Comparison of Treatment Modalities for Dry Eye in Primary Sjögren’s Syndrome
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Clinical Assessments
2.3. Statistical Analysis
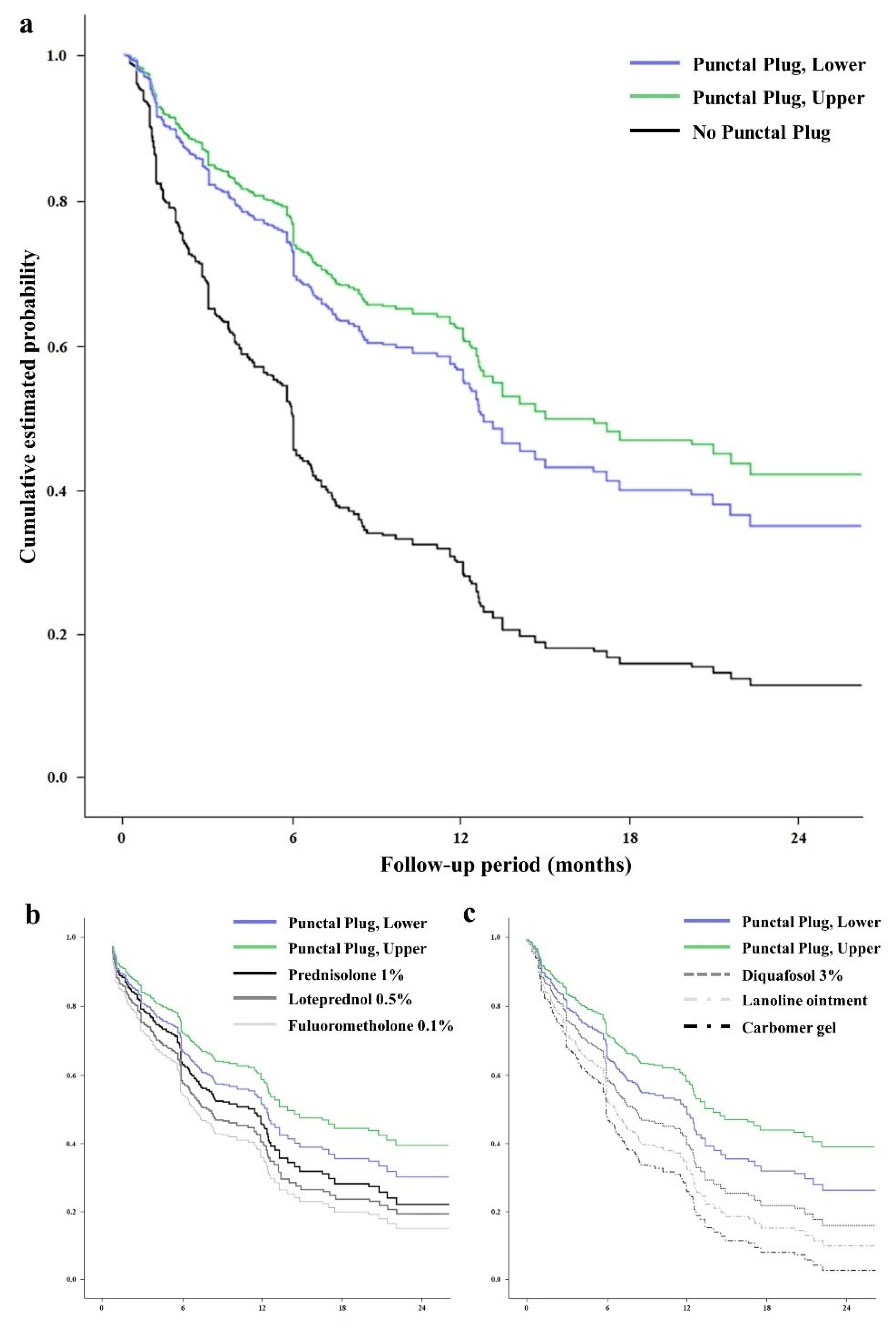
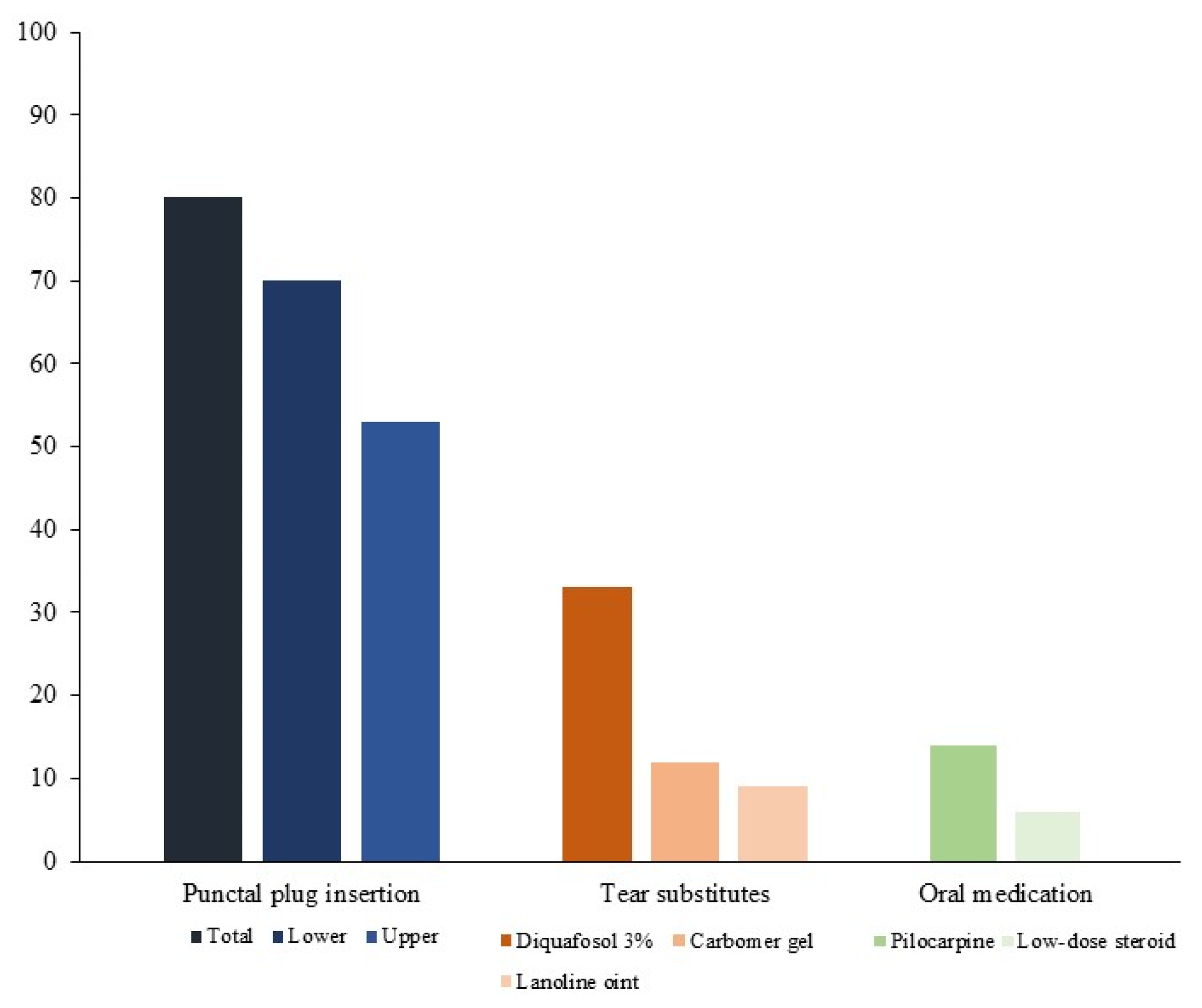
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjögren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef]
- Helmick, C.G.; Felson, D.T.; Lawrence, R.C.; Gabriel, S.; Hirsch, R.; Kwoh, C.K.; Liang, M.H.; Kremers, H.M.; Mayes, M.D.; Merkel, P.A.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008, 58, 15–25. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Acar-Denizli, N.; Zeher, M.; Rasmussen, A.; Seror, R.; Theander, E.; Li, X.; Baldini, C.; Gottenberg, J.; Danda, D.; et al. Influence of geolocation and ethnicity on the phenotypic expression of primary Sjögren’s syndrome at diagnosis in 8310 patients: A cross-sectional study from the Big Data Sjögren Project Consortium. Ann. Rheum. Dis. 2017, 76, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Han, S.J.; Nam, S.M.; Yoon, S.; Ahn, J.M.; Kim, T.-I.; Kim, E.K.; Seo, K.Y. Analysis of Tear Cytokines and Clinical Correlations in Sjögren Syndrome Dry Eye Patients and Non–Sjögren Syndrome Dry Eye Patients. Am. J. Ophthalmol. 2013, 156, 247–253.e1. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Retamozo, S.; Kostov, B.; Baldini, C.; Bootsma, H.; De Vita, S.; Dörner, T.; Gottenberg, J.-E.; Kruize, A.A.; Mandl, T.; et al. Efficacy and safety of topical and systemic medications: A systematic literature review informing the EULAR recommendations for the management of Sjögren’s syndrome. RMD Open 2019, 5, e001064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, K.C.; Lun, C.N.; Jhanji, V.; Thong, B.Y.-H.; Tong, L. Systematic review of randomized controlled trials in the treatment of dry eye disease in Sjogren syndrome. J. Inflamm. 2017, 14, 26. [Google Scholar] [CrossRef] [Green Version]
- Foulks, G.N.; Forstot, S.L.; Donshik, P.C.; Forstot, J.Z.; Goldstein, M.H.; Lemp, M.A.; Nelson, J.D.; Nichols, K.K.; Pflugfelder, S.C.; Tanzer, J.M.; et al. Clinical Guidelines for Management of Dry Eye Associated with Sjögren Disease. Ocul. Surf. 2015, 13, 118–132. [Google Scholar] [CrossRef]
