Circulating Blood-Based Biomarkers in Pulmonary Hypertension
Abstract
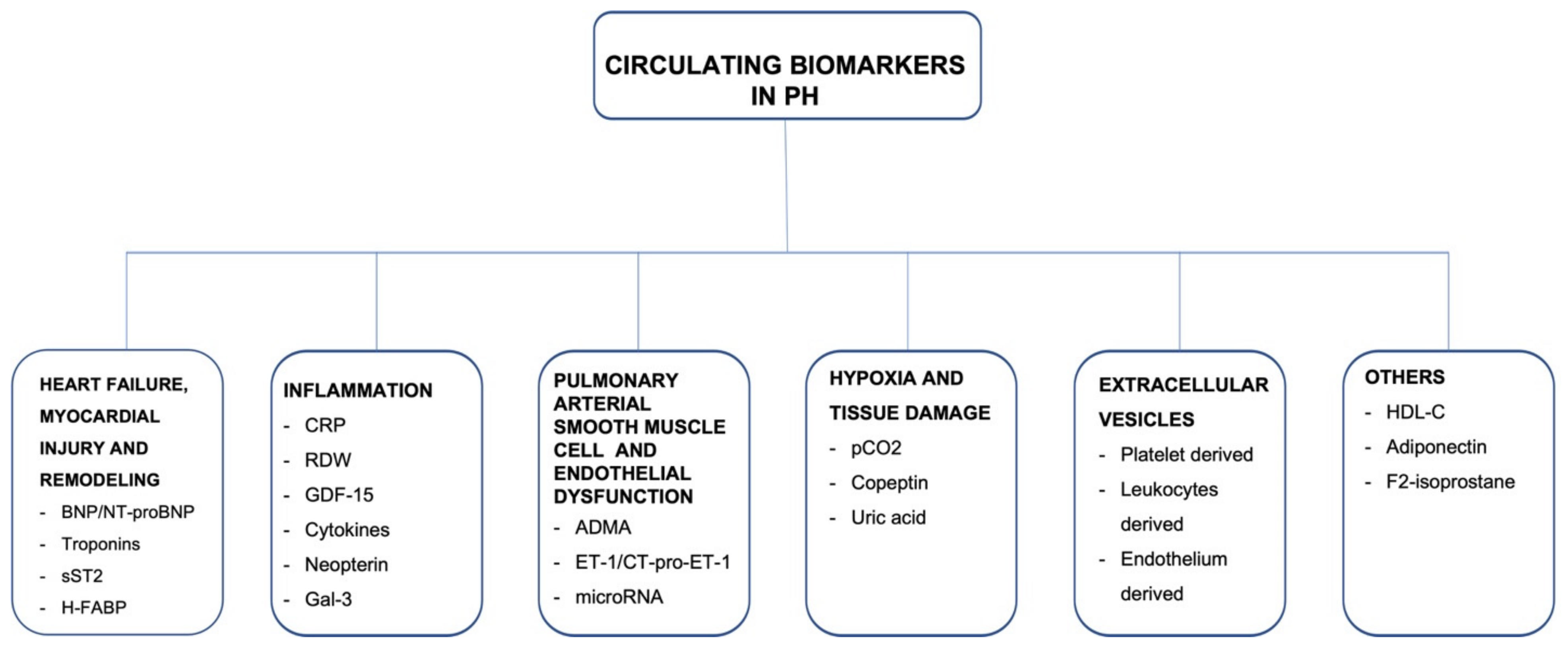
1. Introduction
2. Biomarkers Related to Heart Failure, Myocardial Injury, and Remodeling
2.1. Natriuretic Peptides
2.2. Cardiac Troponins
2.3. Soluble ST2
2.4. Heart-Type Fatty Acid-Binding Protein
3. Markers of Inflammation
3.1. C-Reactive Protein
3.2. Red Blood Cell Distribution Width
3.3. Growth Differentiation Factor-15
3.4. Cytokines
3.5. Neopterin
3.6. Galectin 3
4. Biomarkers Related to Pulmonary Arterial Smooth Muscle Cell and Endothelial Dysfunction
4.1. Asymmetric Dimethylarginine
4.2. Endothelin-I and COOH-Terminal Pro Endothelin 1
4.3. MicroRNAs as Biomarkers in PAH
5. Markers of Hypoxia and Tissue Damage
5.1. pCO2
5.2. Uric Acid
5.3. Copeptin
6. Extracellular Vesicles
7. Other Biomarkers
7.1. High-Density Lipoprotein Cholesterol
7.2. Adiponectin
7.3. F(2)-Isoprostane
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galie, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1802148. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef]
- Leuchte, H.H.; El Nounou, M.; Tuerpe, J.C.; Hartmann, B.; Baumgartner, R.A.; Vogeser, M.; Muehling, O.; Behr, J. N-terminal pro-brain natriuretic peptide and renal insufficiency as predictors of mortality in pulmonary hypertension. Chest 2007, 131, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.H.; Handler, C.E.; Akram, R.; Smith, C.J.; Das, C.; Smee, J.; Nair, D.; Denton, C.P.; Black, C.M.; Coghlan, J.G. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur. Heart J. 2006, 27, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Berghaus, T.M.; Kutsch, J.; Faul, C.; von Scheidt, W.; Schwaiblmair, M. The association of N-terminal pro-brain-type natriuretic peptide with hemodynamics and functional capacity in therapy-naive precapillary pulmonary hypertension: Results from a cohort study. BMC Pulm. Med. 2017, 17, 167. [Google Scholar] [CrossRef]
- Souza, R.; Jardim, C.; Fernandes, C.J.C.; Lapa, M.S.; Rabelo, R.; Humbert, M. NT-proBNP as a tool to stratify disease severity in pulmonary arterial hypertension. Respir. Med. 2007, 101, 69–75. [Google Scholar] [CrossRef][Green Version]
- Fijalkowska, A.; Kurzyna, M.; Torbicki, A.; Szewczyk, G.; Florczyk, M.; Pruszczyk, P.; Szturmowicz, M. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest 2006, 129, 1313–1321. [Google Scholar] [CrossRef]
- Benza, R.L.; Gomberg-Maitland, M.; Elliott, C.G.; Farber, H.W.; Foreman, A.J.; Frost, A.E.; McGoon, M.D.; Pasta, D.J.; Selej, M.; Burger, C.D.; et al. Predicting Survival in Patients With Pulmonary Arterial Hypertension: The REVEAL Risk Score Calculator 2.0 and Comparison With ESC/ERS-Based Risk Assessment Strategies. Chest 2019, 156, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Miller, D.P.; Barst, R.J.; Badesch, D.B.; Frost, A.E.; McGoon, M.D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012, 142, 448–456. [Google Scholar] [CrossRef]
- Chin, K.M.; Rubin, L.J.; Channick, R.; Di Scala, L.; Gaine, S.; Galie, N.; Ghofrani, H.A.; Hoeper, M.M.; Lang, I.M.; McLaughlin, V.V.; et al. Association of N-Terminal Pro Brain Natriuretic Peptide and Long-Term Outcome in Patients With Pulmonary Arterial Hypertension. Circulation 2019, 139, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Jansa, P.; Pulido, T.; Channick, R.N.; Delcroix, M.; Ghofrani, H.A.; Le Brun, F.O.; Mehta, S.; Perchenet, L.; Rubin, L.J.; et al. SERAPHIN haemodynamic substudy: The effect of the dual endothelin receptor antagonist macitentan on haemodynamic parameters and NT-proBNP levels and their association with disease progression in patients with pulmonary arterial hypertension. Eur. Heart J. 2017, 38, 1147–1155. [Google Scholar] [CrossRef]
- Nagaya, N.; Ando, M.; Oya, H.; Ohkita, Y.; Kyotani, S.; Sakamaki, F.; Nakanishi, N. Plasma brain natriuretic peptide as a noninvasive marker for efficacy of pulmonary thromboendarterectomy. Ann. Thorac. Surg. 2002, 74, 180–184. [Google Scholar] [CrossRef]
- Surie, S.; Reesink, H.J.; van der Plas, M.N.; Hardziyenka, M.; Kloek, J.J.; Zwinderman, A.H.; Bresser, P. Plasma brain natriuretic peptide as a biomarker for haemodynamic outcome and mortality following pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Kriechbaum, S.D.; Wiedenroth, C.B.; Wolter, J.S.; Hutz, R.; Haas, M.; Breithecker, A.; Roller, F.C.; Keller, T.; Guth, S.; Rolf, A.; et al. N-terminal pro-B-type natriuretic peptide for monitoring after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. 2018, 37, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Sadushi-Kolici, R.; Jansa, P.; Kopec, G.; Torbicki, A.; Skoro-Sajer, N.; Campean, I.A.; Halank, M.; Simkova, I.; Karlocai, K.; Steringer-Mascherbauer, R.; et al. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): A double-blind, phase 3, randomised controlled trial. Lancet Respir. Med. 2019, 7, 239–248. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Galie, N.; Grimminger, F.; Grunig, E.; Humbert, M.; Jing, Z.C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef]
