Use of CytoSorb© Hemoadsorption in Patients on Veno-Venous ECMO Support for Severe Acute Respiratory Distress Syndrome: A Systematic Review
Abstract
:1. Introduction
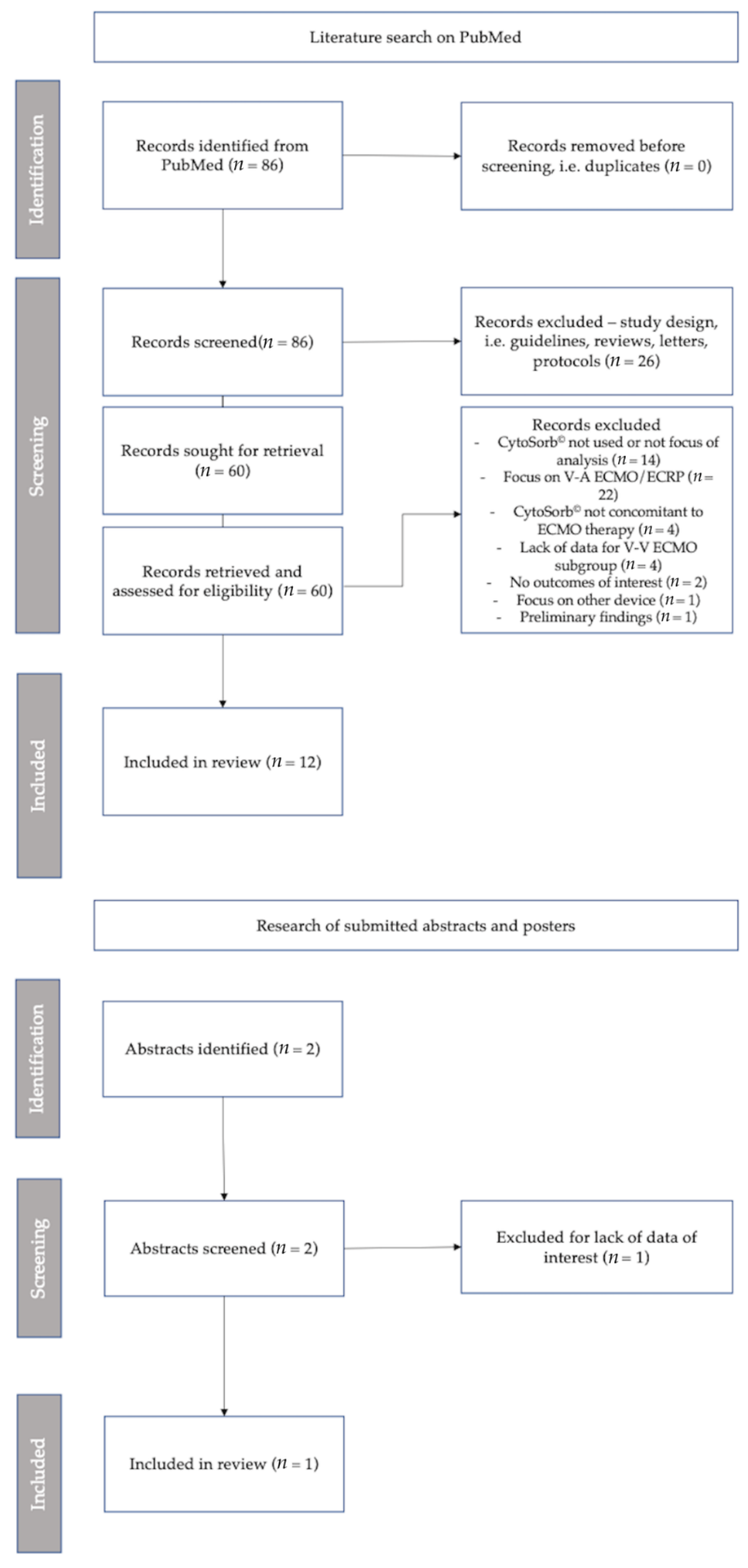
2. Materials and Methods
3. Results
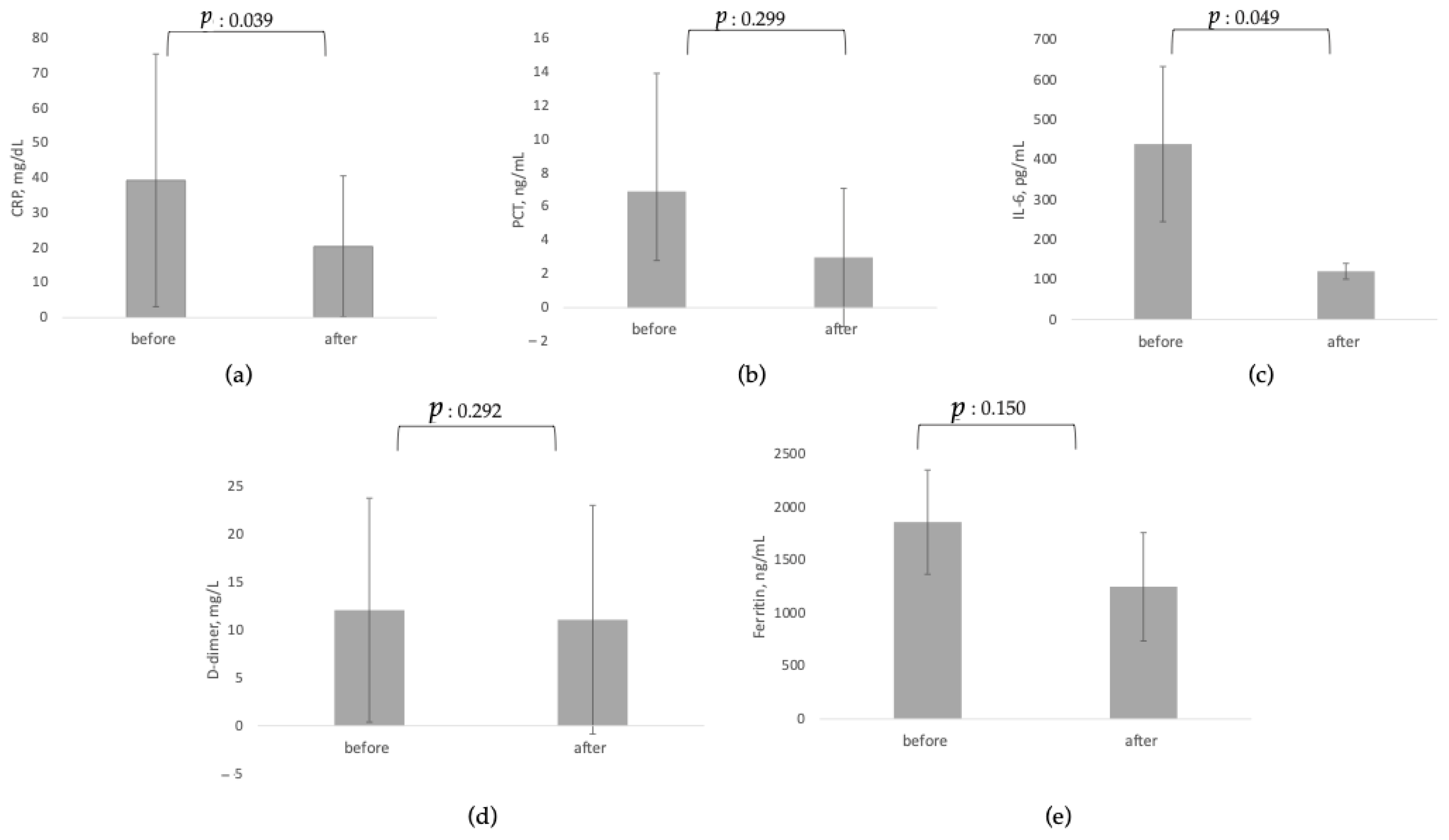
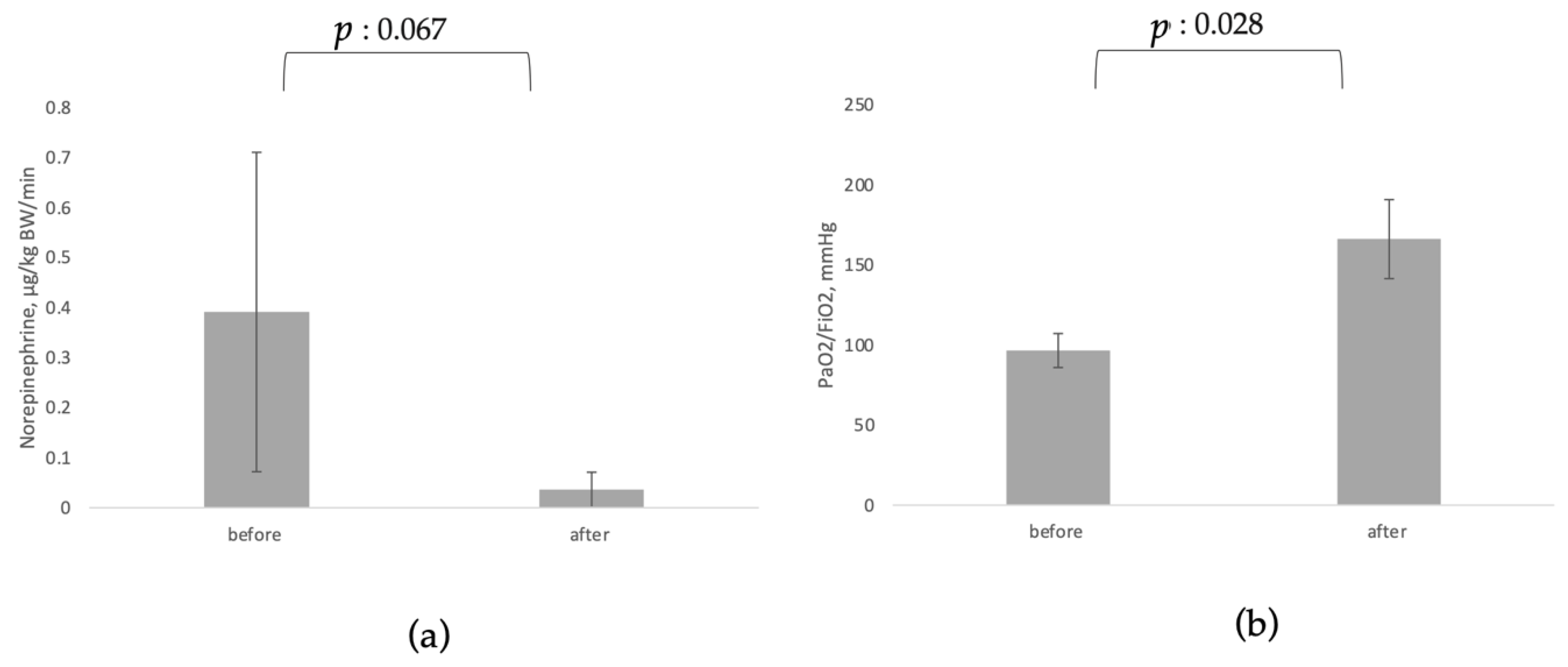
3.1. Effects on Circulating Biomarkers, Organ Function and Organ Support
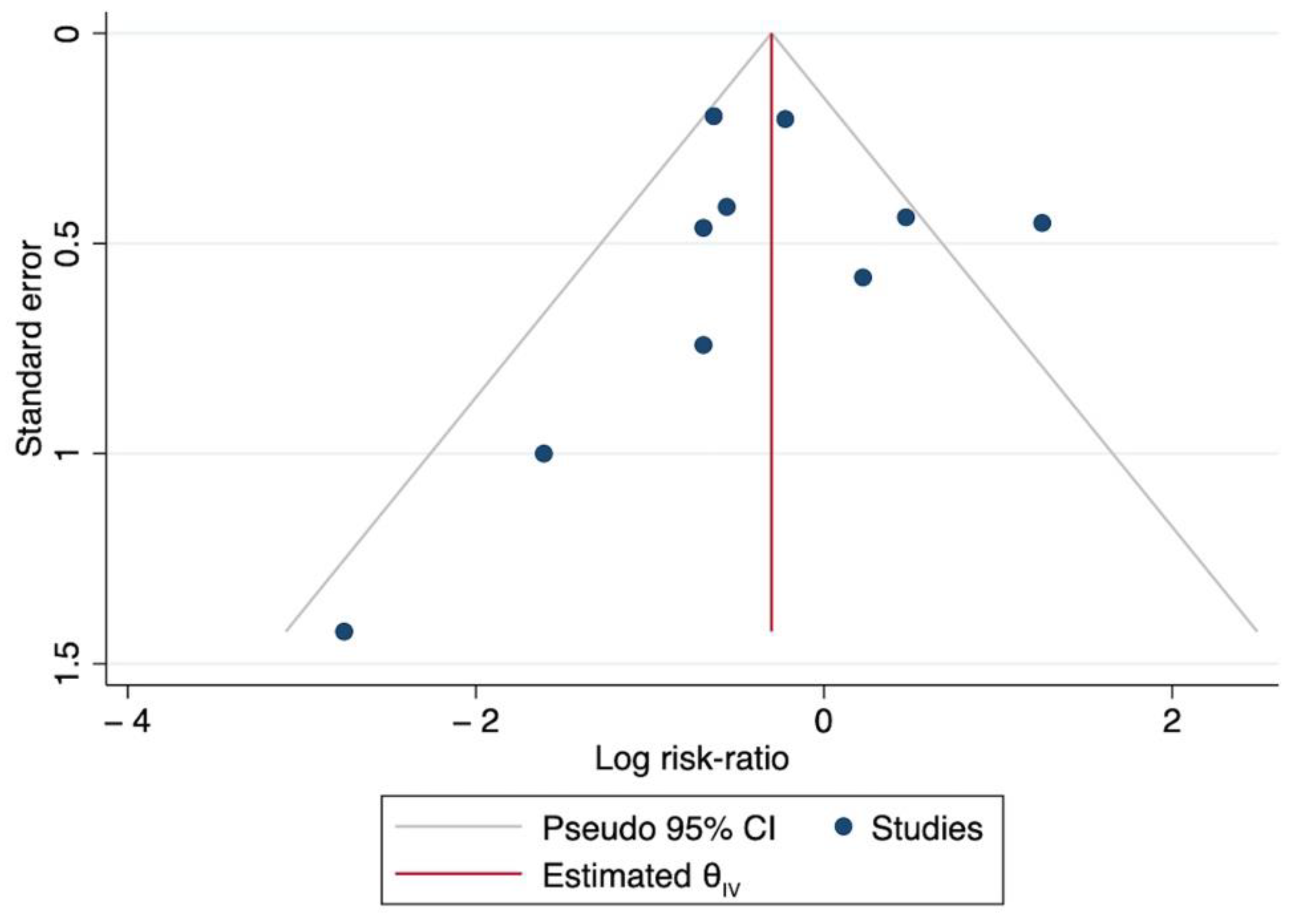
3.2. Effect on Mortality
CytoSorb© and V-V ECMO in COVID-19 Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reference | Indication | Patients Treated n | Controls n | Mortality Reported At | Inflammatory Markers | Norepinephrine Dosage Reported | PaO2/FiO2 Ratio Reported |
|---|---|---|---|---|---|---|---|
| Akil et al., Thorac Cardiovasc Surg 2021; 69(3):246–251 [80] | ARDS | 13 | 7 | 30 days | PCT, CRP | ✓ | - |
| Song et al. Front Med 2021; 8:773461 [interim analysis] [89] | ARDS/COVID | 52 | ICU, 30 days, 90 days | CRP, ILs-6, D-dimer | - | ✓ | |
| Kogelmann et al., J Intensive Care Society 2020: 21(2):183–190 [13] | ARDS | 7 | 28 days, ICU and hospital | - | ✓ | ||
| Hayanga et al., 2022 Abstract No 000494, The European Society of Intensive Care Medicine (ESICM) 2022 [79] | ARDS/COVID | 100 | 90 days | PCT, CRP, IL-6, D-dimer | - | (✓) | |
| Geraci et al., J Cardiac Surg 2021; 36(11):4256–4264 [84] | ARDS/COVID | 10 | Overall | PCT, CRP, IL6, D-dimer | - | - | |
| Pieri et al., Int J Artif Organs. 2022; 45(2):216–220 [83] | ARDS/COVID | 15 | ICU | CRP | - | ✓ | |
| Supady et al., Lan Resp Med 2021; 9(7): 755–762 [20] | ARDS/COVID | 17 | 17 | 30 days | IL-6, D-dimer | ✓ | - |
| Rieder et al., Artif Organs 2021; 45(2):191–194 [86] | ARDS/COVID | 1 | - | IL-6 D-dimer | ✓ | - | |
| Rodeia et al., Blood Purif 2021; epub [87] | ARDS/COVID | 5 | Overall/unspecified | PCT, CRP, IL-6 | - | - | |
| Rieder et al., ASAIO J. 2021 Mar 1;67(3):332–338 [82] | ARDS | 9 | 9 | ICU | - | ✓ | - |
| Paisey et al., Int J Artif Organs. 2021 Oct;44(10):664–674 [85] | ARDS/COVID | 10 | ICU | PCT, CRP, IL-6, D-dimer, Ferritin | - | - | |
| Stockmann et al., Crit Care Med. 2022;50(6):964–976 [81] | COVID | 9 | 7 | 30 days | - | - | - |
| Akil et al., Int J Artif Organs 2022 Jul;45(7):615–622 [88] | ARDS/COVID | 16 | 10 | 90 days | IL-6 | ✓ | ✓ |
References
- Auriemma, C.L.; Delucchi, K.; Liu, K.D.; Calfee, C.S. The attributable mortality of acute respiratory distress syndrome. Intensive Care Med. 2020, 46, 1510–1511. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Tsai, Y.F.; Pan, Y.L.; Hwang, T.L. Understanding the role of neutrophils in acute respiratory distress syndrome. Biomed. J. 2021, 44, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mallampalli, R.K. The acute respiratory distress syndrome: From mechanism to translation. J. Immunol. 2015, 194, 855–860. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.Y.; Hong, S.B. Sepsis and Acute Respiratory Distress Syndrome: Recent Update. Tuberc. Respir. Dis. 2016, 79, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Pelosi, P.; Rocco, P.R.M.; Gama de Abreu, M. Close down the lungs and keep them resting to minimize ventilator-induced lung injury. Crit. Care 2018, 22, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gattinoni, L.; Chiumello, D.; Rossi, S. COVID-19 pneumonia: ARDS or not? Crit. Care 2020, 24, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perico, L.; Benigni, A.; Casiraghi, F.; Ng, L.F.P.; Renia, L.; Remuzzi, G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021, 17, 46–64. [Google Scholar] [CrossRef]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef] [Green Version]
- Thiagarajan, R.R.; Barbaro, R.P.; Rycus, P.T.; McMullan, D.M.; Conrad, S.A.; Fortenberry, J.D.; Paden, M.L.; ELSO Member Centers. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017, 63, 60–67. [Google Scholar] [CrossRef]
- Schultz, P.; Schwier, E.; Eickmeyer, C.; Henzler, D.; Köhler, T. High-dose CytoSorb hemoadsorption is associated with improved survival in patients with septic shock: A retrospective cohort study. J. Crit. Care 2021, 64, 184–192. [Google Scholar] [CrossRef]
- Rimmele, T.; Kellum, J.A. Clinical review: Blood purification for sepsis. Crit. Care 2011, 15, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronco, C.; Tetta, C.; Mariano, F.; Wratten, M.L.; Bonello, M.; Bordoni, V.; Cardona, X.; Inguaggiato, P.; Pilotto, L.; d’Intini, V.; et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: The peak concentration hypothesis. Artif. Organs. 2003, 27, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Kogelmann, K.; Scheller, M.; Druner, M.; Jarczak, D. Use of hemoadsorption in sepsis-associated ECMO-dependent severe ARDS: A case series. J. Intensive Care Soc. 2020, 21, 183–190. [Google Scholar] [CrossRef]
- Gruda, M.C.; Ruggeberg, K.G.; O’Sullivan, P.; Guliashvili, T.; Scheirer, A.R.; Golobish, T.D.; Capponi, V.J.; Chan, P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb(R) sorbent porous polymer beads. PLoS ONE 2018, 13, e0191676. [Google Scholar] [CrossRef]
- Kumar, V. Inflammation research sails through the sea of immunology to reach immunometabolism. Int. Immunopharmacol. 2019, 73, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Friesecke, S.; Stecher, S.S.; Gross, S.; Felix, S.B.; Nierhaus, A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: A prospective single-center study. J. Artif. Organs. 2017, 20, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, W.P.; Duran, S.; Kuijper, M.; Ince, C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: A propensity-score-weighted retrospective study. Crit. Care 2019, 23, 317. [Google Scholar] [CrossRef] [Green Version]