- Kawashima, M.; The DECS-J Study Group; Yamada, M.; Suwaki, K.; Shigeyasu, C.; Uchino, M.; Hiratsuka, Y.; Yokoi, N.; Tsubota, K. A Clinic-based Survey of Clinical Characteristics and Practice Pattern of Dry Eye in Japan. Adv. Ther. 2017, 34, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C. Classification criteria for Sjogren’s syndrome. Ann. Rheum. Dis. 2003, 62, 94–95. [Google Scholar] [CrossRef] [Green Version]
- Vitali, C.; Del Papa, N. Classification criteria for Sjögren’s syndrome. In Sjögren’s Syndrome; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 47–60. [Google Scholar] [CrossRef]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of Corneal and Conjunctival Staining in the Context of Other Dry Eye Tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Whitcher, J.P.; Shiboski, C.H.; Shiboski, S.C.; Heidenreich, A.M.; Kitagawa, K.; Zhang, S.; Hamann, S.; Larkin, G.; McNamara, N.A.; Greenspan, J.S.; et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren’s Syndrome International Registry. Am. J. Ophthalmol. 2010, 149, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Efron, N.; Morgan, P.B.; Katsara, S.S. Validation of grading scales for contact lens complications. Ophthalmic Physiol. Opt. 2001, 21, 17–29. [Google Scholar]
- Ferrari, G.; Rabiolo, A.; Bignami, F.; Sizzano, F.; Palini, A.; Villa, C.; Rama, P. Quantifying Ocular Surface Inflammation and Correlating It with Inflammatory Cell Infiltration In Vivo: A Novel Method. Investig. Opthalmol. Vis. Sci. 2015, 56, 7067–7075. [Google Scholar] [CrossRef] [Green Version]
- Beigi, B.; Gupta, D.; Luo, Y.H.L.; Saldana, M.; Georgalas, I.; Kalantzis, G.; El-Hindy, N. Punctal function in lacrimal drainage: The ‘pipette sign’ and functional ectropion. Clin. Exp. Optom. 2015, 98, 366–369. [Google Scholar] [CrossRef]
