Could Bone Biomarkers Predict Bone Turnover after Kidney Transplantation?—A Proof-of-Concept Study
Abstract
1. Introduction
1.1. Wnt Signaling Pathway: Sclerostin and Dkk-1
1.2. Rank/Rank L/Opg System
2. Methods
2.1. Patients
2.2. Bone Biomarkers
2.3. Biochemical Follow-Up
2.4. Bone Histomorphometry
2.5. Statistical Analysis
3. Results
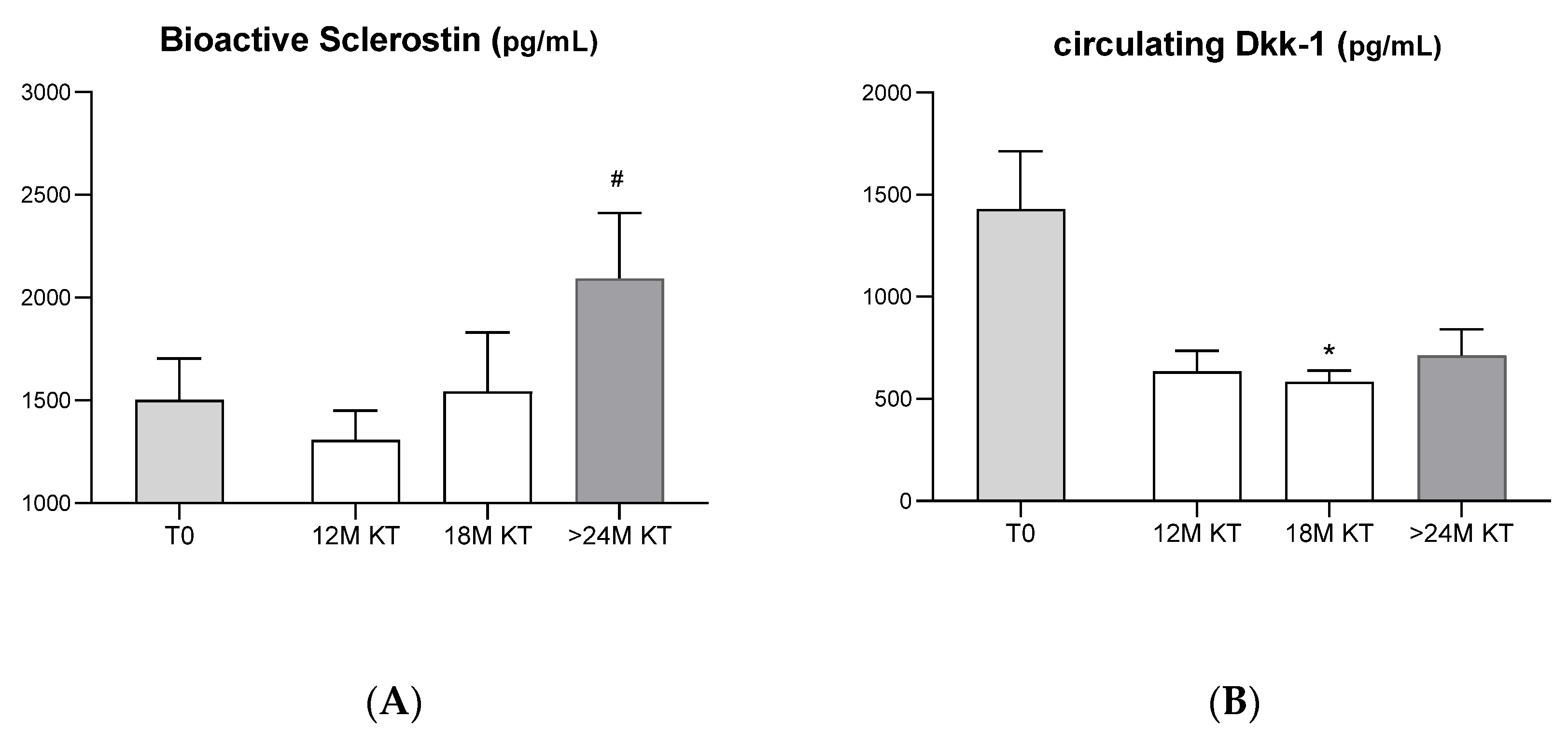
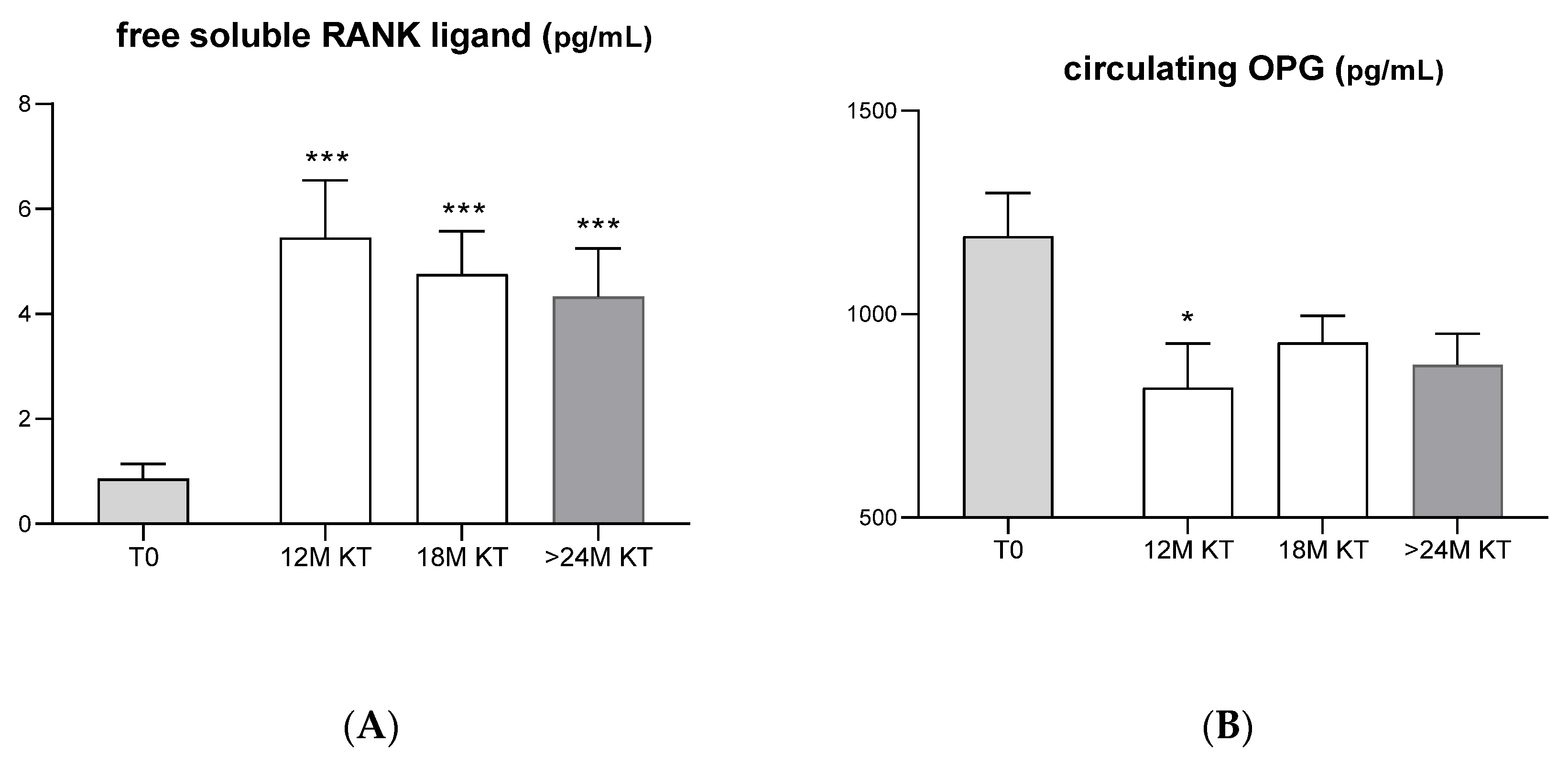