- Kogelmann, K.; Jarczak, D.; Scheller, M.; Druner, M. Hemoadsorption by CytoSorb in septic patients: A case series. Crit. Care 2017, 21, 74. [Google Scholar] [CrossRef] [Green Version]
- Napp, L.C.; Lebreton, G.; De Somer, F.; Supady, A.; Pappalardo, F. Opportunities, controversies, and challenges of extracorporeal hemoadsorption with CytoSorb during ECMO. Artif. Organs. 2021, 45, 1240–1249. [Google Scholar] [CrossRef]
- Supady, A.; Weber, E.; Rieder, M.; Lother, A.; Niklaus, T.; Zahn, T.; Frech, F.; Muller, S.; Kuhl, M.; Benk, C.; et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): A single centre, open-label, randomised, controlled trial. Lancet. Respir. Med. 2021, 9, 755–762. [Google Scholar] [CrossRef]
- Hawchar, F.; Laszlo, I.; Oveges, N.; Trasy, D.; Ondrik, Z.; Molnar, Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J. Crit. Care 2019, 49, 172–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawchar, F.; Rao, C.; Akil, A.; Mehta, Y.; Rugg, C.; Scheier, J.; Adamson, H.; Deliargyris, E.; Molnar, Z. The Potential Role of Extracorporeal Cytokine Removal in Hemodynamic Stabilization in Hyperinflammatory Shock. Biomedicines 2021, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Leonardis, F.; De Angelis, V.; Frisardi, F.; Pietrafitta, C.; Riva, I.; Valetti, T.M.; Broletti, V.; Marchesi, G.; Menato, L.; Nani, R.; et al. Effect of Hemoadsorption for Cytokine Removal in Pneumococcal and Meningococcal Sepsis. Case. Rep. Crit. Care 2018, 2018, 1205613. [Google Scholar] [CrossRef] [PubMed]
- Dimski, T.; Brandenburger, T.; Slowinski, T.; Kindgen-Milles, D. Feasibility and safety of combined cytokine adsorption and continuous veno-venous hemodialysis with regional citrate anticoagulation in patients with septic shock. Int. J. Artif. Organs. 2020, 43, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Poli, E.C.; Rimmele, T.; Schneider, A.G. Hemoadsorption with CytoSorb((R)). Intensive Care Med. 2019, 45, 236–239. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ Open 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Rosner, B.; Glynn, R.J.; Lee, M.L.T. The Wilcoxon signed rank test for paired comparisons of clustered data. Biometrics 2006, 62, 185–192. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2019.
- Perez, M.H.; Maitre, G.; Longchamp, D.; Amiet, V.; Natterer, J.; Ferry, T.; Schneider, A.; Plaza Wuthrich, S.; Di Bernardo, S. CytoSorb((R)) hemoadsorption and mechanical circulatory support in a newborn with refractory shock after congenital heart surgery. Int. J. Artif. Organs. 2019, 42, 521–524. [Google Scholar] [CrossRef]