- Vu, C.H.V.; Kawashima, M.; Yamada, M.; Suwaki, K.; Uchino, M.; Shigeyasu, C.; Hiratsuka, Y.; Yokoi, N.; Tsubota, K. Influence of Meibomian Gland Dysfunction and Friction-Related Disease on the Severity of Dry Eye. Ophthalmology 2018, 125, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.; Pflugfelder, S.C. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren syndrome. Ophthalmology 1999, 106, 811–816. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Maskin, S.L.; Anderson, B.; Chodosh, J.; Holland, E.J.; de Paiva, C.; Bartels, S.P.; Micuda, T.; Proskin, H.M.; Vogel, R. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am. J. Ophthalmol. 2004, 138, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Sendrowski, D.P. Anti-Inflammatory Drugs. In Clinical Ocular Pharmacology, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2008. [Google Scholar]
- Samudre, S.S.; Lattanzio, F.A., Jr.; Williams, P.B.; Sheppard, J.D., Jr. Comparison of topical steroids for acute anterior uveitis. J. Ocular Pharm. Ther. 2004, 20, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- Koroloff, N.; Boots, R.; Lipman, J.; Thomas, P.; Rickard, C.; Coyer, F. A randomised controlled study of the efficacy of hypromellose and Lacri-Lube combination versus polyethylene/Cling wrap to prevent corneal epithelial breakdown in the semiconscious intensive care patient. Intensive Care Med. 2004, 30, 1122–1126. [Google Scholar] [CrossRef]
- Fujihara, T.; Murakami, T.; Nagano, T.; Nakamura, M.; Nakata, K. INS365 Suppresses Loss of Corneal Epithelial Integrity by Secretion of Mucin-like Glycoprotein in a Rabbit Short-term Dry Eye Model. J. Ocul. Pharmacol. Ther. 2002, 18, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Takaoka-Shichijo, Y.; Sakamoto, A.; Nakamura, M. Effect of diquafosol tetrasodium on MUC5AC secretion by rabbit conjunctival tissues. Atarashii Ganka (J. Eye) 2011, 28, 261–265. [Google Scholar]
- Takaoka-Shichijo, Y.; Nakamura, M. Stimulatory effect of diquafosol tetrasodium on the expression of membrane-binding mucin genes in cultured human corneal epithelial cells. J. Eye 2011, 28, 425–429. [Google Scholar]
- Takamura, E.; Tsubota, K.; Watanabe, H.; Ohashi, Y. The Diquafosol Ophthalmic Solution Phase 3 Study Group A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br. J. Ophthalmol. 2012, 96, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Soong, S.J.; Balch, C.M. Multivariate analysis. JAMA 1983, 250, 2471–2472. [Google Scholar] [CrossRef]