3.1. Evolution of Serum Bone-Related Biomarkers after Kidney Transplantation
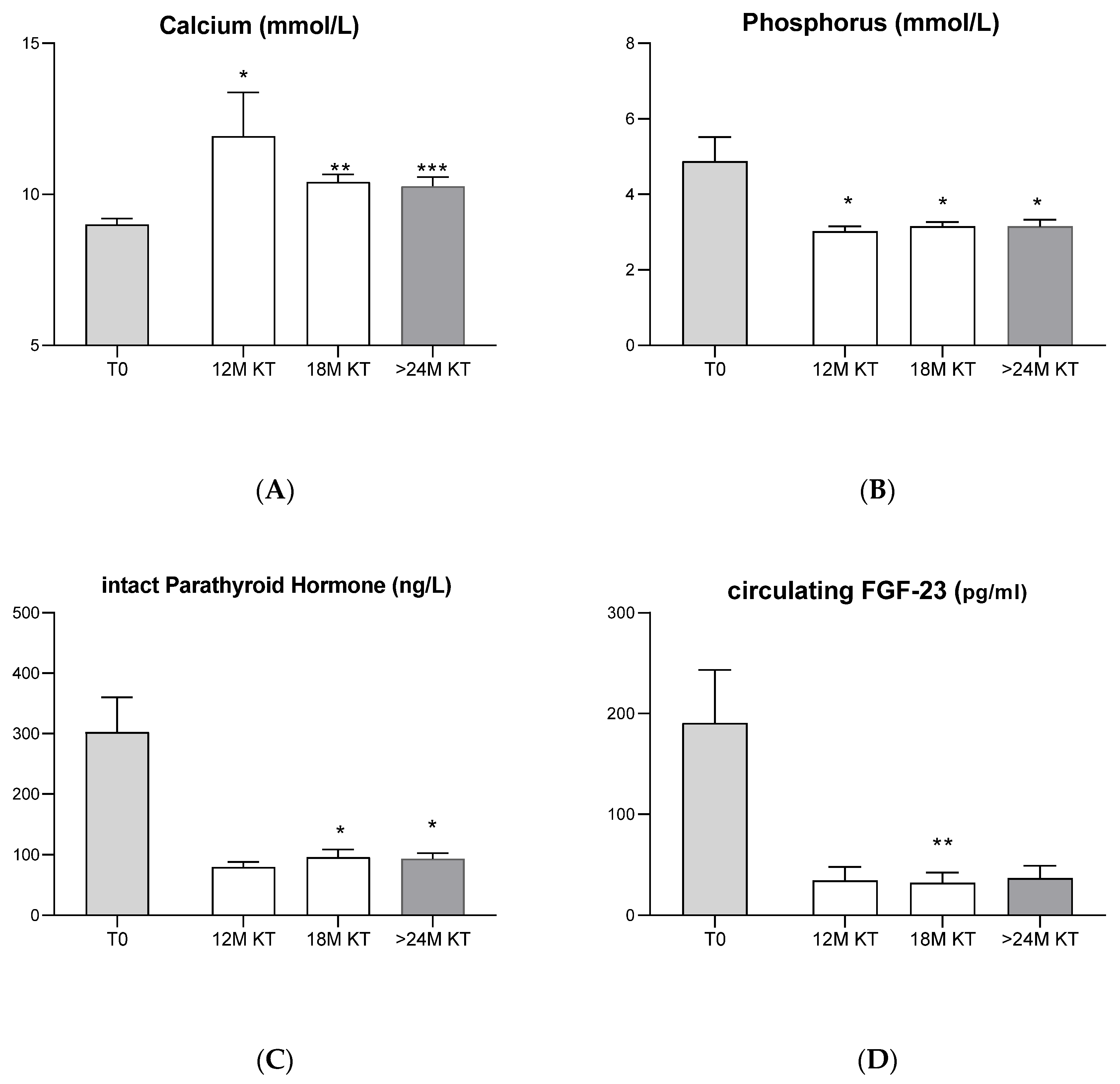
3.2. Evolution of Classic Bone Biomarkers after Kidney Transplantation
3.3. Correlation between Novel Bone Biomarkers and Classic Bone Biomarkers
3.4. Correlation between Bone-Related Biomarkers and Bone Biopsy Parameters
3.5. Histologic Analysis
4. Discussion
4.1. Evolution of Classic Bone Biomarkers after Kidney Transplantation
4.2. Evolution of Serum Bone-Related Biomarkers after Kidney Transplantation
- (1)
- Wnt signaling pathway: Sclerostin and Dkk-1
- (2)
- RANK/RANK L/OPG system
4.3. Serum Bone-Related Biomarkers vs. Histomorphometric Analysis of Bone Biopsy
5. Conclusions
6. Highlights
- Our study shows a significantly increase in the circulating levels of bioactive sclerostin after kidney transplant.
