Atrial Functional Tricuspid Regurgitation as a Distinct Pathophysiological and Clinical Entity: No Idiopathic Tricuspid Regurgitation Anymore
Abstract
:1. Introduction
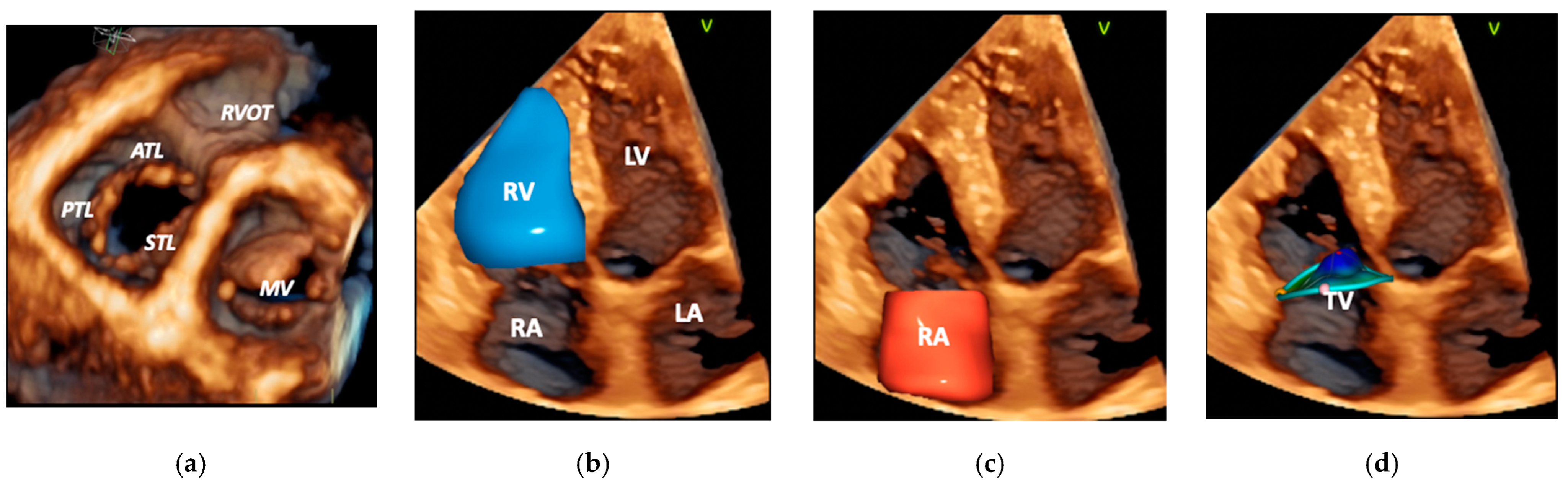
2. Anatomy and Pathophysiology of A-FTR
3. TTVIs in A-FTR
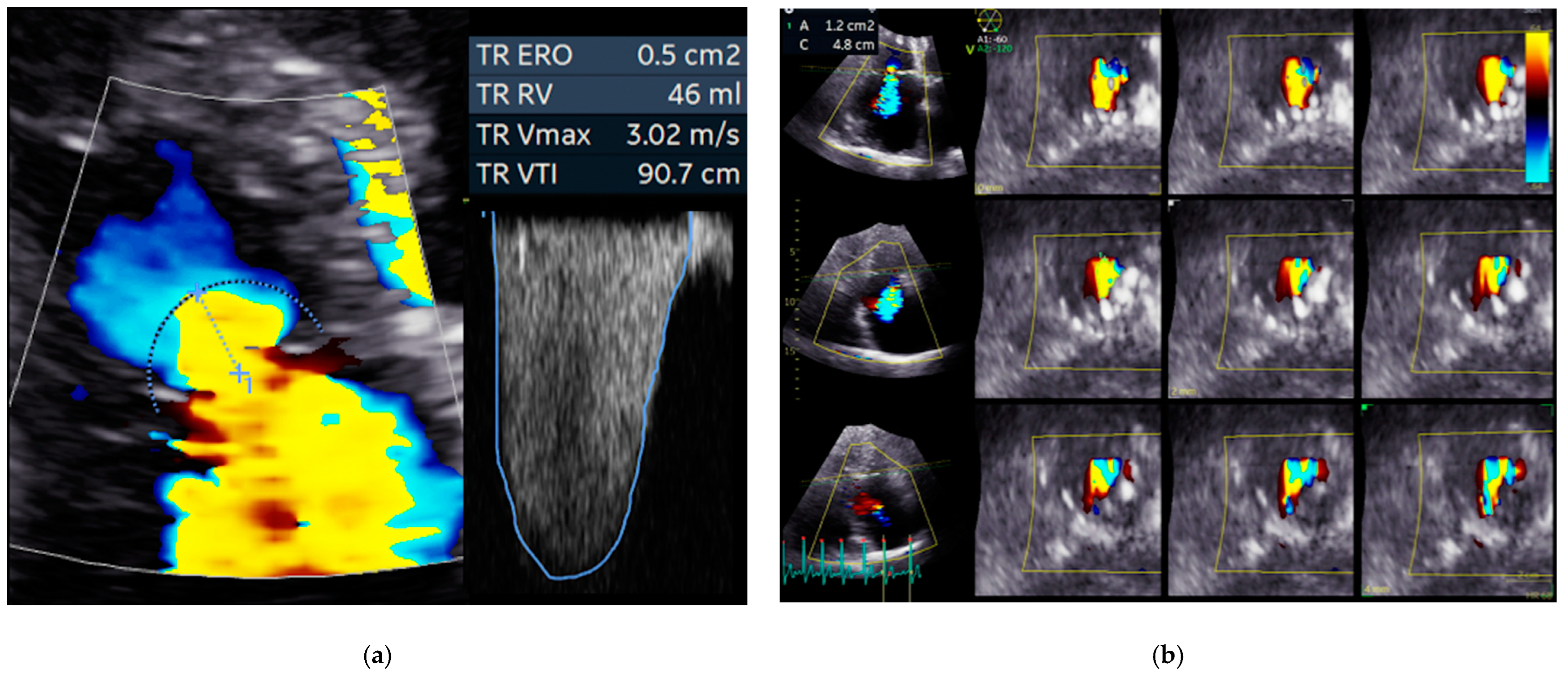
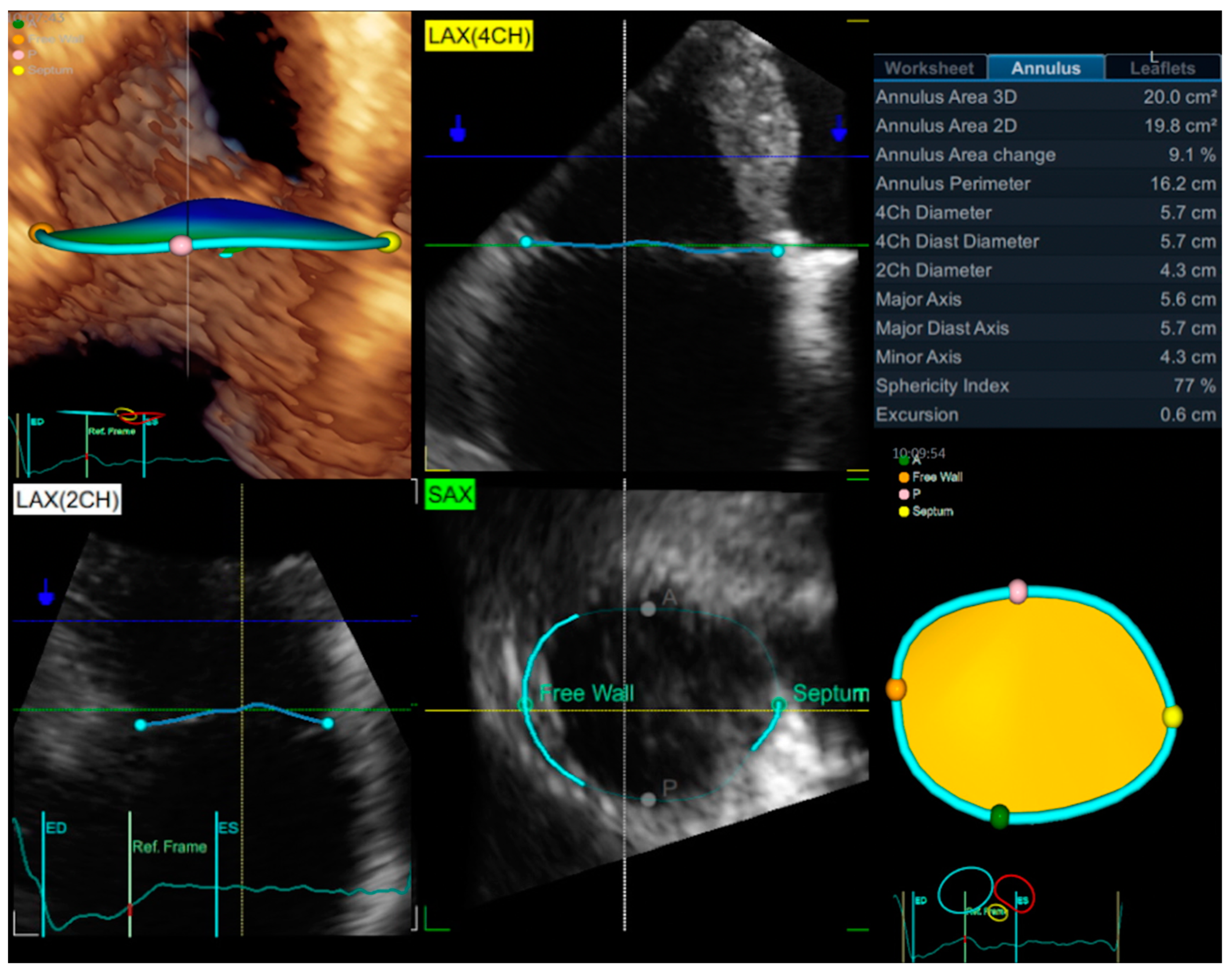
3.1. Echocardiography
3.2. Cardiac Magnetic Resonance, Cardiac Computed Tomography, and Fusion Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badano, L.P.; Muraru, D.; Enriquez-Sarano, M. Assessment of functional tricuspid regurgitation. Eur. Heart J. 2013, 34, 1875–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asmarats, L.; Taramasso, M.; Rodés-Cabau, J. Tricuspid valve disease: Diagnosis, prognosis and management of a rapidly evolving field. Nat. Rev. Cardiol. 2019, 16, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Prihadi, E.A.; Delgado, V.; Leon, M.B.; Enriquez-Sarano, M.; Topilsky, Y.; Bax, J.J. Morphologic Types of Tricuspid Regurgitation: Characteristics and Prognostic Implications. JACC Cardiovasc. Imaging 2019, 12, 491–499. [Google Scholar] [CrossRef]
- Benfari, G.; Antoine, C.; Miller, W.L.; Thapa, P.; Topilsky, Y.; Rossi, A.; Michelena, H.I.; Pislaru, S.; Enriquez-Sarano, M. Excess Mortality Associated with Functional Tricuspid Regurgitation Complicating Heart Failure With Reduced Ejection Fraction. Circulation 2019, 140, 196–206. [Google Scholar] [CrossRef]
- Prihadi, E.A.; van der Bijl, P.; Gursoy, E.; Abou, R.; Mara Vollema, E.; Hahn, R.T.; Stone, G.W.; Leon, M.B.; Ajmone Marsan, N.; Delgado, V.; et al. Development of significant tricuspid regurgitation over time and prognostic implications: New insights into natural history. Eur. Heart J. 2018, 39, 3574–3581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Fulcher, J.; Abeysuriya, N.; McGrady, M.; Wilcox, I.; Celermajer, D.; Lal, S. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: A systematic review and meta-analysis. Eur. Heart J. 2019, 40, 476–484. [Google Scholar] [CrossRef]
- Chorin, E.; Rozenbaum, Z.; Topilsky, Y.; Konigstein, M.; Ziv-Baran, T.; Richert, E.; Keren, G.; Banai, S. Tricuspid regurgitation and long-term clinical outcomes. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 157–165. [Google Scholar] [CrossRef]
- Spinka, G.; Bartko, P.E.; Heitzinger, G.; Prausmüller, S.; Pavo, N.; Frey, M.K.; Arfsten, H.; Genger, M.; Hengstenberg, C.; Hülsmann, M.; et al. Natural Course of Nonsevere Secondary Tricuspid Regurgitation. J. Am. Soc. Echocardiogr. 2021, 34, 13–19. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D.; Verta, P.; Gregson, J.; Pocock, S.J.; Boero, I.; Feldman, T.E.; Abraham, W.T.; Lindenfeld, J.; Bax, J.; Leon, M.; et al. Impact of tricuspid regurgitation on survival in patients with heart failure: A large electronic health record patient-level database analysis. Eur. J. Heart Fail. 2020, 22, 1803–1813. [Google Scholar] [CrossRef]
- Nath, J.; Foster, E.; Heidenreich, P.A. Impact of Tricuspid Regurgitation on Long-Term Survival. J. Am. Coll. Cardiol. 2004, 43, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Voci, D.; Pozzoli, A.; Miura, M.; Gavazzoni, M.; Gülmez, G.; Scianna, S.; Zuber, M.; Maisano, F.; Taramasso, M. Developments in transcatheter tricuspid valve therapies. Expert Rev. Cardiovasc. Ther. 2019, 17, 841–856. [Google Scholar] [CrossRef]
- Kebed, K.Y.; Addetia, K.; Henry, M.; Yamat, M.; Weinert, L.; Besser, S.A.; Mor-Avi, V.; Lang, R.M. Refining Severe Tricuspid Regurgitation Definition by Echocardiography with a New Outcomes-Based “Massive” Grade. J. Am. Soc. Echocardiogr. 2020, 33, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Marco Del Castillo, A.; González-Gómez, A.; Monteagudo, J.M.; Hinojar, R.; Lorente, A.; Abellás, M.; Vieitez, J.M.; Garcia Martìn, A.; Casas Rojo, E.; et al. Mid-term outcome of severe tricuspid regurgitation: Are there any differences according to mechanism and severity? Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1035–1042. [Google Scholar] [CrossRef] [Green Version]