| Characteristic | (n = 191) |
|---|---|
| Age (years, mean ± SD) | 52.3 ± 12.2 (range 35 to 90) |
| Sex (Female, %) | 95 |
| Observation period (years, mean ±SD) | 5.4 ± 3.1 (range 2.0 to 14.1) |
| Ocular Surface Inflammation period (%) | 17.8, of entire observational period (29.1, of treatment plans) |
| Corneal Staining Score * (%) | |
| Grade 1 | 20.1 |
| Grade 2 | 46.0 |
| Grade 3 | 33.9 |
| The Symptom Scale (%) | |
| Satisfactory | 18.5 |
| Poor | 53.8 |
| Very poor | 27.7 |
| 95% CI | ||||
|---|---|---|---|---|
| Treatments | ß | Lower | Upper | p |
| Oral pilocarpine | 1.017 | 0.995 | 1.039 | 0.141 |
| Low-dose oral steroid | 1.220 | 0.724 | 2.057 | 0.455 |
| Tear substitutes | ||||
| Carbomer gel | 1.035 | 1.002 | 1.069 | 0.037 * |
| Lanoline ointment | 1.142 | 1.008 | 1.274 | 0.033 * |
| Diquafosol 3% | 1.203 | 1.060 | 1.475 | 0.005 * |
| Topical cyclosporine | 1.082 | 0.980 | 1.191 | 0.099 |
| Steroid eye drops (frequency dependent) | ||||
| Prednisolone 1% | 1.255 | 1.058 | 1.487 | 0.009 * |
| Loteprednol 0.5% | 1.229 | 1.002 | 1.486 | 0.042 * |
| Fluorometholone 0.1% | 1.188 | 1.012 | 1.395 | 0.035 * |
| Punctal plug insertion | ||||
| Lower eyelid | 2.697 | 2.161 | 3.365 | <0.001 * |
| Upper eyelid | 1.801 | 1.369 | 2.370 | <0.001 * |
| 95% CI | ||||
|---|---|---|---|---|
| Treatments | ß | Lower | Upper | p |
| Oral pilocarpine | 0.987 | 0.962 | 1.013 | 0.987 |
| Low-dose oral steroid | 0.972 | 0.882 | 1.070 | 0.972 |
| Tear substitutes | ||||
| Carbomer gel | 1.030 | 0.996 | 1.065 | 0.089 |
| Lanoline ointment | 1.040 | 1.006 | 1.077 | 0.023 * |
| Diquafosol 3% | 1.027 | 1.003 | 1.052 | 0.022 * |
| Topical cyclosporine | 0.920 | 0.836 | 1.013 | 0.136 |
| Steroid eye drops (frequency dependent) | ||||
| Prednisolone 1% | 1.297 | 1.174 | 1.434 | <0.001 * |
| Loteprednol 0.5% | 1.228 | 1.034 | 1.542 | 0.017 * |
| Fluorometholone 0.1% | 1.228 | 1.124 | 1.343 | <0.001 * |
| Punctal plug insertion | ||||
| Lower eyelid | 2.867 | 2.248 | 3.655 | <0.001 * |