- Traeger, K.; Skrabal, C.; Fischer, G.; Schroeder, J.; Marenski, L.; Liebold, A.; Reinelt, H.; Datzmann, T. Hemoadsorption treatment with CytoSorb((R)) in patients with extracorporeal life support therapy: A case series. Int. J. Artif. Organs. 2020, 43, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Calabro, M.G.; Febres, D.; Recca, G.; Lembo, R.; Fominskiy, E.; Scandroglio, A.M.; Zangrillo, A.; Pappalardo, F. Blood Purification With CytoSorb in Critically Ill Patients: Single-Center Preliminary Experience. Artif. Organs. 2019, 43, 189–194. [Google Scholar] [CrossRef]
- Peyneau, M.; de Chaisemartin, L.; Faille, D.; Messika, J.; Mal, H.; Castier, Y.; Mordant, P.; Carrasco, J.L.; Tanaka, S.; Lortat Jacob, B.; et al. First Experience With Extracorporeal Cytokine Adsorption Therapy After Lung Transplantation. Transpl. Int. 2022, 35, 10319. [Google Scholar] [CrossRef]
- Supady, A.; Zahn, T.; Kuhl, M.; Maier, S.; Benk, C.; Kaier, K.; Bottiger, B.W.; Bode, C.; Lother, A.; Staudacher, D.L.; et al. Cytokine adsorption in patients with post-cardiac arrest syndrome after extracorporeal cardiopulmonary resuscitation (CYTER)—A single-centre, open-label, randomised, controlled trial. Resuscitation 2022, 173, 169–178. [Google Scholar] [CrossRef]
- Supady, A.; Zahn, T.; Rieder, M.; Benk, C.; Lother, A.; Bode, C.; Wengenmayer, T.; Staudacher, D.; Kellum, J.A.; Duerschmied, D. Effect of Cytokine Adsorption on Survival and Circulatory Stabilization in Patients Receiving Extracorporeal Cardiopulmonary Resuscitation. ASAIO J. 2022, 68, 64–72. [Google Scholar] [CrossRef]
- Eid, M.; Fouquet, O.; Darreau, C.; Pierrot, M.; Kouatchet, A.; Mercat, A.; Baufreton, C. Successfully treated necrotizing fasciitis using extracorporeal life support combined with hemoadsorption device and continuous renal replacement therapy. Int. J. Artif. Organs. 2018, 41, 178–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruenger, F.; Kizner, L.; Weile, J.; Morshuis, M.; Gummert, J.F. First successful combination of ECMO with cytokine removal therapy in cardiogenic septic shock: A case report. Int. J. Artif. Organs. 2015, 38, 113–116. [Google Scholar] [CrossRef]
- Bemtgen, X.; Zotzmann, V.; Benk, C.; Rilinger, J.; Steiner, K.; Asmussen, A.; Bode, C.; Wengenmayer, T.; Maier, S.; Staudacher, D.L. Thrombotic circuit complications during venovenous extracorporeal membrane oxygenation in COVID-19. J. Thromb. Thrombolysis 2021, 51, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.F.; Hon, K.L.; Lun, K.S.; Leung, K.K.Y.; Cheung, W.L.; Leung, A.K.C. Successful Treatment of Rhabdomyolysis-Associated Acute Kidney Injury with Haemoadsorption and Continuous Renal Replacement Therapy. Case. Rep. Pediatr. 2021, 2021, 2148024. [Google Scholar] [CrossRef] [PubMed]
- Rybalko, A.; Pytal, A.; Kaabak, M.; Rappoport, N.; Bidzhiev, A.; Lastovka, V. Case Report: Successful Use of Extracorporeal Therapies After ECMO Resuscitation in a Pediatric Kidney Transplant Recipient. Front. Pediatr. 2020, 8, 593123. [Google Scholar] [CrossRef]