- Vieitez, J.M.; Monteagudo, J.M.; Mahia, P.; Perez, L.; Lopez, T.; Marco, I.; Perone, F.; González, T.; Sitges, M.; Bouzas, A.; et al. New insights of tricuspid regurgitation: A large-scale prospective cohort study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Previtero, M.; Ochoa-Jimenez, R.C.; Guta, A.C.; Figliozzi, S.; Gregori, D.; Bottigliengo, D.; Parati, G.; Badano, L.P. Prognostic validation of partition values for quantitative parameters to grade functional tricuspid regurgitation severity by conventional echocardiography. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 60, 727–800. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease. J. Am. Coll. Cardiol. 2021, 212, e131–e135. [Google Scholar] [CrossRef]
- Muraru, D.; Guta, A.C.; Ochoa-Jimenez, R.C.; Bartos, D.; Aruta, P.; Mihaila, S.; Popescu, B.A.; Iliceto, S.; Basso, C.; Badano, L. Functional Regurgitation of Atrioventricular Valves and Atrial Fibrillation: An Elusive Pathophysiological Link Deserving Further Attention. J. Am. Soc. Echocardiogr. 2020, 33, 42–53. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Harada, Y.; Susawa, H.; Ueda, Y.; Izumi, K.; Itakura, K.; Hidaka, T.; Shiota, T.; Nakano, Y.; Kihara, Y. Tricuspid valve geometry and right heart remodelling: Insights into the mechanism of atrial functional tricuspid regurgitation. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1068–1078. [Google Scholar] [CrossRef]
- Silbiger, J.J. Atrial functional tricuspid regurgitation: An underappreciated cause of secondary tricuspid regurgitation. Echocardiography 2019, 36, 954–957. [Google Scholar] [CrossRef]
- Guta, A.C.; Badano, L.P.; Tomaselli, M.; Mihalcea, D.; Bartos, D.; Parati, G.; Muraru, D. The pathophysiological link between right atrial remodeling and functional tricuspid regurgitation in patients with atrial fibrillation. A three-dimensional echocardiography study. J. Am. Soc. Echocardiogr. 2021, 34, 585–594.e1. [Google Scholar] [CrossRef]
- Praz, F.; Muraru, D.; Kreidel, F.; Lurz, P.; Hahn, R.T.; Delgado, V.; Senni, M.; von Bardeleben, R.S.; Nickenig, G.; Hausleiter, J.; et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention 2021, 17, 791–808. [Google Scholar] [CrossRef]
- Florescu, D.R.; Muraru, D.; Florescu, C.; Volpato, V.; Caravita, S.; Perger, E.; Bălșeanu, T.A.; Parati, G.; Badano, L.P. Right heart chambers geometry and function in patients with the atrial and the ventricular phenotypes of functional tricuspid regurgitation. Eur. Heart J. Cardiovasc. Imaging 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Caravita, S.; Guta, A.C.; Muraru, D.; Caravita, S.; Guta, A.C.; Mihalcea, D.; Branzi, G.; Parati, G.; Badano, L.P. Functional Tricuspid Regurgitation and Atrial Fibrillation: Which Comes First, the Chicken or the Egg? Case 2020, 4, 458. [Google Scholar] [CrossRef]
- Ortiz-Leon, X.A.; Posada-Martinez, E.L.; Trejo-Paredes, M.C.; Ivey-Miranda, J.B.; Pereira, J.; Crandall, I.; DaSilva, P.; Bouman, E.; Brooks, A.; Gerardi, C.; et al. Understanding tricuspid valve remodelling in atrial fibrillation using three-dimensional echocardiography. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 747–755. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [Green Version]
- Lancellotti, P.; Moura, L.; Pierard, L.A.; Agricola, E.; Popescu, B.A.; Tribouilloy, C.; Hagendorff, A.; Monin, J.L.; Badano, L.; Zamorano, J.L. European association of echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 307–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, T.; Takahashi, Y.; Fujii, H.; Morisaki, A.; Abe, Y. Surgical considerations for atrial functional regurgitation of the mitral and tricuspid valves based on the etiological mechanism. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. J. Cardio-Thorac. Surg. 2017, 52, 616–664. [Google Scholar] [CrossRef]