- Sugimura, K.; Fukumoto, Y.; Satoh, K.; Nochioka, K.; Miura, Y.; Aoki, T.; Tatebe, S.; Miyamichi-Yamamoto, S.; Shimokawa, H. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ. J. 2012, 76, 485–488. [Google Scholar] [CrossRef]
- Kimura, M.; Kohno, T.; Kawakami, T.; Kataoka, M.; Inohara, T.; Takei, M.; Tsugu, T.; Murata, M.; Maekawa, Y.; Fukuda, K. Balloon pulmonary angioplasty attenuates ongoing myocardial damage in patients with chronic thromboembolic pulmonary hypertension. Int. J. Cardiol. 2016, 207, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Ogo, T.; Fukuda, T.; Tsuji, A.; Fukui, S.; Ueda, J.; Sanda, Y.; Morita, Y.; Asano, R.; Konagai, N.; Yasuda, S. Efficacy and safety of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension guided by cone-beam computed tomography and electrocardiogram-gated area detector computed tomography. Eur. J. Radiol. 2017, 89, 270–276. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Nagao, M.; Abe, K.; Hosokawa, K.; Kawanami, S.; Kamitani, T.; Yamanouchi, T.; Horimoto, K.; Yabuuchi, H.; Honda, H. Balloon pulmonary angioplasty improves interventricular dyssynchrony in patients with inoperable chronic thromboembolic pulmonary hypertension: A cardiac MR imaging study. Int. J. Cardiovasc. Imaging 2017, 33, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Sugimura, K.; Nochioka, K.; Miura, M.; Tatebe, S.; Yamamoto, S.; Yaoita, N.; Suzuki, H.; Sato, H.; Kozu, K.; et al. Effects of Balloon Pulmonary Angioplasty on Oxygenation in Patients With Chronic Thromboembolic Pulmonary Hypertension- Importance of Intrapulmonary Shunt. Circ. J. 2016, 80, 2227–2234. [Google Scholar] [CrossRef]
- Inami, T.; Kataoka, M.; Shimura, N.; Ishiguro, H.; Yanagisawa, R.; Fukuda, K.; Yoshino, H.; Satoh, T. Pressure-wire-guided percutaneous transluminal pulmonary angioplasty: A breakthrough in catheter-interventional therapy for chronic thromboembolic pulmonary hypertension. JACC Cardiovasc. Interv. 2014, 7, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Kurzyna, M.; Darocha, S.; Pietura, R.; Pietrasik, A.; Norwa, J.; Manczak, R.; Wieteska, M.; Biederman, A.; Matsubara, H.; Torbicki, A. Changing the strategy of balloon pulmonary angioplasty resulted in a reduced complication rate in patients with chronic thromboembolic pulmonary hypertension. A single-centre European experience. Kardiol. Pol. 2017, 75, 645–654. [Google Scholar] [CrossRef]
- Olsson, K.M.; Wiedenroth, C.B.; Kamp, J.C.; Breithecker, A.; Fuge, J.; Krombach, G.A.; Haas, M.; Hamm, C.; Kramm, T.; Guth, S.; et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: The initial German experience. Eur. Respir. J. 2017, 49, 1602409. [Google Scholar] [CrossRef] [PubMed]
- Araszkiewicz, A.; Darocha, S.; Pietrasik, A.; Pietura, R.; Jankiewicz, S.; Banaszkiewicz, M.; Slawek-Szmyt, S.; Biederman, A.; Mularek-Kubzdela, T.; Lesiak, M.; et al. Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int. J. Cardiol. 2019, 278, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Darocha, S.; Araszkiewicz, A.; Kurzyna, M.; Banaszkiewicz, M.; Jankiewicz, S.; Dobosiewicz, A.; Slawek-Szmyt, S.; Janus, M.; Grymuza, M.; Pietrasik, A.; et al. Balloon Pulmonary Angioplasty in Technically Operable and Technically Inoperable Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2021, 10, 1038. [Google Scholar] [CrossRef]
- Gerges, C.; Friewald, R.; Gerges, M.; Shafran, I.; Sadushi-Kolici, R.; Skoro-Sajer, N.; Moser, B.; Taghavi, S.; Klepetko, W.; Lang, I.M. Efficacy and Safety of Percutaneous Pulmonary Artery Subtotal Occlusion and Chronic Total Occlusion Intervention in Chronic Thromboembolic Pulmonary Hypertension. Circ. Cardiovasc. Interv. 2021, 14, e010243. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev. Esp. Cardiol. 2021, 74, 544. [Google Scholar] [CrossRef]
- Eggers, K.M.; Lagerqvist, B.; Venge, P.; Wallentin, L.; Lindahl, B. Persistent cardiac troponin I elevation in stabilized patients after an episode of acute coronary syndrome predicts long-term mortality. Circulation 2007, 116, 1907–1914. [Google Scholar] [CrossRef]
- de Lemos, J.A.; Drazner, M.H.; Omland, T.; Ayers, C.R.; Khera, A.; Rohatgi, A.; Hashim, I.; Berry, J.D.; Das, S.R.; Morrow, D.A.; et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010, 304, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Murakami, M.M.; Pearce, L.A.; Herzog, C.A. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 2002, 106, 2941–2945. [Google Scholar] [CrossRef] [PubMed]
- Horwich, T.B.; Patel, J.; MacLellan, W.R.; Fonarow, G.C. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003, 108, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Velez-Martinez, M.; Ayers, C.; Mishkin, J.D.; Bartolome, S.B.; Garcia, C.K.; Torres, F.; Drazner, M.H.; de Lemos, J.A.; Turer, A.T.; Chin, K.M. Association of cardiac troponin I with disease severity and outcomes in patients with pulmonary hypertension. Am. J. Cardiol. 2013, 111, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Torbicki, A.; Kurzyna, M.; Kuca, P.; Fijalkowska, A.; Sikora, J.; Florczyk, M.; Pruszczyk, P.; Burakowski, J.; Wawrzynska, L. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation 2003, 108, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Heresi, G.A.; Tang, W.H.; Aytekin, M.; Hammel, J.; Hazen, S.L.; Dweik, R.A. Sensitive cardiac troponin I predicts poor outcomes in pulmonary arterial hypertension. Eur. Respir. J. 2012, 39, 939–944. [Google Scholar] [CrossRef]