- Our prospective study reinforces the view that the loss in bone volume observed after kidney transplantation could be mainly related to the inhibition of bone formation mediated by sclerostin changes.
- Our data also reinforce the view that the enhanced bone resorption observed in the follow-up of kidney transplant appears to be mediated by the elevated circulating levels of sRANKL.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malluche, H.H.; Monier-Faugere, M.C.; Herberth, J. Bone disease after renal transplantation. Nat. Rev. Nephrol. 2010, 6, 32–40. [Google Scholar] [CrossRef]
- Bouquegneau, A.; Salam, S.; Delanaye, P.; Eastell, R.; Khwaja, A. Bone Disease after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2016, 11, 1282–1296. [Google Scholar] [CrossRef]
- Carvalho, C.; Magalhaes, J.; Pereira, L.; Simoes-Silva, L.; Castro-Ferreira, I.; Frazao, J.M. Evolution of bone disease after kidney transplantation: A prospective histomorphometric analysis of trabecular and cortical bone. Nephrology 2016, 21, 55–61. [Google Scholar] [CrossRef]
- Rojas, E.; Carlini, R.G.; Clesca, P.; Arminio, A.; Suniaga, O.; De Elguezabal, K.; Weisinger, J.R.; Hruska, K.A.; Bellorin-Font, E. The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int. 2003, 63, 1915–1923. [Google Scholar] [CrossRef]
- Keronen, S.; Martola, L.; Finne, P.; Burton, I.S.; Kroger, H.; Honkanen, E. Changes in Bone Histomorphometry after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2019, 14, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Alves, C.M.; Frazao, J.M. The role of bone biopsy for the diagnosis of renal osteodystrophy: A short overview and future perspectives. J. Nephrol. 2016, 29, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Souberbielle, J.C.; Lafage-Proust, M.H.; Jean, G.; Cavalier, E. Can we use circulating biomarkers to monitor bone turnover in CKD haemodialysis patients? Hypotheses and facts. Nephrol. Dial. Transplant. 2014, 29, 997–1004. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alderson, H.V.; Ritchie, J.P.; Green, D.; Chiu, D.; Kalra, P.A. Potential for biomarkers of chronic kidney disease-mineral bone disorder to improve patient care. Nephron. Clin. Pract. 2013, 124, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The osteocyte: An endocrine cell ... and more. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.M. Sclerostin and Wnt signaling--the pathway to bone strength. J. Clin. Endocrinol. Metab. 2005, 90, 6741–6743. [Google Scholar] [CrossRef] [PubMed]
- Semenov, M.; Tamai, K.; He, X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005, 280, 26770–26775. [Google Scholar] [CrossRef] [PubMed]
- Gittoes, N. Osteoporosis: Pathophysiology and Clinical Management. Clin. Endocrinol. 2003, 59, 826–827. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D.; Herberth, J.; Branscum, A.J.; Fardo, D.W.; Monier-Faugere, M.C.; Diarra, D.; Haas, M.; Malluche, H.H. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin. J. Am. Soc. Nephrol. 2011, 6, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S.; Dubourg, L.; Carlier, M.C.; Hadj-Aissa, A.; Fouque, D. The relation between renal function and serum sclerostin in adult patients with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 819–823. [Google Scholar] [CrossRef]