- Badano, L.; Pardo, A.; Muraru, D.; Zamorano, J. Acquired tricuspid valve diseases. In Hurst’s the Heart, 15th ed.; Fuster, V., Harrington, R., Narula, J., Eapen, Z., Eds.; McGraw-Hill Education: New York, NY, USA, 2021. [Google Scholar]
- Prihadi, E.A.; Delgado, V.; Hahn, R.T.; Leipsic, J.; Min, J.K.; Bax, J.J. Imaging Needs in Novel Transcatheter Tricuspid Valve Interventions. JACC Cardiovasc. Imaging 2018, 11, 736–754. [Google Scholar] [CrossRef]
- Agricola, E.; Asmarats, L.; Maisano, F.; Cavalcante, J.L.; Liu, S.; Milla, F.; Meduri, C.; Rodés-Cabau, J.; Vannan, M.; Pibarot, P. Imaging for Tricuspid Valve Repair and Replacement. JACC Cardiovasc. Imaging 2021, 14, 61–111. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Nabauer, M.; Zuber, M.; Nazif, T.M.; Hausleiter, J.; Taramasso, M.; Pozzoli, A.; George, I.; Kodali, S.; Bapat, V.; et al. Intraprocedural Imaging of Transcatheter Tricuspid Valve Interventions. JACC Cardiovasc. Imaging 2019, 12, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Saracino, G.; Matsumura, Y.; Daimon, M.; Tran, H.; Greenberg, N.L.; Hozumi, T.; Yoshikawa, J.; Thomas, J.D.; Shiota, T. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: A real-time, 3-dimensional echocardiographic study. Circulation 2006, 114, I492–I498. [Google Scholar] [CrossRef] [Green Version]
- Anwar, A.M.; Geleijnse, M.L.; Soliman, O.I.I.; McGhie, J.S.; Frowijn, R.; Nemes, A.; van den Bosch, A.E.; Galema, T.W.; Ten Cate, F.J. Assessment of normal tricuspid valve anatomy in adults by real-time three-dimensional echocardiography. Int. J. Cardiovasc. Imaging 2007, 23, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addetia, K.; Muraru, D.; Veronesi, F.; Jenei, C.; Cavalli, G.; Besser, S.A.; Mor-Avi, V.; Lang, R.M.; Badano, L.P. 3-Dimensional Echocardiographic Analysis of the Tricuspid Annulus Provides New Insights Into Tricuspid Valve Geometry and Dynamics. JACC Cardiovasc. Imaging 2019, 12, 401–412. [Google Scholar] [CrossRef]
- Khalique, O.K.; Cavalcante, J.L.; Shah, D.; Guta, A.C.; Zhan, Y.; Piazza, N.; Muraru, D. Multimodality Imaging of the Tricuspid Valve and Right Heart Anatomy. JACC Cardiovasc. Imaging 2019, 12, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Topilsky, Y.; Maltais, S.; Medina Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc. Imaging 2019, 12, 433–442. [Google Scholar] [CrossRef]
- Afilalo, J.; Grapsa, J.; Nihoyannopoulos, P.; Beaudoin, J.; Gibbs, J.S.; Channick, R.N.; Langleben, D.; Rudski, L.G.; Hua, L.; Handschumacher, M.D.; et al. Leaflet Area as a Determinant of Tricuspid Regurgitation Severity in Patients with Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2015, 8, e002714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badano, L.P.; Hahn, R.; Zanella, H.; Araiza Garaygordobil, D.; Ochoa-Jimenez, R.C.; Muraru, D. Morphological Assessment of the Tricuspid Apparatus and Grading Regurgitation Severity in Patients With Functional Tricuspid Regurgitation: Thinking Outside the Box. JACC Cardiovasc. Imaging 2019, 12, 652–664. [Google Scholar] [CrossRef]
- Yamasaki, N.; Kondo, F.; Kubo, T.; Okawa, M.; Matsumura, Y.; Kitaoka, H.; Yabe, T.; Furuno, T.; Doi, Y. Severe tricuspid regurgitation in the aged: Atrial remodeling associated with long-standing atrial fibrillation. J. Cardiol. 2006, 48, 315–323. [Google Scholar]