- Kriechbaum, S.D.; Wiedenroth, C.B.; Keller, T.; Wolter, J.S.; Ajnwojner, R.; Peters, K.; Haas, M.A.; Roller, F.C.; Breithecker, A.; Rieth, A.J.; et al. Dynamics of high-sensitivity cardiac troponin T during therapy with balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. PLoS ONE 2018, 13, e0204683. [Google Scholar] [CrossRef]
- Weinberg, E.O.; Shimpo, M.; De Keulenaer, G.W.; MacGillivray, C.; Tominaga, S.; Solomon, S.D.; Rouleau, J.L.; Lee, R.T. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002, 106, 2961–2966. [Google Scholar] [CrossRef]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Peacock, W.F.; Maisel, A.S.; Chae, C.U.; Jesse, R.L.; Baggish, A.L.; O’Donoghue, M.; Sakhuja, R.; Chen, A.A.; van Kimmenade, R.R.; et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: Results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J. Am. Coll. Cardiol. 2007, 50, 607–613. [Google Scholar] [CrossRef]
- Dieplinger, B.; Mueller, T. Soluble ST2 in heart failure. Clin. Chim. Acta 2015, 443, 57–70. [Google Scholar] [CrossRef]
- Ky, B.; French, B.; McCloskey, K.; Rame, J.E.; McIntosh, E.; Shahi, P.; Dries, D.L.; Tang, W.H.; Wu, A.H.; Fang, J.C.; et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ. Heart Fail. 2011, 4, 180–187. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Manzano-Fernandez, S.; Boronat, M.; Casas, T.; Garrido, I.P.; Bonaque, J.C.; Pastor-Perez, F.; Valdes, M.; Januzzi, J.L. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: Complementary role for risk stratification in acutely decompensated heart failure. Eur. J. Heart Fail. 2011, 13, 718–725. [Google Scholar] [CrossRef]
- Felker, G.M.; Fiuzat, M.; Thompson, V.; Shaw, L.K.; Neely, M.L.; Adams, K.F.; Whellan, D.J.; Donahue, M.P.; Ahmad, T.; Kitzman, D.W.; et al. Soluble ST2 in ambulatory patients with heart failure: Association with functional capacity and long-term outcomes. Circ. Heart Fail. 2013, 6, 1172–1179. [Google Scholar] [CrossRef]
- Lassus, J.; Gayat, E.; Mueller, C.; Peacock, W.F.; Spinar, J.; Harjola, V.P.; van Kimmenade, R.; Pathak, A.; Mueller, T.; Disomma, S.; et al. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: The Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int. J. Cardiol. 2013, 168, 2186–2194. [Google Scholar] [CrossRef]
- Lu, J.; Snider, J.V.; Grenache, D.G. Establishment of reference intervals for soluble ST2 from a United States population. Clin. Chim. Acta 2010, 411, 1825–1826. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, G.; Messalli, G.; Melillo, R.M.; Stanziola, A.A.; Visciano, C.; Mercurio, V.; Imbriaco, M.; Ghio, S.; Sofia, M.; Bonaduce, D.; et al. Serum soluble ST2 and interleukin-33 levels in patients with pulmonary arterial hypertension. Int. J. Cardiol. 2013, 168, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
- Banaszkiewicz, M.; Pietrasik, A.; Darocha, S.; Piłka, M.; Florczyk, M.; Dobosiewicz, A.; Kędzierski, P.; Pędzich-Placha, E.; Kochman, J.; Opolski, G.; et al. Soluble ST2 protein as a new biomarker in patientswith precapillary pulmonary hypertension. Arch. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Geenen, L.W.; Baggen, V.J.M.; Kauling, R.M.; Koudstaal, T.; Boomars, K.A.; Boersma, E.; Roos-Hesselink, J.W.; van den Bosch, A.E. The Prognostic Value of Soluble ST2 in Adults with Pulmonary Hypertension. J. Clin. Med. 2019, 8, 1517. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Yang, T.; He, J.G.; Chen, G.; Liu, Z.H.; Xiong, C.M.; Gu, Q.; Ni, X.H.; Zhao, Z.H. Plasma soluble ST2 levels correlate with disease severity and predict clinical worsening in patients with pulmonary arterial hypertension. Clin. Cardiol. 2014, 37, 365–370. [Google Scholar] [CrossRef]
- Kriechbaum, S.D.; Wiedenroth, C.B.; Peters, K.; Barde, M.A.; Ajnwojner, R.; Wolter, J.S.; Haas, M.; Roller, F.C.; Guth, S.; Rieth, A.J.; et al. Galectin-3, GDF-15, and sST2 for the assessment of disease severity and therapy response in patients suffering from inoperable chronic thromboembolic pulmonary hypertension. Biomarkers 2020, 25, 578–586. [Google Scholar] [CrossRef]
- Banaszkiewicz, M.; Pietrasik, A.; Florczyk, M.; Kedzierski, P.; Pilka, M.; Manczak, R.; Kochman, J.; Opolski, G.; Torbicki, A.; Kurzyna, M.; et al. Soluble ST2 as a Biomarker for Early Complications in Patients with Chronic Thromboembolic Pulmonary Hypertension Treated with Balloon Pulmonary Angioplasty. Diagnostics 2021, 11, 133. [Google Scholar] [CrossRef]
- Carroll, C.; Al Khalaf, M.; Stevens, J.W.; Leaviss, J.; Goodacre, S.; Collinson, P.O.; Wang, J. Heart-type fatty acid binding protein as an early marker for myocardial infarction: Systematic review and meta-analysis. Emerg. Med. J. 2013, 30, 280–286. [Google Scholar] [CrossRef]