- Malluche, H.H.; Davenport, D.L.; Cantor, T.; Monier-Faugere, M.C. Bone mineral density and serum biochemical predictors of bone loss in patients with CKD on dialysis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1254–1262. [Google Scholar] [CrossRef]
- Pinzone, J.J.; Hall, B.M.; Thudi, N.K.; Vonau, M.; Qiang, Y.W.; Rosol, T.J.; Shaughnessy, J.D., Jr. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 2009, 113, 517–525. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, L.; Liu, A. Dickkopf-1: Current knowledge and related diseases. Life Sci. 2018, 209, 249–254. [Google Scholar] [CrossRef]
- Korvala, J.; Juppner, H.; Makitie, O.; Sochett, E.; Schnabel, D.; Mora, S.; Bartels, C.F.; Warman, M.L.; Deraska, D.; Cole, W.G.; et al. Mutations in LRP5 cause primary osteoporosis without features of OI by reducing Wnt signaling activity. BMC Med. Genet. 2012, 13, 26. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Joiner, D.M.; Oyserman, S.M.; Sharma, P.; Goldstein, S.A.; He, X.; Hauschka, P.V. Bone mass is inversely proportional to Dkk1 levels in mice. Bone 2007, 41, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef]
- Ozkaya, O.; Buyan, N.; Bideci, A.; Gonen, S.; Ortac, E.; Fidan, K.; Cinaz, P.; Söylemezoğlu, O. Osteoprotegerin and RANKL serum levels and their relationship with serum ghrelin in children with chronic renal failure and on dialysis. Nephron. Clin. Pract. 2007, 105, c153–c158. [Google Scholar] [CrossRef]
- Naumnik, B.; Klejna, K.; Koc-Zorawska, E.; Mysliwiec, M. Age and gender predict OPG level and OPG/sRANKL ratio in maintenance hemodialysis patients. Adv. Med. Sci. 2013, 58, 382–387. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Hernandez, J.D.; Wesseling, K.; Pereira, R.; Gales, B.; Harrison, R.; Salusky, I.B. Technical approach to iliac crest biopsy. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S164–S169. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]
- Evenepoel, P. Recovery versus persistence of disordered mineral metabolism in kidney transplant recipients. Semin. Nephrol. 2013, 33, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Behets, G.J.; Viaene, L.; D’Haese, P.C. Bone histomorphometry in de novo renal transplant recipients indicates a further decline in bone resorption 1 year posttransplantation. Kidney Int. 2017, 91, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Frazao, J.M. The bone-vessel axis in chronic kidney disease: An update on biochemical players and its future role in laboratory medicine. Clin. Chim. Acta 2020, 508, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Bonani, M.; Rodriguez, D.; Fehr, T.; Mohebbi, N.; Brockmann, J.; Blum, M.; Graf, N.; Frey, D.; Wüthrich, R.P. Sclerostin blood levels before and after kidney transplantation. Kidney Blood Press Res. 2014, 39, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; Bacelar Marques, I.D.; Graciolli, F.G.; Fukuhara, L.; Machado Dos Reis, L.; Custodio, M.; Jorgetti, V.; Elias, R.M.; David-Neto, E.; Moysés, R.M. Comparison of serum levels with bone content and gene expression indicate a contradictory effect of kidney transplantation on sclerostin. Kidney Int. 2019, 96, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Calle, J.; Arozamena, J.; Garcia-Renedo, R.; Garcia-Ibarbia, C.; Pascual-Carra, M.A.; Gonzalez-Macias, J.; Riancho, J.A. Osteocyte deficiency in hip fractures. Calcif. Tissue Int. 2011, 89, 327–334. [Google Scholar] [CrossRef]