- Muraru, D.; Addetia, K.; Guta, A.C.; Ochoa-Jimenez, R.C.; Genovese, D.; Veronesi, F.; Basso, C.; Iliceto, S.; Badano, L.P.; Lang, R.M. Right atrial volume is a major determinant of tricuspid annulus area in functional tricuspid regurgitation: A three-dimensional echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2020, 22, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Itabashi, Y.; Mihara, H.; Berdejo, J.; Kobayashi, S.; Siegel, R.J.; Shiota, T. Functional Tricuspid Regurgitation Caused by Chronic Atrial Fibrillation: A Real-Time 3-Dimensional Transesophageal Echocardiography Study. Circ. Cardiovasc. Imaging 2017, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Najib, M.Q.; Vinales, K.L.; Vittala, S.S.; Challa, S.; Lee, H.R.; Chaliki, H.P. Predictors for the Development of Severe Tricuspid Regurgitation with Anatomically Normal Valve in Patients with Atrial Fibrillation. Echocardiography 2012, 29, 140–146. [Google Scholar] [CrossRef]
- Nemoto, N.; Lesser, J.R.; Pedersen, W.R.; Sorajja, P.; Spinner, E.; Garberich, R.F.; Vock, D.M.; Schwartz, R.S. Pathogenic structural heart changes in early tricuspid regurgitation. J. Thorac. Cardiovasc. Surg. 2015, 150, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef]
- Federico Fortuni, M.D.; Marlieke FDietz, M.D.; Edgard APrihadi, M.D.; Pieter van der Bijl, M.D.; Gaetano MDe Ferrari, M.D.; Jeroen JBax, M.D.; Victoria Delgado, M.D.; Nina Ajmone Marsan, M.P. Ratio between vena contracta width and tricuspid annular diameter: Prognostic value in secondary tricuspid regurgitation. J. Am. Soc. Echocardiogr. 2021, 34, 944–954. [Google Scholar] [CrossRef]
- Taramasso, M.; Benfari, G.; van der Bijl, P.; Alessandrini, H.; Attinger-Toller, A.; Biasco, L.; Lurz, P.; Braun, D.; Brochet, E.; Connelly, K.A.; et al. Transcatheter Versus Medical Treatment of Patients With Symptomatic Severe Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 2998–3008. [Google Scholar] [CrossRef]
- Fender, E.A.; Zack, C.J.; Nishimura, R.A. Isolated tricuspid regurgitation: Outcomes and therapeutic interventions. Heart 2018, 104, 798–806. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Ye, Q.; Ma, X.; Zhao, Y.; Han, J.; Li, Y.; Zheng, S.; Liu, K.; He, M.; et al. Catheter ablation or surgical therapy in moderate-severe tricuspid regurgitation caused by long-standing persistent atrial fibrillation. Propensity score analysis. J. Cardiothorac. Surg. 2020, 15, 277. [Google Scholar] [CrossRef]
- Markman, T.M.; Plappert, T.; de Feria Alsina, A.; Levin, M.; Amankwah, N.; Sheth, S.; Gertz, Z.M.; Schaller, R.D.; Marchlinski, F.E.; Rame, J.E.; et al. Improvement in tricuspid regurgitation following catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2020, 31, 2883–2888. [Google Scholar] [CrossRef] [PubMed]
- Itakura, K.; Hidaka, T.; Nakano, Y.; Utsunomiya, H.; Kinoshita, M.; Susawa, H.; Harada, Y.; Izumi, K.; Kihara, Y. Successful catheter ablation of persistent atrial fibrillation is associated with improvement in functional tricuspid regurgitation and right heart reverse remodeling. Heart Vessel. 2020, 35, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Meduri, C.U.; Davidson, C.J.; Lim, S.; Nazif, T.M.; Ricciardi, M.J.; Rajagopal, V.; Ailawadi, G.; Vannan, M.A.; Thomas, J.D.; et al. Early Feasibility Study of a Transcatheter Tricuspid Valve Annuloplasty: SCOUT Trial 30-Day Results. J. Am. Coll. Cardiol. 2017, 69, 1795–1806. [Google Scholar] [CrossRef]
- Miura, M.; Alessandrini, H.; Alkhodair, A.; Attinger-Toller, A.; Biasco, L.; Lurz, P.; Braun, D.; Brochet, E.; Connelly, K.A.; de Bruijn, S.; et al. Impact of Massive or Torrential Tricuspid Regurgitation in Patients Undergoing Transcatheter Tricuspid Valve Intervention. JACC Cardiovasc. Interv. 2020, 13, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Hahn, R.T.; Latib, A.; Laule, M.; Lauten, A.; Maisano, F.; Schofer, J.; Campelo-Parada, F.; Puri, R.; Vahanian, A. Transcatheter Therapies for Treating Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2016, 67, 1829–1845. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Zamorano, J.L. The need for a new tricuspid regurgitation grading scheme. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1342–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, Y.Y.; Dulgheru, R.; Lancellotti, P. The Conundrum of Tricuspid Regurgitation Grading. Front. Cardiovasc. Med. 2018, 5, 3–6. [Google Scholar] [CrossRef]