| Upper eyelid | 2.102 | 1.535 | 2.877 | <0.001 * |
| 95% CI | ||||
|---|---|---|---|---|
| Treatments | ß | Lower | Upper | p |
| Oral pilocarpine | 1.050 | 1.000 | 1.102 | 0.092 |
| Low-dose oral steroid | 1.250 | 0.875 | 1.745 | 0.278 |
| Tear substitutes | ||||
| Carbomer gel | 0.986 | 0.888 | 1.094 | 0.786 |
| Lanoline ointment | 1.097 | 0.948 | 1.269 | 0.214 |
| Diquafosol 3% | 1.012 | 0.924 | 1.108 | 0.800 |
| Topical cyclosporine | 1.084 | 0.980 | 1.199 | 0.098 |
| Steroid eye drops (frequency dependent) | ||||
| Prednisolone 1% | 1.477 | 1.292 | 1.688 | <0.001 * |
| Loteprednol 0.5% | 1.390 | 1.076 | 1.798 | 0.012 * |
| Fluorometholone 0.1% | 1.245 | 1.149 | 1.350 | <0.001 * |
| Punctal plug insertion | ||||
| Lower eyelid | 2.257 | 1.927 | 2.643 | <0.001 * |
| Upper eyelid | 1.702 | 1.402 | 2.067 | 0.002 * |
| 95% CI | ||||
|---|---|---|---|---|
| Treatments | ß | Lower | Upper | p |
| Oral pilocarpine | 0.991 | 0.962 | 1.022 | 0.578 |
| Low-dose oral steroid | 0.992 | 0.901 | 1.091 | 0.862 |
| Tear substitutes | ||||
| Carbomer gel | 1.060 | 1.019 | 1.102 | 0.003 * |
| Lanoline ointment | 1.171 | 1.134 | 1.211 | <0.001 * |
| Diquafosol 3% | 1.372 | 1.116 | 1.685 | 0.003 * |
| Topical cyclosporine | 0.861 | 0.686 | 1.080 | 0.196 |
| Punctal plug insertion | ||||
| Lower eyelid | 2.967 | 2.259 | 3.897 | <0.001 * |
| Upper eyelid | 1.848 | 1.315 | 2.598 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, H.; Ji, Y.W.; Jun, I.; Kim, T.-i.; Lee, H.K.; Seo, K.Y. Comparison of Treatment Modalities for Dry Eye in Primary Sjögren’s Syndrome. J. Clin. Med. 2022, 11, 463. https://doi.org/10.3390/jcm11020463
Ahn H, Ji YW, Jun I, Kim T-i, Lee HK, Seo KY. Comparison of Treatment Modalities for Dry Eye in Primary Sjögren’s Syndrome. Journal of Clinical Medicine. 2022; 11(2):463. https://doi.org/10.3390/jcm11020463
Chicago/Turabian StyleAhn, Hyunmin, Yong Woo Ji, Ikhyun Jun, Tae-im Kim, Hyung Keun Lee, and Kyoung Yul Seo. 2022. "Comparison of Treatment Modalities for Dry Eye in Primary Sjögren’s Syndrome" Journal of Clinical Medicine 11, no. 2: 463. https://doi.org/10.3390/jcm11020463