- Chang, M.K.; Kramer, I.; Huber, T.; Kinzel, B.; Guth-Gundel, S.; Leupin, O.; Kneissel, M. Disruption of Lrp4 function by genetic deletion or pharmacological blockade increases bone mass and serum sclerostin levels. Proc. Natl. Acad. Sci. USA 2014, 111, E5187–E5195. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D.; Marculescu, R.; Kozakowski, N.; Plischke, M.; Reiter, T.; Gessl, A.; Haas, M. Renal elimination of sclerostin increases with declining kidney function. J. Clin. Endocrinol. Metab. 2014, 99, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Claes, K.; Viaene, L.; Bammens, B.; Meijers, B.; Naesens, M.; Sprangers, B.; Kuypers, D. Decreased Circulating Sclerostin Levels in Renal Transplant Recipients With Persistent Hyperparathyroidism. Transplantation 2016, 100, 2188–2193. [Google Scholar] [CrossRef]
- Gifre, L.; Ruiz-Gaspa, S.; Monegal, A.; Nomdedeu, B.; Filella, X.; Guanabens, N.; Peris, P. Effect of glucocorticoid treatment on Wnt signalling antagonists (sclerostin and Dkk-1) and their relationship with bone turnover. Bone 2013, 57, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Boltenstal, H.; Qureshi, A.R.; Behets, G.J.; Lindholm, B.; Stenvinkel, P.; D’Haese, P.C.; Haarhaus, M. Association of Serum Sclerostin with Bone Sclerostin in Chronic Kidney Disease is Lost in Glucocorticoid Treated Patients. Calcif. Tissue Int. 2019, 104, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.Y.; Cregor, M.; Delgado-Calle, J.; Condon, K.W.; Allen, M.R.; Peacock, M.; Plotkin, L.I.; Bellido, T. Protection From Glucocorticoid-Induced Osteoporosis by Anti-Catabolic Signaling in the Absence of Sost/Sclerostin. J. Bone Miner. Res. 2016, 31, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Mare, A.; Verhulst, A.; Cavalier, E.; Delanaye, P.; Behets, G.J.; Meijers, B.; Kuypers, D.; D’Haese, P.C.; Evenepoel, P. Clinical Inference of Serum and Bone Sclerostin Levels in Patients with End-Stage Kidney Disease. J. Clin. Med. 2019, 8, 2027. [Google Scholar] [CrossRef]
- Witcher, P.C.; Miner, S.E.; Horan, D.J.; Bullock, W.A.; Lim, K.E.; Kang, K.S.; Adaniya, A.L.; Ross, R.D.; Loots, G.; Robling, A.G. Sclerostin neutralization unleashes the osteoanabolic effects of Dkk1 inhibition. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Keller, H.; Kneissel, M. SOST is a target gene for PTH in bone. Bone 2005, 37, 148–158. [Google Scholar] [CrossRef]
- Tu, X.; Delgado-Calle, J.; Condon, K.W.; Maycas, M.; Zhang, H.; Carlesso, N.; Taketo, M.M.; Burr, D.B.; Plotkin, L.I.; Bellido, T. Osteocytes mediate the anabolic actions of canonical Wnt/beta-catenin signaling in bone. Proc. Natl. Acad. Sci. USA 2015, 112, E478–E486. [Google Scholar] [CrossRef]
- Wijenayaka, A.R.; Kogawa, M.; Lim, H.P.; Bonewald, L.F.; Findlay, D.M.; Atkins, G.J. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS ONE. 2011, 6, e25900. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S. Clinical practice. Glucocorticoid-induced bone disease. N. Engl. J. Med. 2011, 365, 62–70. [Google Scholar] [CrossRef]