- Puls, M.; Dellas, C.; Lankeit, M.; Olschewski, M.; Binder, L.; Geibel, A.; Reiner, C.; Schafer, K.; Hasenfuss, G.; Konstantinides, S. Heart-type fatty acid-binding protein permits early risk stratification of pulmonary embolism. Eur. Heart J. 2007, 28, 224–229. [Google Scholar] [CrossRef]
- Lankeit, M.; Dellas, C.; Panzenbock, A.; Skoro-Sajer, N.; Bonderman, D.; Olschewski, M.; Schafer, K.; Puls, M.; Konstantinides, S.; Lang, I.M. Heart-type fatty acid-binding protein for risk assessment of chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2008, 31, 1024–1029. [Google Scholar] [CrossRef]
- Mirna, M.; Rohm, I.; Jirak, P.; Wernly, B.; Baz, L.; Paar, V.; Kretzschmar, D.; Hoppe, U.C.; Schulze, P.C.; Lichtenauer, M.; et al. Analysis of Novel Cardiovascular Biomarkers in Patients With Pulmonary Hypertension (PH). Heart Lung Circ. 2020, 29, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Quarck, R.; Nawrot, T.; Meyns, B.; Delcroix, M. C-reactive protein: A new predictor of adverse outcome in pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009, 53, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, G.; Kempny, A.; Price, L.C.; Alonso-Gonzalez, R.; Marino, P.; Swan, L.; D’Alto, M.; Hooper, J.; Gatzoulis, M.A.; Dimopoulos, K.; et al. C-reactive protein in adults with pulmonary arterial hypertension associated with congenital heart disease and its prognostic value. Heart 2014, 100, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Wynants, M.; Quarck, R.; Ronisz, A.; Alfaro-Moreno, E.; Van Raemdonck, D.; Meyns, B.; Delcroix, M. Effects of C-reactive protein on human pulmonary vascular cells in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2012, 40, 886–894. [Google Scholar] [CrossRef]
- Yang, M.; Deng, C.; Wu, D.; Zhong, Z.; Lv, X.; Huang, Z.; Lian, N.; Liu, K.; Zhang, Q. The role of mononuclear cell tissue factor and inflammatory cytokines in patients with chronic thromboembolic pulmonary hypertension. J. Thromb. Thrombolysis 2016, 42, 38–45. [Google Scholar] [CrossRef]
- Lippi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 2009, 133, 628–632. [Google Scholar] [CrossRef]
- Osadnik, T.; Strzelczyk, J.; Hawranek, M.; Lekston, A.; Wasilewski, J.; Kurek, A.; Gutowski, A.R.; Wilczek, K.; Dyrbus, K.; Gierlotka, M.; et al. Red cell distribution width is associated with long-term prognosis in patients with stable coronary artery disease. BMC Cardiovasc. Disord. 2013, 13, 113. [Google Scholar] [CrossRef]
- Felker, G.M.; Allen, L.A.; Pocock, S.J.; Shaw, L.K.; McMurray, J.J.; Pfeffer, M.A.; Swedberg, K.; Wang, D.; Yusuf, S.; Michelson, E.L.; et al. Red cell distribution width as a novel prognostic marker in heart failure: Data from the CHARM Program and the Duke Databank. J. Am. Coll. Cardiol. 2007, 50, 40–47. [Google Scholar] [CrossRef]
- Zorlu, A.; Bektasoglu, G.; Guven, F.M.; Dogan, O.T.; Gucuk, E.; Ege, M.R.; Altay, H.; Cinar, Z.; Tandogan, I.; Yilmaz, M.B. Usefulness of admission red cell distribution width as a predictor of early mortality in patients with acute pulmonary embolism. Am. J. Cardiol. 2012, 109, 128–134. [Google Scholar] [CrossRef]
- Rhodes, C.J.; Wharton, J.; Howard, L.S.; Gibbs, J.S.; Wilkins, M.R. Red cell distribution width outperforms other potential circulating biomarkers in predicting survival in idiopathic pulmonary arterial hypertension. Heart 2011, 97, 1054–1060. [Google Scholar] [CrossRef]
- Hampole, C.V.; Mehrotra, A.K.; Thenappan, T.; Gomberg-Maitland, M.; Shah, S.J. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am. J. Cardiol. 2009, 104, 868–872. [Google Scholar] [CrossRef]
- Smukowska-Gorynia, A.; Tomaszewska, I.; Malaczynska-Rajpold, K.; Marcinkowska, J.; Komosa, A.; Janus, M.; Olasinska-Wisniewska, A.; Slawek, S.; Araszkiewicz, A.; Jankiewicz, S.; et al. Red Blood Cells Distribution Width as a Potential Prognostic Biomarker in Patients With Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension. Heart Lung Circ. 2018, 27, 842–848. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Yang, Y.H.; Zhai, Z.G.; Wang, C.; Wang, J. Red cell distribution width is increased in chronic thromboembolic pulmonary hypertension. Clin. Respir. J. 2016, 10, 54–60. [Google Scholar] [CrossRef]
- Nickel, N.; Jonigk, D.; Kempf, T.; Bockmeyer, C.L.; Maegel, L.; Rische, J.; Laenger, F.; Lehmann, U.; Sauer, C.; Greer, M.; et al. GDF-15 is abundantly expressed in plexiform lesions in patients with pulmonary arterial hypertension and affects proliferation and apoptosis of pulmonary endothelial cells. Respir. Res. 2011, 12, 62. [Google Scholar] [CrossRef]
- Mueller, T.; Leitner, I.; Egger, M.; Haltmayer, M.; Dieplinger, B. Association of the biomarkers soluble ST2, galectin-3 and growth-differentiation factor-15 with heart failure and other non-cardiac diseases. Clin. Chim. Acta 2015, 445, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin. Chim. 2017, 63, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Nickel, N.; Kempf, T.; Tapken, H.; Tongers, J.; Laenger, F.; Lehmann, U.; Golpon, H.; Olsson, K.; Wilkins, M.R.; Gibbs, J.S.; et al. Growth differentiation factor-15 in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2008, 178, 534–541. [Google Scholar] [CrossRef]