- Zickler, D.; Nee, J.; Arnold, T.; Schroder, T.; Slowinski, T.; Eckardt, K.U.; Korner, R.; Kruse, J.M. Use of Hemoadsorption in Patients With Severe Intoxication Requiring Extracorporeal Cardiopulmonary Support-A Case Series. ASAIO J. 2021, 67, e186–e190. [Google Scholar] [CrossRef]
- Steurer, L.M.; Schlager, G.; Sadeghi, K.; Golej, J.; Wiedemann, D.; Hermon, M. Hemadsorption as rescue therapy for patients with multisystem organ failure in pediatric intensive care-Report of two cases reports and review of the literature. Artif. Organs. 2021, 45, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, I.; Zoller, M.; Angstwurm, M.; Kur, F.; Frey, L. Venlafaxine intoxication with development of takotsubo cardiomyopathy: Successful use of extracorporeal life support, intravenous lipid emulsion and CytoSorb(R). Int. J. Artif. Organs. 2017, 40, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Dogan, G.; Hanke, J.; Puntigam, J.; Haverich, A.; Schmitto, J.D. Hemoadsorption in cardiac shock with bi ventricular failure and giant-cell myocarditis: A case report. Int. J. Artif. Organs. 2018, 41, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Szigeti, S.; Varga, T.; Daroczi, L.; Barati, Z.; Merkely, B.; Gal, J. Continuous cytokine haemoadsorption incorporated into a venoarterial ECMO circuit for the management of postcardiotomy cardiogenic and septic shock—A case report. Perfusion 2018, 33, 593–596. [Google Scholar] [CrossRef]
- Traeger, K.; Schutz, C.; Fischer, G.; Schroder, J.; Skrabal, C.; Liebold, A.; Reinelt, H. Cytokine Reduction in the Setting of an ARDS-Associated Inflammatory Response with Multiple Organ Failure. Case. Rep. Crit. Care 2016, 2016, 9852073. [Google Scholar] [CrossRef] [Green Version]
- Marek, S.; Gamper, G.; Reining, G.; Bergmann, P.; Mayr, H.; Kliegel, A. ECMO and cytokine removal for bridging to surgery in a patient with ischemic ventricular septal defect—A case report. Int. J. Artif. Organs. 2017, 40, 526–529. [Google Scholar] [CrossRef]
- De Rosa, S.; Samoni, S.; Ronco, C. Sequential Extracorporeal Therapy Collaborative Device and Timely Support for Endotoxic, Septic, and Cardiac Shock: A Case Report. Blood. Purif. 2020, 49, 502–508. [Google Scholar] [CrossRef]
- Poli, E.C.; Simoni, C.; Andre, P.; Buclin, T.; Longchamp, D.; Perez, M.H.; Ferry, T.; Schneider, A.G. Clindamycin clearance during Cytosorb((R)) hemoadsorption: A case report and pharmacokinetic study. Int. J. Artif. Organs. 2019, 42, 258–262. [Google Scholar] [CrossRef]
- Bemtgen, X.; Klingel, K.; Hufnagel, M.; Janda, A.; Bode, C.; Staudacher, D.L.; Supady, A.; Jandova, I. Case Report: Lymphohistiocytic Myocarditis With Severe Cardiogenic Shock Requiring Mechanical Cardiocirculatory Support in Multisystem Inflammatory Syndrome Following SARS-CoV-2 Infection. Front. Cardiovasc. Med. 2021, 8, 1091. [Google Scholar] [CrossRef]