- Peri, Y.; Sadeh, B.; Sherez, C.; Hochstadt, A.; Biner, S.; Aviram, G.; Ingbir, M.; Nachmany, I.; Topaz, G.; Flint, N.; et al. Quantitative assessment of effective regurgitant orifice: Impact on risk stratification, and cut-off for severe and torrential tricuspid regurgitation grade. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, F.; Dietz, M.F.; Prihadi, E.A.; van der Bijl, P.; De Ferrari, G.M.; Knuuti, J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Prognostic Implications of a Novel Algorithm to Grade Secondary Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, M.; Gavazzoni, M.; Pozzoli, A.; Dreyfus, G.D.; Bolling, S.F.; George, I.; Kapos, I.; Tanner, F.C.; Zuber, M.; Maisano, F. Tricuspid Regurgitation: Predicting the Need for Intervention, Procedural Success, and Recurrence of Disease. JACC Cardiovasc. Imaging 2019, 12, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, M.; Alessandrini, H.; Latib, A.; Asami, M.; Attinger-Toller, A.; Biasco, L.; Braun, D.; Brochet, E.; Connelly, K.A.; Denti, P.; et al. Outcomes After Current Transcatheter Tricuspid Valve Intervention: Mid-Term Results From the International TriValve Registry. JACC Cardiovasc. Interv. 2019, 12, 155–165. [Google Scholar] [CrossRef]
- Volpato, V.; Badano, L.P.; Figliozzi, S.; Florescu, D.R.; Parati, G.; Muraru, D. Multimodality cardiac imaging and new display options to broaden our understanding of the tricuspid valve. Curr. Opin. Cardiol. 2021, 36, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Caravita, S.; Figliozzi, S.; Florescu, D.R.; Volpato, V.; Oliverio, G.; Tomaselli, M.; Torlasco, C.; Muscogiuri, G.; Cernigliaro, F.; Parati, G.; et al. Recent advances in multimodality imaging of the tricuspid valve. Expert Rev. Med. Devices 2021, 18, 1069–1081. [Google Scholar] [CrossRef]
- Praz, F.; Khalique, O.K.; dos Reis Macedo, L.G.; Pulerwitz, T.C.; Jantz, J.; Wu, I.Y.; Kantor, A.; Patel, A.; Vahl, T.; Bapat, V.; et al. Comparison between Three-Dimensional Echocardiography and Computed Tomography for Comprehensive Tricuspid Annulus and Valve Assessment in Severe Tricuspid Regurgitation: Implications for Tricuspid Regurgitation Grading and Transcatheter Therapies. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2018, 31, 1190–1202.e3. [Google Scholar] [CrossRef]
- Hahn, R.T. State-of-the-art review of echocardiographic imaging in the evaluation and treatment of functional tricuspid regurgitation. Circ. Cardiovasc. Imaging 2016, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Asmarats, L.; Puri, R.; Latib, A.; Navia, J.L.; Rodés-Cabau, J. Transcatheter Tricuspid Valve Interventions. J. Am. Coll. Cardiol. 2018, 71, 2935–2956. [Google Scholar] [CrossRef]
- Patrizio, L.; Luis, Z.J.; Habib Gilbert, B.L. The EACVI Textbook of Echocardiography; Oxford University Press: Oxford, UK, 2017; Available online: https://www.oupjapan.co.jp/en/node/16697 (accessed on 8 October 2021).
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Zaidi, A.; Oxborough, D.; Augustine, D.X.; Bedair, R.; Harkness, A.; Rana, B.; Robinson, S.; Badano, L.P. Echocardiographic assessment of the tricuspid and pulmonary valves: A practical guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, G95–G122. [Google Scholar] [CrossRef]
- Hahn, R.T.; Thomas, J.D.; Khalique, O.K.; Cavalcante, J.L.; Praz, F.; Zoghbi, W.A. Imaging Assessment of Tricuspid Regurgitation Severity. JACC Cardiovasc. Imaging 2019, 12, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Abdellaziz, D.; Geraldine, O.; Nadira, H.; Eleonora, A.; Jing, Y.; Hahn, R.T. Quantifying Tricuspid Regurgitation Severity. JACC Cardiovasc. Imaging 2019, 12, 560–562. [Google Scholar] [CrossRef]