- Meadows, C.A.; Risbano, M.G.; Zhang, L.; Geraci, M.W.; Tuder, R.M.; Collier, D.H.; Bull, T.M. Increased expression of growth differentiation factor-15 in systemic sclerosis-associated pulmonary arterial hypertension. Chest 2011, 139, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Soon, E.; Holmes, A.M.; Treacy, C.M.; Doughty, N.J.; Southgate, L.; Machado, R.D.; Trembath, R.C.; Jennings, S.; Barker, L.; Nicklin, P.; et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010, 122, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Selimovic, N.; Bergh, C.H.; Andersson, B.; Sakiniene, E.; Carlsten, H.; Rundqvist, B. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur. Respir. J. 2009, 34, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Langer, F.; Schramm, R.; Bauer, M.; Tscholl, D.; Kunihara, T.; Schafers, H.J. Cytokine response to pulmonary thromboendarterectomy. Chest 2004, 126, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zabini, D.; Heinemann, A.; Foris, V.; Nagaraj, C.; Nierlich, P.; Balint, Z.; Kwapiszewska, G.; Lang, I.M.; Klepetko, W.; Olschewski, H.; et al. Comprehensive analysis of inflammatory markers in chronic thromboembolic pulmonary hypertension patients. Eur. Respir. J. 2014, 44, 951–962. [Google Scholar] [CrossRef]
- Nathan, C.F. Peroxide and pteridine: A hypothesis on the regulation of macrophage antimicrobial activity by interferon gamma. Interferon 1986, 7, 125–143. [Google Scholar] [PubMed]
- Hoffmann, G.; Wirleitner, B.; Fuchs, D. Potential role of immune system activation-associated production of neopterin derivatives in humans. Inflamm. Res. 2003, 52, 313–321. [Google Scholar] [CrossRef]
- Yamamoto, E.; Hirata, Y.; Tokitsu, T.; Kusaka, H.; Tabata, N.; Tsujita, K.; Yamamuro, M.; Kaikita, K.; Watanabe, H.; Hokimoto, S.; et al. The clinical significance of plasma neopterin in heart failure with preserved left ventricular ejection fraction. ESC Heart Fail. 2016, 3, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Morrow, D.A.; Sabatine, M.S.; Shui, A.; Rifai, N.; Cannon, C.P.; Braunwald, E. Long-term prognostic value of neopterin: A novel marker of monocyte activation in patients with acute coronary syndrome. Circulation 2007, 115, 3071–3078. [Google Scholar] [CrossRef] [PubMed]
- Smukowska-Gorynia, A.; Marcinkowska, J.; Chmara, E.; Malaczynska-Rajpold, K.; Slawek-Szmyt, S.; Cieslewicz, A.; Janus, M.; Araszkiewicz, A.; Jankiewicz, S.; Komosa, A.; et al. Neopterin as a Biomarker in Patients with Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension. Respiration 2018, 96, 222–230. [Google Scholar] [CrossRef]
- Meijers, W.C.; van der Velde, A.R.; Pascual-Figal, D.A.; de Boer, R.A. Galectin-3 and post-myocardial infarction cardiac remodeling. Eur. J. Pharmacol. 2015, 763, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Rabinovich, G.A.; Liu, F.T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef]
- de Boer, R.A.; Voors, A.A.; Muntendam, P.; van Gilst, W.H.; van Veldhuisen, D.J. Galectin-3: A novel mediator of heart failure development and progression. Eur. J. Heart Fail. 2009, 11, 811–817. [Google Scholar] [CrossRef]
- Meijers, W.C.; Januzzi, J.L.; deFilippi, C.; Adourian, A.S.; Shah, S.J.; van Veldhuisen, D.J.; de Boer, R.A. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: A pooled analysis of 3 clinical trials. Am. Heart J. 2014, 167, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Gullestad, L.; Ueland, T.; Kjekshus, J.; Nymo, S.H.; Hulthe, J.; Muntendam, P.; McMurray, J.J.; Wikstrand, J.; Aukrust, P. The predictive value of galectin-3 for mortality and cardiovascular events in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Am. Heart J. 2012, 164, 878–883. [Google Scholar] [CrossRef]
- van der Velde, A.R.; Gullestad, L.; Ueland, T.; Aukrust, P.; Guo, Y.; Adourian, A.; Muntendam, P.; van Veldhuisen, D.J.; de Boer, R.A. Prognostic value of changes in galectin-3 levels over time in patients with heart failure: Data from CORONA and COACH. Circ. Heart Fail. 2013, 6, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Fenster, B.E.; Lasalvia, L.; Schroeder, J.D.; Smyser, J.; Silveira, L.J.; Buckner, J.K.; Brown, K.K. Galectin-3 levels are associated with right ventricular functional and morphologic changes in pulmonary arterial hypertension. Heart Vessels 2016, 31, 939–946. [Google Scholar] [CrossRef]
- Calvier, L.; Legchenko, E.; Grimm, L.; Sallmon, H.; Hatch, A.; Plouffe, B.D.; Schroeder, C.; Bauersachs, J.; Murthy, S.K.; Hansmann, G. Galectin-3 and aldosterone as potential tandem biomarkers in pulmonary arterial hypertension. Heart 2016, 102, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, J.A.; Horne, B.D.; Saeed, W.; Sardar, M.R.; Zolty, R. Galectin-3 Levels Are Elevated and Predictive of Mortality in Pulmonary Hypertension. Heart Lung Circ. 2017, 26, 1208–1215. [Google Scholar] [CrossRef]