- Lees, N.J.; Rosenberg, A.; Hurtado-Doce, A.I.; Jones, J.; Marczin, N.; Zeriouh, M.; Weymann, A.; Sabashnikov, A.; Simon, A.R.; Popov, A.F. Combination of ECMO and cytokine adsorption therapy for severe sepsis with cardiogenic shock and ARDS due to Panton-Valentine leukocidin-positive Staphylococcus aureus pneumonia and H1N1. J. Artif. Organs. 2016, 19, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Roedl, K.; Kahn, A.; Jarczak, D.; Fischer, M.; Boenisch, O.; de Heer, G.; Burdelski, C.; Frings, D.; Sensen, B.; Nierhaus, A.; et al. Clinical Characteristics, Complications and Outcomes of Patients with Severe Acute Respiratory Distress Syndrome Related to COVID-19 or Influenza Requiring Extracorporeal Membrane Oxygenation-A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 5440. [Google Scholar] [CrossRef] [PubMed]
- Zurl, C.; Waller, M.; Schwameis, F.; Muhr, T.; Bauer, N.; Zollner-Schwetz, I.; Valentin, T.; Meinitzer, A.; Ullrich, E.; Wunsch, S.; et al. Isavuconazole Treatment in a Mixed Patient Cohort with Invasive Fungal Infections: Outcome, Tolerability and Clinical Implications of Isavuconazole Plasma Concentrations. J. Fungi. 2020, 6, 90. [Google Scholar] [CrossRef]
- Schrage, B.; Becher, P.M.; Gossling, A.; Savarese, G.; Dabboura, S.; Yan, I.; Beer, B.; Soffker, G.; Seiffert, M.; Kluge, S.; et al. Temporal trends in incidence, causes, use of mechanical circulatory support and mortality in cardiogenic shock. ESC Heart. Fail. 2021, 8, 1295–1303. [Google Scholar] [CrossRef]
- Roedl, K.; Jarczak, D.; Drolz, A.; Wichmann, D.; Boenisch, O.; de Heer, G.; Burdelski, C.; Frings, D.; Sensen, B.; Nierhaus, A.; et al. Severe liver dysfunction complicating course of COVID-19 in the critically ill: Multifactorial cause or direct viral effect? Ann. Intensive Care 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Ghandili, S.; Pfefferle, S.; Roedl, K.; Sonnemann, P.; Karagiannis, P.; Boenisch, O.; Kluge, S.; Schmiedel, S.; Ittrich, H.; Rohde, H.; et al. Challenges in treatment of patients with acute leukemia and COVID-19: A series of 12 patients. Blood. Adv. 2020, 4, 5936–5941. [Google Scholar] [CrossRef]
- Seeliger, B.; Doebler, M.; Hofmaenner, D.A.; Wendel-Garcia, P.D.; Schuepbach, R.A.; Schmidt, J.J.; Welte, T.; Hoeper, M.M.; Gillmann, H.J.; Kuehn, C.; et al. Intracranial Hemorrhages on Extracorporeal Membrane Oxygenation: Differences Between COVID-19 and Other Viral Acute Respiratory Distress Syndrome. Crit. Care Med. 2022, 50, e526–e538. [Google Scholar] [CrossRef]
- Widmeier, E.; Wengenmayer, T.; Maier, S.; Benk, C.; Zotzmann, V.; Staudacher, D.L.; Supady, A. Extracorporeal membrane oxygenation during the first three waves of the coronavirus disease 2019 pandemic: A retrospective single-center registry study. Artif. Organs. 2022, 46, 1876–1885. [Google Scholar] [CrossRef]