- Addetia, K.; Muraru, D.; Badano, L.P.; Lang, R.M. New Directions in Right Ventricular Assessment Using 3-Dimensional Echocardiography. JAMA Cardiol. 2019, 4, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Hahn, R.T.; Soliman, O.I.; Faletra, F.F.; Basso, C.; Badano, L.P. 3-Dimensional Echocardiography in Imaging the Tricuspid Valve. JACC Cardiovasc. Imaging 2019, 12, 500–515. [Google Scholar] [CrossRef]
- Hahn, R.T.; Weckbach, L.T.; Noack, T.; Hamid, N.; Kitamura, M.; Bae, R.; Lurz, P.; Kodali, S.K.; Sorajja, P.; Hausleiter, J.; et al. Proposal for a Standard Echocardiographic Tricuspid Valve Nomenclature. JACC Cardiovasc. Imaging 2021, 14, 1299–1305. [Google Scholar] [CrossRef]
- Muraru, D.; Badano, L.P.; Sarais, C.; Soldà, E.; Iliceto, S. Evaluation of tricuspid valve morphology and function by transthoracic three-dimensional echocardiography. Curr. Cardiol. Rep. 2011, 13, 242–249. [Google Scholar] [CrossRef]
- Karagodin, I.; Yamat, M.; Addetia, K.; Lang, R.M. Visualization of Number of Tricuspid Valve Leaflets Using Three-Dimensional Transthoracic Echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2021, 34, 449–450. [Google Scholar] [CrossRef]
- Miglioranza, M.H.; Mihăilă, S.; Muraru, D.; Cucchini, U.; Iliceto, S.; Badano, L.P. Dynamic changes in tricuspid annular diameter measurement in relation to the echocardiographic view and timing during the cardiac cycle. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 226–235. [Google Scholar] [CrossRef]
- Miglioranza, M.H.; Mihăilă, S.; Muraru, D.; Cucchini, U.; Iliceto, S.; Badano, L.P. Variability of Tricuspid Annulus Diameter Measurement in Healthy Volunteers. JACC Cardiovasc. Imaging 2015, 8, 864–866. [Google Scholar] [CrossRef] [PubMed]
- Ton-Nu, T.T.; Levine, R.A.; Handschumacher, M.D.; Dorer, D.J.; Yosefy, C.; Fan, D.; Hua, L.; Jiang, L.; Hung, J. Geometric determinants of functional tricuspid regurgitation: Insights from 3-dimensional echocardiography. Circulation 2006, 114, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpato, V.; Lang, R.M.; Yamat, M.; Veronesi, F.; Weinert, L.; Tamborini, G.; Muratori, M.; Fusini, L.; Pepi, M.; Genovese, D.; et al. Echocardiographic Assessment of the Tricuspid Annulus: The Effects of the Third Dimension and Measurement Methodology. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2019, 32, 238–247. [Google Scholar] [CrossRef]
- Badano, L.; Caravita, S.; Rella, V.; Guida, V.; Parati, G.; Muraru, D. The Added Value of 3-Dimensional Echocardiography to Understand the Pathophysiology of Functional Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 683–689. [Google Scholar] [CrossRef]
- Mihalcea, D.; Guta, A.C.; Caravita, S.; Parati, G.; Vinereanu, D.; Badano, L.P.; Muraru, D. Sex, body size and right atrial volume are the main determinants of tricuspid annulus geometry in healthy volunteers. A 3D echo study using a novel, commercially-available dedicated software package. Eur. Heart J. 2020, 41 (Suppl. 2). [Google Scholar] [CrossRef]
- Muraru, D.; Spadotto, V.; Cecchetto, A.; Romeo, G.; Aruta, P.; Ermacora, D.; Jenei, C.; Cucchini, U.; Iliceto, S.; Badano, L.P. New speckle-tracking algorithm for right ventricular volume analysis from three-dimensional echocardiographic data sets: Validation with cardiac magnetic resonance and comparison with the previous analysis tool. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1279–1289. [Google Scholar] [CrossRef]