- Weinstein, R.S. Glucocorticoid-induced osteoporosis. Rev. Endocr. Metab. Disord. 2001, 2, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I. Apoptotic osteocytes and the control of targeted bone resorption. Curr. Osteoporos. Rep. 2014, 12, 121–126. [Google Scholar] [CrossRef]
- Ru, J.Y.; Wang, Y.F. Osteocyte apoptosis: The roles and key molecular mechanisms in resorption-related bone diseases. Cell Death Dis. 2020, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; Noble, B.; Weinstein, R.S. Osteocyte apoptosis. Bone 2013, 54, 264–271. [Google Scholar] [CrossRef]
- Rogers, A.; Eastell, R. Circulating osteoprotegerin and receptor activator for nuclear factor kappaB ligand: Clinical utility in metabolic bone disease assessment. J. Clin. Endocrinol. Metab. 2005, 90, 6323–6331. [Google Scholar] [CrossRef]
- Coen, G.; Ballanti, P.; Balducci, A.; Calabria, S.; Fischer, M.S.; Jankovic, L.; Manni, M.; Morosetti, M.; Moscaritolo, E.; Sardella, D.; et al. Serum osteoprotegerin and renal osteodystrophy. Nephrol. Dial. Transplant. 2002, 17, 233–238. [Google Scholar] [CrossRef]
- Honma, M.; Ikebuchi, Y.; Kariya, Y.; Hayashi, M.; Hayashi, N.; Aoki, S.; Suzuki, H. RANKL subcellular trafficking and regulatory mechanisms in osteocytes. J. Bone Miner. Res. 2013, 28, 1936–1949. [Google Scholar] [CrossRef]
- Xiong, J.; Cawley, K.; Piemontese, M.; Fujiwara, Y.; Zhao, H.; Goellner, J.J.; O’Brien, C.A. Soluble RANKL contributes to osteoclast formation in adult mice but not ovariectomy-induced bone loss. Nat. Commun. 2018, 9, 2909. [Google Scholar] [CrossRef] [PubMed]

| Patient | Sex | Age (Years) | Dialysis Mode | Primary Disease | Pcr (mg/dL) T0 | Bone Related Pre-Transplant Medication | Immunosuppression | Pcr (mg/dL) T24 | eGFR (mL/min) T24 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 60 | PD | HTN | 1.83 | Cinac | CsA + MMF + Pred | 1.15 | 64 |
| 2 | F | 57 | HD | HTN | 1.54 | Cinac + α-Calcid | CsA + MMF + Pred | 0.91 | 48 |
| 3 | M | 59 | HD | GN | 1.74 | - | CsA + MMF + Pred | 1.5 | 48 |
| 4 | M | 48 | HD | GN | 1.93 | - | CsA + MMF + Pred | * | * |
| 5 | M | 34 | HD | Unknown | 2.45 | Calcitriol | TAC + MMF + Pred | 1.7 | 46 |
| 6 | M | 50 | HD | IgAN | 2.27 | Cinac | CsA + MMF + Pred | 1.9 | 37 |
| 7 | F | 64 | HD | Unknown | 1.24 | Calcitriol | CsA + MMF + Pred | * | * |
| 8 | F | 63 | HD | Unknown | 1.68 | Cinac | CsA + MMF + Pred | * | * |
| 9 | M | 53 | HD | Unknown | 1.29 | - | CsA + MMF + Pred | * | * |
| 10 | M | 55 | PH | Diab NP | 2.18 | Calcitriol | CsA + MMF + Pred | ** | ** |
| 11 | F | 43 | HD | GN | 1.03 | - | TAC + MMF + Pred | * | * |
| 12 | M | 59 | HD | Diab NP | 1.98 | α-Calcid | CsA + MMF + Pred | * | * |
| 13 | M | 33 | HD | Unknown | 2.03 | - | TAC + MMF + Pred | 1.24 | 65 |
| Novel Bone Biomarkers | ||||
|---|---|---|---|---|
| Classic Bone Biomarkers | Sclerostin | Dkk-1 | sRANK-L | OPG |
| Ca | r = −0.22; p = 0.25 | r = −0.60; p = 0.0002 *** | r = 0.66; p < 0.0001 **** | r = −0.43; p = 0.02 * |
| Pi | r = −0.10; p = 0.75 | r = 0.23; p = 0.1893 | r = −0.44; p = 0.01 * | r = −0.10; p = 0.63 |