- Geenen, L.W.; Baggen, V.J.M.; Koudstaal, T.; Boomars, K.A.; Eindhoven, J.A.; Boersma, E.; Roos-Hesselink, J.W.; van den Bosch, A.E. The prognostic value of various biomarkers in adults with pulmonary hypertension; a multi-biomarker approach. Am. Heart J. 2019, 208, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, T.; Xu, X.; Wang, M.; Zhong, L.; Yang, Y.; Zhai, Z.; Xiao, F.; Wang, C. Oxidative stress and nitric oxide signaling related biomarkers in patients with pulmonary hypertension: A case control study. BMC Pulm. Med. 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Gasecka, A.; Szwed, P.; Jasinska, K.; Fidali, O.; Klebukowska, A.; Eyileten, C.; Postula, M.; Szarpak, L.; Mazurek, T.; Opolski, G.; et al. Symmetric Dimethylarginine is Altered in Patients After Myocardial Infarction and Predicts Adverse Outcomes. J. Inflamm. Res. 2021, 14, 3797–3808. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Bode-Boger, S.M.; Hesse, G.; Martens-Lobenhoffer, J.; Takacs, A.; Fliser, D.; Hoeper, M.M. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Skoro-Sajer, N.; Mittermayer, F.; Panzenboeck, A.; Bonderman, D.; Sadushi, R.; Hitsch, R.; Jakowitsch, J.; Klepetko, W.; Kneussl, M.P.; Wolzt, M.; et al. Asymmetric dimethylarginine is increased in chronic thromboembolic pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2007, 176, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Sanli, C.; Oguz, D.; Olgunturk, R.; Tunaoglu, F.S.; Kula, S.; Pasaoglu, H.; Gulbahar, O.; Cevik, A. Elevated homocysteine and asymmetric dimethyl arginine levels in pulmonary hypertension associated with congenital heart disease. Pediatr. Cardiol. 2012, 33, 1323–1331. [Google Scholar] [CrossRef]
- Giaid, A.; Yanagisawa, M.; Langleben, D.; Michel, R.P.; Levy, R.; Shennib, H.; Kimura, S.; Masaki, T.; Duguid, W.P.; Stewart, D.J. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1993, 328, 1732–1739. [Google Scholar] [CrossRef]
- Meoli, D.F.; White, R.J. Endothelin-1 induces pulmonary but not aortic smooth muscle cell migration by activating ERK1/2 MAP kinase. Can. J. Physiol. Pharmacol. 2010, 88, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Park, J.E.; Wort, S.J. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol. Res. 2011, 63, 504–511. [Google Scholar] [CrossRef]
- Rubens, C.; Ewert, R.; Halank, M.; Wensel, R.; Orzechowski, H.D.; Schultheiss, H.P.; Hoeffken, G. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest 2001, 120, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Langer, F.; Bauer, M.; Tscholl, D.; Schramm, R.; Kunihara, T.; Lausberg, H.; Georg, T.; Wilkens, H.; Schafers, H.J. Circulating big endothelin-1: An active role in pulmonary thromboendarterectomy? J. Thorac. Cardiovasc. Surg. 2005, 130, 1342–1347. [Google Scholar] [CrossRef][Green Version]
- Reesink, H.J.; Meijer, R.C.; Lutter, R.; Boomsma, F.; Jansen, H.M.; Kloek, J.J.; Bresser, P. Hemodynamic and clinical correlates of endothelin-1 in chronic thromboembolic pulmonary hypertension. Circ. J. 2006, 70, 1058–1063. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cacoub, P.; Dorent, R.; Nataf, P.; Carayon, A.; Riquet, M.; Noe, E.; Piette, J.C.; Godeau, P.; Gandjbakhch, I. Endothelin-1 in the lungs of patients with pulmonary hypertension. Cardiovasc. Res. 1997, 33, 196–200. [Google Scholar] [CrossRef]
- Struck, J.; Morgenthaler, N.G.; Bergmann, A. Proteolytic processing pattern of the endothelin-1 precursor in vivo. Peptides 2005, 26, 2482–2486. [Google Scholar] [CrossRef] [PubMed]
- Silva Marques, J.; Martins, S.R.; Calisto, C.; Goncalves, S.; Almeida, A.G.; de Sousa, J.C.; Pinto, F.J.; Diogo, A.N. An exploratory panel of biomarkers for risk prediction in pulmonary hypertension: Emerging role of CT-proET-1. J. Heart Lung Transplant. 2013, 32, 1214–1221. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Wang, G.K.; Zhu, J.Q.; Zhang, J.T.; Li, Q.; Li, Y.; He, J.; Qin, Y.W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef]
- Di Stefano, V.; Zaccagnini, G.; Capogrossi, M.C.; Martelli, F. microRNAs as peripheral blood biomarkers of cardiovascular disease. Vasc. Pharmacol. 2011, 55, 111–118. [Google Scholar] [CrossRef]
- Lee, A.; McLean, D.; Choi, J.; Kang, H.; Chang, W.; Kim, J. Therapeutic implications of microRNAs in pulmonary arterial hypertension. BMB Rep. 2014, 47, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.M.; Arnold, N.D.; Pickworth, J.A.; Iremonger, J.; Ciuclan, L.; Allen, R.M.; Guth-Gundel, S.; Southwood, M.; Morrell, N.W.; Thomas, M.; et al. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J. Clin. Investig. 2016, 126, 2495–2508. [Google Scholar] [CrossRef] [PubMed]
- Sarrion, I.; Milian, L.; Juan, G.; Ramon, M.; Furest, I.; Carda, C.; Cortijo Gimeno, J.; Mata Roig, M. Role of circulating miRNAs as biomarkers in idiopathic pulmonary arterial hypertension: Possible relevance of miR-23a. Oxid. Med. Cell Longev. 2015, 2015, 792846. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Pletz, M.W.; Golpon, H.; Welte, T. Prognostic value of blood gas analyses in patients with idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2007, 29, 944–950. [Google Scholar] [CrossRef]
- Valentin, S.; Maurac, A.; Sitbon, O.; Beurnier, A.; Gomez, E.; Guillaumot, A.; Textoris, L.; Fay, R.; Savale, L.; Jais, X.; et al. Outcomes of patients with decreased arterial oxyhaemoglobin saturation on pulmonary arterial hypertension drugs. Eur. Respir. J. 2021, 58. [Google Scholar] [CrossRef]