- Moreno, J.; de Isla, L.P.; Campos, N.; Guinea, J.; Domínguez-Perez, L.; Saltijeral, A.; Lennie, V.; Quezada, M.; de Agustín, A.; Marcos-Alberca, P.; et al. Right atrial indexed volume in healthy adult population: Reference values for two-dimensional and three-dimensional echocardiographic measurements. Echocardiography 2013, 30, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Besler, C.; Orban, M.; Rommel, K.P.; Braun, D.; Patel, M.; Hagl, C.; Borger, M.; Nabauer, M.; Massberg, S.; Thiele, H.; et al. Predictors of Procedural and Clinical Outcomes in Patients With Symptomatic Tricuspid Regurgitation Undergoing Transcatheter Edge-to-Edge Repair. JACC Cardiovasc. Interv. 2018, 11, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Lurz, P.; Besler, C.; Noack, T.; Forner, A.F.; Bevilacqua, C.; Seeburger, J.; Rommel, K.P.; Blazek, S.; Hartung, P.; Zimmer, M.; et al. Transcatheter treatment of tricuspid regurgitation using edge-to-edge repair: Procedural results, clinical implications and predictors of success. EuroIntervention 2018, 14, e290–e297. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Kresoja, K.P.; Besler, C.; Leontyev, S.; Kiefer, P.; Rommel, K.P.; Otto, W.; Forner, A.F.; Ender, J.; Holzhey, D.M.; et al. Impact of Tricuspid Valve Morphology on Clinical Outcomes After Transcatheter Edge-to-Edge Repair. JACC Cardiovasc. Interv. 2021, 14, 1616–1618. [Google Scholar] [CrossRef]
- Karam, N.; Mehr, M.; Taramasso, M.; Besler, C.; Ruf, T.; Connelly, K.A.; Weber, M.; Yzeiraj, E.; Schiavi, D.; Mangieri, A.; et al. Value of Echocardiographic Right Ventricular and Pulmonary Pressure Assessment in Predicting Transcatheter Tricuspid Repair Outcome. JACC Cardiovasc. Interv. 2020, 13, 1251–1261. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Gatehouse, P.; Rolf, M.; Graves, M.; Hofman, M.B.; Totman, J.; Werner, B.; Quest, R.A.; Liu, Y.; von Spiczak, J.; Dieringer, M. Flow measurement by cardiovascular magnetic resonance: A multi-centre multi-vendor study of background phase offset errors that can compromise the accuracy of derived regurgitant or shunt flow measurements. J. Cardiovasc. Magn. Reson. 2010, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Feneis, J.F.; Kyubwa, E.; Atianzar, K.; Cheng, J.Y.; Alley, M.T.; Vasanawala, S.S.; Demaria, A.N.; Hsiao, A. 4D flow MRI quantification of mitral and tricuspid regurgitation: Reproducibility and consistency relative to conventional MRI. J. Magn. Reson. Imaging JMRI 2018, 48, 1147–1158. [Google Scholar] [CrossRef] [Green Version]
- Kamphuis, V.P.; Westenberg, J.J.M.; van den Boogaard, P.J.; Clur, S.A.B.; Roest, A.A.W. Direct assessment of tricuspid regurgitation by 4D flow cardiovascular magnetic resonance in a patient with Ebstein’s anomaly. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 587. [Google Scholar] [CrossRef]
- Kabasawa, M.; Kohno, H.; Ishizaka, T.; Ishida, K.; Funabashi, N.; Kataoka, A.; Matsumiya, G. Assessment of functional tricuspid regurgitation using 320-detector-row multislice computed tomography: Risk factor analysis for recurrent regurgitation after tricuspid annuloplasty. J. Thorac. Cardiovasc. Surg. 2014, 147, 312–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, B.B.C.; Hashimoto, G.; Bapat, V.N.; Sorajja, P.; Scherer, M.D.; Cavalcante, J.L. Cardiac Computed Tomography and Magnetic Resonance Imaging of the Tricuspid Valve: Preprocedural Planning and Postprocedural Follow-up. Interv. Cardiol. Clin. 2022, 11, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Therkelsen, S.K.; Groenning, B.A.; Svendsen, J.H.; Jensen, G.B. Atrial and ventricular volume and function in persistent and permanent atrial fibrillation, a magnetic resonance imaging study. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2005, 7, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Kocaoglu, M.; Pednekar, A.S.; Wang, H.; Alsaied, T.; Taylor, M.D.; Rattan, M.S. Breath-hold and free-breathing quantitative assessment of biventricular volume and function using compressed SENSE: A clinical validation in children and young adults. J. Cardiovasc. Magn. Reson. 