| PTHi | r = −0.33; p = 0.15 | r = 0.50; p = 0.01 * | r = −0.57; p = 0.005 ** | r = 0.40; p = 0.07 |
| 25-OH-VitD | r = 0.52; p = 0.20 | r = −0.59; p = 0.08 | r = −0.00; p > 0.9999 | r = −0.64; p = 0.07 |
| FGF-23 | r = 0.10; p = 0.73 | r = 0.13; p = 0.46 | r = −0.33; p = 0.07 | r = −0.10; p = 0.58 |
| Novel Bone Biomarkers | ||||
|---|---|---|---|---|
| Bone Biopsy Parameters | Sclerostin | Dkk-1 | sRANK-L | OPG |
| Bone volume (BV/TV) | r = −0.42; p = 0.18 | r = −0.43; p = 0.11 | r = 0.16; p = 0.58 | r = −0.60; p = 0.02 * |
| Osteoblast surface (Ob.S/BS) | r = −0.35; p = 0.22 | r = 0.003; p = 0.99 | r = −0.50; p = 0.07 | r = 0.10; p = 0.70 |
| Osteoclast surface (Oc.S/BS) | r = 0.29; p = 0.32 | r = −0.001; p = 0.99 | r = −0.60; p = 0.02 * | r = 0.21; p = 0.44 |
| Trabecular Separation (TbSp) | r = 0.76; p = 0.0055 ** | r = 0.47; p = 0.08 | r = −0.01; p = 0.97 | r = 0.27; p = 0.34 |
| Trabecular Number (TbN) | r = −0.78; p = 0.0043 ** | r = −0.47; p = 0.08 | r = 0.06; p = 0.83 | r = −0.25; p = 0.39 |
| Bone Formation Rate (BFR/BS) | r = 0.42; p = 0.27 | r = 0.13; p = 0.70 | r = −0.43; p = 0.21 | r = −0.03; p = 0.94 |
| Spearman’s Correlations | Spearman’s Rho | p |
|---|---|---|
| Sclerostin-trabecular separation (TbSp) | 0.762 | 0.006 |
| Sclerostin-TbSp conditioned on variables: Ca | 0.756 | 0.007 |
| Sclerostin-TbSp conditioned on variables: Pi | 0.795 | 0.003 |
| Sclerostin-TbSp conditioned on variables: PTHi | 0.703 | 0.035 |
| Sclerostin-TbSp conditioned on variables: Pcreatinine | 0.765 | 0.006 |
| Sclerostin-Trabecular Number (TbN) | −0.776 | 0.005 |
| Sclerostin-TbN conditioned on variables: Ca | −0.768 | 0.006 |
| Sclerostin-TbN conditioned on variables: Pi | −0.807 | 0.003 |
| Sclerostin-TbN conditioned on variables: PTHi | −0.742 | 0.022 |
| Sclerostin-TbN conditioned on variables: Pcreatinine | −0.785 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, J.; Quelhas-Santos, J.; Pereira, L.; Neto, R.; Castro-Ferreira, I.; Martins, S.; Frazão, J.M.; Carvalho, C. Could Bone Biomarkers Predict Bone Turnover after Kidney Transplantation?—A Proof-of-Concept Study. J. Clin. Med. 2022, 11, 457. https://doi.org/10.3390/jcm11020457
Magalhães J, Quelhas-Santos J, Pereira L, Neto R, Castro-Ferreira I, Martins S, Frazão JM, Carvalho C. Could Bone Biomarkers Predict Bone Turnover after Kidney Transplantation?—A Proof-of-Concept Study. Journal of Clinical Medicine. 2022; 11(2):457. https://doi.org/10.3390/jcm11020457
Chicago/Turabian StyleMagalhães, Juliana, Janete Quelhas-Santos, Luciano Pereira, Ricardo Neto, Inês Castro-Ferreira, Sandra Martins, João Miguel Frazão, and Catarina Carvalho. 2022. "Could Bone Biomarkers Predict Bone Turnover after Kidney Transplantation?—A Proof-of-Concept Study" Journal of Clinical Medicine 11, no. 2: 457. https://doi.org/10.3390/jcm11020457
APA StyleMagalhães, J., Quelhas-Santos, J., Pereira, L., Neto, R., Castro-Ferreira, I., Martins, S., Frazão, J. M., & Carvalho, C. (2022). Could Bone Biomarkers Predict Bone Turnover after Kidney Transplantation?—A Proof-of-Concept Study. Journal of Clinical Medicine, 11(2), 457. https://doi.org/10.3390/jcm11020457