- American Thoracic Society. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Weatherald, J.; Farina, S.; Bruno, N.; Laveneziana, P. Cardiopulmonary Exercise Testing in Pulmonary Hypertension. Ann. Am. Thorac. Soc. 2017, 14, S84–S92. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Hohlfeld, J.M.; Fabel, H. Hyperuricaemia in patients with right or left heart failure. Eur. Respir. J. 1999, 13, 682–685. [Google Scholar] [CrossRef]
- Nagaya, N.; Uematsu, M.; Satoh, T.; Kyotani, S.; Sakamaki, F.; Nakanishi, N.; Yamagishi, M.; Kunieda, T.; Miyatake, K. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1999, 160, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, M.A.; Wynne, K.M.; Badesch, D.B.; Groves, B.M.; Voelkel, N.F. Hyperuricemia in severe pulmonary hypertension. Chest 2000, 117, 19–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Wang, Y.; Li, X.; Huang, Y.; Sun, X.; Wang, Q.; Zhang, M. Serum uric acid is associated with disease severity and may predict clinical outcome in patients of pulmonary arterial hypertension secondary to connective tissue disease in Chinese: A single-center retrospective study. BMC Pulm. Med. 2020, 20, 272. [Google Scholar] [CrossRef] [PubMed]
- Van Albada, M.E.; Loot, F.G.; Fokkema, R.; Roofthooft, M.T.; Berger, R.M. Biological serum markers in the management of pediatric pulmonary arterial hypertension. Pediatr. Res. 2008, 63, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.; Tzikas, S.; Zeller, T.; Czyz, E.; Lillpopp, L.; Ojeda, F.M.; Roth, A.; Bickel, C.; Baldus, S.; Sinning, C.R.; et al. Copeptin improves early diagnosis of acute myocardial infarction. J. Am. Coll. Cardiol. 2010, 55, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Neuhold, S.; Huelsmann, M.; Strunk, G.; Stoiser, B.; Struck, J.; Morgenthaler, N.G.; Bergmann, A.; Moertl, D.; Berger, R.; Pacher, R. Comparison of copeptin, B-type natriuretic peptide, and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure: Prediction of death at different stages of the disease. J. Am. Coll. Cardiol. 2008, 52, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, N.G.; Struck, J.; Jochberger, S.; Dunser, M.W. Copeptin: Clinical use of a new biomarker. Trends Endocrinol. Metab. 2008, 19, 43–49. [Google Scholar] [CrossRef]
- Alehagen, U.; Dahlstrom, U.; Rehfeld, J.F.; Goetze, J.P. Association of copeptin and N-terminal proBNP concentrations with risk of cardiovascular death in older patients with symptoms of heart failure. JAMA 2011, 305, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Kernis, J.T.; et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef]
- Shah, S.J.; Thenappan, T.; Rich, S.; Tian, L.; Archer, S.L.; Gomberg-Maitland, M. Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation 2008, 117, 2475–2483. [Google Scholar] [CrossRef]
- Benza, R.L.; Miller, D.P.; Gomberg-Maitland, M.; Frantz, R.P.; Foreman, A.J.; Coffey, C.S.; Frost, A.; Barst, R.J.; Badesch, D.B.; Elliott, C.G.; et al. Predicting survival in pulmonary arterial hypertension: Insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010, 122, 164–172. [Google Scholar] [CrossRef]
- Nickel, N.P.; Lichtinghagen, R.; Golpon, H.; Olsson, K.M.; Brand, K.; Welte, T.; Hoeper, M.M. Circulating levels of copeptin predict outcome in patients with pulmonary arterial hypertension. Respir. Res. 2013, 14, 130. [Google Scholar] [CrossRef]
- Diehl, P.; Aleker, M.; Helbing, T.; Sossong, V.; Germann, M.; Sorichter, S.; Bode, C.; Moser, M. Increased platelet, leukocyte and endothelial microparticles predict enhanced coagulation and vascular inflammation in pulmonary hypertension. J. Thromb. Thrombolysis 2011, 31, 173–179. [Google Scholar] [CrossRef]
- Amabile, N.; Heiss, C.; Real, W.M.; Minasi, P.; McGlothlin, D.; Rame, E.J.; Grossman, W.; De Marco, T.; Yeghiazarians, Y. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2008, 177, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Amabile, N.; Rautou, P.E.; Tedgui, A.; Boulanger, C.M. Microparticles: Key protagonists in cardiovascular disorders. Semin. Thromb. Hemost. 2010, 36, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Amabile, N.; Heiss, C.; Chang, V.; Angeli, F.S.; Damon, L.; Rame, E.J.; McGlothlin, D.; Grossman, W.; De Marco, T.; Yeghiazarians, Y. Increased CD62e(+) endothelial microparticle levels predict poor outcome in pulmonary hypertension patients. J. Heart Lung Transplant. 2009, 28, 1081–1086. [Google Scholar] [CrossRef]
- Gasecka, A.; Banaszkiewicz, M.; Nieuwland, R.; van der Pol, E.; Hajji, N.; Mutwil, H.; Rogula, S.; Rutkowska, W.; Pluta, K.; Eyileten, C.; et al. Prostacyclin Analogues Inhibit Platelet Reactivity, Extracellular Vesicle Release and Thrombus Formation in Patients with Pulmonary Arterial Hypertension. J. Clin. Med. 2021, 10, 1024. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.M.; McCully, R.B.; Murphy, J.G.; Kushwaha, S.S.; Frantz, R.P.; Kane, G.C. Usefulness of High-Density Lipoprotein Cholesterol to Predict Survival in Pulmonary Arterial Hypertension. Am. J. Cardiol. 2016, 118, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Heresi, G.A.; Aytekin, M.; Newman, J.; DiDonato, J.; Dweik, R.A. Plasma levels of high-density lipoprotein cholesterol and outcomes in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2010, 182, 661–668. [Google Scholar] [CrossRef]