- Roedl, K.; Jarczak, D.; Thasler, L.; Bachmann, M.; Schulte, F.; Bein, B.; Weber, C.F.; Schafer, U.; Veit, C.; Hauber, H.P.; et al. Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: A multicentric study in Germany. Aust. Crit. Care 2021, 34, 167–175. [Google Scholar] [CrossRef]
- Hayanga, J.W.A.; Kakuturu, J.; Dhamija, A.; Asad, F.; McCarthy, P.; Sappington, P.; Badhwar, V. Cannulate, extubate, ambulate approach for extracorporeal membrane oxygenation for COVID-19. J. Thorac. Cardiovasc. Surg. 2022; in press. [Google Scholar] [CrossRef]
- Scherer, C.; Lusebrink, E.; Joskowiak, D.; Feuchtgruber, V.; Petzold, T.; Hausleiter, J.; Peterss, S.; Massberg, S.; Hagl, C.; Orban, M. Mortality in Cardiogenic Shock Patients Is Predicted by Pao2/Fio2 (Horowitz Index) Measured on ICU After Venoarterial Extracorporeal Membrane Oxygenation Implantation. Crit. Care Explor. 2021, 3, e0540. [Google Scholar] [CrossRef] [PubMed]
- Lüsebrink, E.; Massberg, S.; Orban, M. Combined extracorporeal membrane oxygenation and microaxial pump-when left ventricular preload is too low to unload in cardiogenic shock. Health. Sci. Rep. 2021, 4, e321. [Google Scholar] [CrossRef]
- Scherer, C.; Stremmel, C.; Lüsebrink, E.; Stocker, T.J.; Stark, K.; Schönegger, C.; Kellnar, A.; Kleeberger, J.; Hanuna, M.; Petzold, T.; et al. Manual Compression versus Suture-Mediated Closure Device Technique for VA-ECMO Decannulation. J. Interv. Cardiol. 2022, 2022, 9915247. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Miyamoto, K.; Kato, S. Comparison of the circulatory effects of continuous renal replacement therapy using AN69ST and polysulfone membranes in septic shock patients: A retrospective observational study. Apher. Dial. 2020, 24, 561–567. [Google Scholar] [CrossRef]
- Thammavaranucupt, K.; Tassaneyasin, T.; Theerawit, P.; Sutherasan, Y.; Pinsem, P.; Suppadungsuk, S.; Nanthatanti, N.; Kirdlarp, S.; Sungkanuparph, S.; Srichatrapimuk, S. Spontaneous tension hemothorax in a severe COVID-19 patient receiving ECMO therapy: The other side of COVID-19-associated coagulopathy. Respir. Med. Case. Rep. 2022, 37, 101663. [Google Scholar] [CrossRef] [PubMed]
- Scandroglio, A.M.; Pieri, M.; Nardelli, P.; Fominskiy, E.; Calabro, M.G.; Melisurgo, G.; Ajello, S.; Pappalardo, F. Impact of CytoSorb on kinetics of vancomycin and bivalirudin in critically ill patients. Artif. Organs. 2021, 45, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Scharf, C.; Schroeder, I.; Paal, M.; Winkels, M.; Irlbeck, M.; Zoller, M.; Liebchen, U. Can the cytokine adsorber CytoSorb((R)) help to mitigate cytokine storm and reduce mortality in critically ill patients? A propensity score matching analysis. Ann. Intensive Care 2021, 11, 115. [Google Scholar] [CrossRef]
- Scharf, C.; Liebchen, U.; Paal, M.; Irlbeck, M.; Zoller, M.; Schroeder, I. Blood purification with a cytokine adsorber for the elimination of myoglobin in critically ill patients with severe rhabdomyolysis. Crit. Care 2021, 25, 41. [Google Scholar] [CrossRef]
