | Year Published | Treatment (s) | Primary or Secondary | Kidney Outcome | Results |
|---|---|---|---|---|---|
| LEADER [39] | 2016 | Liraglutide vs. placebo | Secondary | Diabetic Nephropathy | HR 0.78 (95% CI 0.67–0.92) |
| SUSTAIN-6 [40] | 2016 | Semaglutide vs. placebo | Secondary | Macroalbuminuria, doubling of serum creatinine, Creatinine clearance ≤ 45 mL/min or KRT | HR 0.64 (95% CI 0.46–0.88) |
| AWARD-7 [41] | 2018 | Dulaglutide vs. insulin glargine | Secondary | eGFR and UACR | A decline in eGFR of the insulin arm but not in the higher-dose dulaglutide arm |
| REWIND [42] | 2019 | Dulaglutide vs. placebo | Secondary | 300 mg/g > UACR in lower baseline concentration, sustained 30% > eGFR decline, KRT | HR 0.85 (95% CI 0.77–0.93) |
| Kristensen et. al. meta-analysis [43] | 2019 | GLP-1′s | --- | New-onset macroalbuminuria, decline in eGFR, progression of kidney disease or death of kidney cause | HR 0.83 (95% CI 0.78–0.89) |
| AMPLITUDE-O [44] | 2021 | Efpeglenatide vs. placebo | Secondary | Incident microalbuminuria > 300 mg/g, increase in UACR of at least 30% from baseline, sustained eGFR decrease > 40% for > 30 days, KRT for 90 days or more, eGFR < 15 for 30 days or more | HR 0.68 (95% CI 0.57–0.79) |
| FLOW | To be completed in 2024 | Semaglutide vs. placebo | Primary | Persistent ≥ 50% reduction in eGFR, reaching ESKD, death from kidney disease or death from CV cause | Ongoing |
| Trial | Year Published | Composite Kidney Outcome | Primary or Secondary End-Point | Findings or Results |
|---|---|---|---|---|
| ARTS [62] | 2013 | Change in serum potassium | Primary | Significant increases in potassium concentrations at 10 mg/day or more |
| Effect eGFR | Secondary | No change in renal impairment | ||
| ARTS-DN [63] | 2015 | Change in UACR | Primary | Dose dependent placebo-corrected mean UACR |
| Potassium and eGFR safety points | Secondary | 1.7–3.2% discontinuation for hyperkalemia in finerenone arm No finerenone discontinuation due to drop in eGFR | ||
| FIDELIO-DKD [64] | 2020 | Kidney failure, >40% decrease in eGFR, death from kidney cause | Primary | HR 0.82 (95% CI 0.73–0.93) |
| FIGARO-DKD [65] | 2021 | Kidney failure, >40% decrease in eGFR, death from kidney cause | Secondary | HR 0.87 (95% CI 0.76–1.01) |
| Trial | Year | Kidney Outcomes | Findings | Notes |
|---|---|---|---|---|
| ASCEND [71] | 2010 | Doubling of serum creatinine, ESKD, death | No significant change in primary outcome composite | Trial ended early due to safety concerns related to volume overload and CHF |
| SONAR [72] | 2019 | Doubling of serum creatinine, ESKD | HR 0.65 (CI 95% 0.49–0.88) | Trial included and “enrichment period” to determine who can tolerate endothelin antagonist prior to randomization |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawaf, H.; Thomas, G.; Taliercio, J.J.; Nakhoul, G.; Vachharajani, T.J.; Mehdi, A. Therapeutic Advances in Diabetic Nephropathy. J. Clin. Med. 2022, 11, 378. https://doi.org/10.3390/jcm11020378
Sawaf H, Thomas G, Taliercio JJ, Nakhoul G, Vachharajani TJ, Mehdi A. Therapeutic Advances in Diabetic Nephropathy. Journal of Clinical Medicine. 2022; 11(2):378. https://doi.org/10.3390/jcm11020378
Chicago/Turabian StyleSawaf, Hanny, George Thomas, Jonathan J. Taliercio, Georges Nakhoul, Tushar J. Vachharajani, and Ali Mehdi. 2022. "Therapeutic Advances in Diabetic Nephropathy" Journal of Clinical Medicine 11, no. 2: 378. https://doi.org/10.3390/jcm11020378
APA StyleSawaf, H., Thomas, G., Taliercio, J. J., Nakhoul, G., Vachharajani, T. J., & Mehdi, A. (2022). Therapeutic Advances in Diabetic Nephropathy. Journal of Clinical Medicine, 11(2), 378. https://doi.org/10.3390/jcm11020378