2020, 22, 54. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Kellman, P.; Larocca, G.; Arai, A.E.; Hansen, M.S. High spatial and temporal resolution retrospective cine cardiovascular magnetic resonance from shortened free breathing real-time acquisitions. J. Cardiovasc. Magn. Reson. 2013, 15, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulerwitz, T.C.; Khalique, O.K.; Leb, J.; Hahn, R.T.; Nazif, T.M.; Leon, M.B.; George, I.; Vahl, T.P.; D’Souza, B.; Bapat, V.N.; et al. Optimizing Cardiac CT Protocols for Comprehensive Acquisition Prior to Percutaneous MV and TV Repair/Replacement. JACC Cardiovasc. Imaging 2020, 13, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, O.A.; Votta, E.; Selmi, M.; Luciani, G.B.; Redaelli, A.; Delgado, V.; Bax, J.J.; Ajmone Marsan, N. 4D MDCT in the assessment of the tricuspid valve and its spatial relationship with the right coronary artery: A customized tool based on computed tomography for the planning of percutaneous procedures. J. Cardiovasc. Comput. Tomogr. 2020, 14, 520–523. [Google Scholar] [CrossRef]
- Surkova, E.; Muraru, D.; Iliceto, S.; Badano, L.P. The use of multimodality cardiovascular imaging to assess right ventricular size and function. Int. J. Cardiol. 2016, 214, 54–69. [Google Scholar] [CrossRef]
- Hell, M.M.; Emrich, T.; Kreidel, F.; Kreitner, K.F.; Schoepf, U.J.; Münzel, T.; von Bardeleben, R.S. Computed tomography imaging needs for novel transcatheter tricuspid valve repair and replacement therapies. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 601–610. [Google Scholar] [CrossRef]
- Faletra, F.F.; Pedrazzini, G.; Pasotti, E.; Murzilli, R.; Leo, L.A.; Moccetti, T. Echocardiography–X-Ray Image Fusion. JACC Cardiovasc. Imaging 2016, 9, 1114–1117. [Google Scholar] [CrossRef]
- Pascual, I.; Pozzoli, A.; Taramasso, M.; Maisano, F.; Ho, E.C. Fusion imaging for transcatheter mitral and tricuspid interventions. Ann. Transl. Med. 2020, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, F.; Marques, A.I.; Bax, J.J.; Ajmone Marsan, N.; Delgado, V. Echocardiography-computed tomography fusion imaging for guidance of transcatheter tricuspid valve annuloplasty. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 937–938. [Google Scholar] [CrossRef] [PubMed]
- Anastasius, M.; Tang, G.H.L.; Love, B.; Krishnamoorthy, P.; Sharma, S.; Kini, A.; Lerakis, S. A Novel Hybrid Imaging Approach for Guidance of Percutaneous Transcatheter Tricuspid Valve Edge-to-Edge Repair. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2021, 34, 567–568. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florescu, D.R.; Muraru, D.; Volpato, V.; Gavazzoni, M.; Caravita, S.; Tomaselli, M.; Ciampi, P.; Florescu, C.; Bălșeanu, T.A.; Parati, G.; et al. Atrial Functional Tricuspid Regurgitation as a Distinct Pathophysiological and Clinical Entity: No Idiopathic Tricuspid Regurgitation Anymore. J. Clin. Med. 2022, 11, 382. https://doi.org/10.3390/jcm11020382
Florescu DR, Muraru D, Volpato V, Gavazzoni M, Caravita S, Tomaselli M, Ciampi P, Florescu C, Bălșeanu TA, Parati G, et al. Atrial Functional Tricuspid Regurgitation as a Distinct Pathophysiological and Clinical Entity: No Idiopathic Tricuspid Regurgitation Anymore. Journal of Clinical Medicine. 2022; 11(2):382. https://doi.org/10.3390/jcm11020382
Chicago/Turabian StyleFlorescu, Diana R., Denisa Muraru, Valentina Volpato, Mara Gavazzoni, Sergio Caravita, Michele Tomaselli, Pellegrino Ciampi, Cristina Florescu, Tudor A. Bălșeanu, Gianfranco Parati, and et al. 2022. "Atrial Functional Tricuspid Regurgitation as a Distinct Pathophysiological and Clinical Entity: No Idiopathic Tricuspid Regurgitation Anymore" Journal of Clinical Medicine 11, no. 2: 382. https://doi.org/10.3390/jcm11020382