- Khirfan, G.; Tejwani, V.; Wang, X.; Li, M.; DiDonato, J.; Dweik, R.A.; Smedira, N.; Heresi, G.A. Plasma levels of high density lipoprotein cholesterol and outcomes in chronic thromboembolic pulmonary hypertension. PLoS ONE 2018, 13, e0197700. [Google Scholar] [CrossRef]
- Takano, H.; Obata, J.E.; Kodama, Y.; Kitta, Y.; Nakamura, T.; Mende, A.; Kawabata, K.; Saito, Y.; Fujioka, D.; Kobayashi, T.; et al. Adiponectin is released from the heart in patients with heart failure. Int. J. Cardiol. 2009, 132, 221–226. [Google Scholar] [CrossRef]
- Perrotta, F.; Nigro, E.; Mollica, M.; Costigliola, A.; D’Agnano, V.; Daniele, A.; Bianco, A.; Guerra, G. Pulmonary Hypertension and Obesity: Focus on Adiponectin. Int. J. Mol. Sci. 2019, 20, 912. [Google Scholar] [CrossRef]
- Isobe, S.; Kataoka, M.; Kawakami, T.; Fukuda, K. Adiponectin in Chronic Thromboembolic Pulmonary Hypertension. Circ. J. 2018, 82, 1466–1468. [Google Scholar] [CrossRef]
- Cracowski, J.L.; Degano, B.; Chabot, F.; Labarere, J.; Schwedhelm, E.; Monneret, D.; Iuliano, L.; Schwebel, C.; Chaouat, A.; Reynaud-Gaubert, M.; et al. Independent association of urinary F2-isoprostanes with survival in pulmonary arterial hypertension. Chest 2012, 142, 869–876. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, M.L.; Fan, Y.F.; Jiang, X.; Zhao, Q.H.; He, J.; Wang, L.; Shailendra, P.K.; Safdar, Z.; Jing, Z.C. Plasma 15-F2t-isoprostane in idiopathic pulmonary arterial hypertension. Int. J. Cardiol. 2014, 175, 268–273. [Google Scholar] [CrossRef] [PubMed]
| Studies | No. of Patients (n) | No. of BPA Sessions (n) | BNP before BPA (pg/mL) | BNP after BPA (pg/mL) | p |
|---|---|---|---|---|---|
| Sugimura et al. [18] | 12 | NR | 335 ± 105 | 16 ± 11 | S |
| Kimura et al. [19] | 66 | 446 | 237.7 ± 475.7 | 45.2 ± 47.6 | S |
| Ogo et al. [20] | 80 | 385 | 227 ± 282 | 48 ± 57 | S |
| Yamasaki et al. [21] | 20 | 2.7 per pt | 66.5 ± 61.3 | 33.8 ± 30.0 | S |
| Aoki et al. [22] | 24 | 113 | 112 (49–199) | 27.5 (14.6–58.4) | S |
| Inami et al. [23] | 103 | 350 | 94 (42–232) | 61 (39–150) | S |
| Studies | No. of Patients (n) | No. of BPA Sessions (n) | NT-proBNP before BPA (pg/mL) | NT-proBNP after BPA (pg/mL) | p |
|---|---|---|---|---|---|
| Kurzyna et al. [24] | 31 | 117 | 2571 ± 2719 | 634 ± 697 | S |
| Olsson et al. [25] | 66 | 446 | 504 (233–1676) | 242 (109–555) | S |
| Araszkiewicz et al. [26] | 15 | 71 | 1554.8 ± 1541.3 | 537 ± 642.6 | S |
| Darocha et al. [27] | 70 | 377 | 1307 (510–3294) | 206 (83–531) | S |
| Gerges et al. [28] | 45 | 6 (4–10) per pt | 579 (182–1385) | 198 (70–429) | S |
| Feature | Soluble ST2 Protein | BNP/NT-proBNP |
|---|---|---|
| Origin | The Toll-like receptor superfamily binding IL-1; Il33–ST2 pathway | Oligopeptide nuerohormones |
| Source of secretion | Cardiomyocytes, endothelial cells, inflammatory cells | Cardiomyocytes |
| Form | Two isoforms: transmembrane ST2-ligand (ST2L) and soluble ST2 (sST2) | N-terminal fragment of prohormone |
| Physiological function | Cardioprotective role, enhancement of Th2-dependent immune response | Cardiovascular homeostasis, vasodilatation |
| Pathophysiological basis | Cardiac remodeling and fibrosis | Hemodynamic condition |
| Secretion factor | Hemodynamic stress and myocardial remodeling; inflammation | Pressure and volume overload |
| Age- and renal function-dependence | NO | YES |
| Role in diagnosis of PH | NO | YES |
| Role in prognosis of PH | YES | YES |
| Correlation with disease severity | YES | YES |
| Correlation with treatment effect | YES | YES |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banaszkiewicz, M.; Gąsecka, A.; Darocha, S.; Florczyk, M.; Pietrasik, A.; Kędzierski, P.; Piłka, M.; Torbicki, A.; Kurzyna, M. Circulating Blood-Based Biomarkers in Pulmonary Hypertension. J. Clin. Med. 2022, 11, 383. https://doi.org/10.3390/jcm11020383
Banaszkiewicz M, Gąsecka A, Darocha S, Florczyk M, Pietrasik A, Kędzierski P, Piłka M, Torbicki A, Kurzyna M. Circulating Blood-Based Biomarkers in Pulmonary Hypertension. Journal of Clinical Medicine. 2022; 11(2):383. https://doi.org/10.3390/jcm11020383
Chicago/Turabian StyleBanaszkiewicz, Marta, Aleksandra Gąsecka, Szymon Darocha, Michał Florczyk, Arkadiusz Pietrasik, Piotr Kędzierski, Michał Piłka, Adam Torbicki, and Marcin Kurzyna. 2022. "Circulating Blood-Based Biomarkers in Pulmonary Hypertension" Journal of Clinical Medicine 11, no. 2: 383. https://doi.org/10.3390/jcm11020383
APA StyleBanaszkiewicz, M., Gąsecka, A., Darocha, S., Florczyk, M., Pietrasik, A., Kędzierski, P., Piłka, M., Torbicki, A., & Kurzyna, M. (2022). Circulating Blood-Based Biomarkers in Pulmonary Hypertension. Journal of Clinical Medicine, 11(2), 383. https://doi.org/10.3390/jcm11020383