- Wunderlich-Sperl, F.; Kautzky, S.; Pickem, C.; Hormann, C. Adjuvant hemoadsorption therapy in patients with severe COVID-19 and related organ failure requiring CRRT or ECMO therapy: A case series. Int. J. Artif. Organs. 2021, 44, 694–702. [Google Scholar] [CrossRef]
- Tampe, D.; Korsten, P.; Bremer, S.C.B.; Winkler, M.S.; Tampe, B. Kinetics of Bilirubin and Ammonia Elimination during Hemadsorption Therapy in Secondary Sclerosing Cholangitis Following ECMO Therapy and Severe COVID-19. Biomedicines 2021, 9, 1841. [Google Scholar] [CrossRef]
- Maritati, F.; Cerutti, E.; Zuccatosta, L.; Fiorentini, A.; Finale, C.; Ficosecco, M.; Cristiano, F.; Capestro, A.; Balestra, E.; Taruscia, D.; et al. SARS-CoV-2 infection in kidney transplant recipients: Experience of the italian marche region. Transpl. Infect. Dis. 2020, 22, e13377. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.F.; Lun, K.S.; Hon, K.L. Single-pass albumin dialysis and hemoadsorption for bilirubin and bile acids removal for a child with hyperbilirubinemia after ventricular assist device implantation. J. Artif. Organs. 2022, 25, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, P.; Kutnik, P.; Potrec-Studzinska, B.; Sysiak-Slawecka, J.; Rypulak, E.; Borys, M.; Czczuwar, M. Hemoadsorption in isolated conjugated hyperbilirubinemia after extracorporeal membrane oxygenation support. Cholestasis of sepsis: A case report and review of the literature on differential causes of jaundice in ICU patient. Int. J. Artif. Organs. 2019, 42, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Gardette, M.; Creteur, J.; Brasseur, A.; Lorent, S.; Grimaldi, D. Hemoadsorption to treat severe iatrogenic intoxication with Patent Blue: A case report. J. Med. Case. Rep. 2021, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.C.; Merchan, C.; Toy, B.; Goldenberg, R.M.; Geraci, T.C.; Chang, S.H.; Galloway, A.C.; Smith, D.E., 3rd; Moazami, N. Impact of CytoSorb Hemoadsorption on Sedation Requirements in Patients With Severe COVID-19 on Venovenous Extracorporeal Membrane Oxygenation. ASAIO J. 2021, 67, 856–861. [Google Scholar] [CrossRef]
- Huang, T.T.; Chien, Y.C.; Wang, C.H.; Chang, S.Y.; Wang, J.T.; Hsieh, S.C.; Yeh, Y.C.; Ku, S.C.; Yu, C.J.; Chiang, B.L.; et al. Successful Treatment of a Critically Ill COVID-19 Patient Using Continuous Renal Replacement Therapy With Enhanced Cytokine Removal and Tocilizumab: A Case Report. Front. Med. 2021, 8, 649583. [Google Scholar] [CrossRef]
- Rieder, M.; Wengenmayer, T.; Staudacher, D.; Duerschmied, D.; Supady, A. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation. Crit. Care 2020, 24, 435. [Google Scholar] [CrossRef]
- Song, T.; Hayanga, J.; Durham, L.; Garrison, L.; Smith, D.; Bartlett, R.; Scheier, J.; Eke-Okoro, C.; Molnar, Z.; Deliargyris, E.N.; et al. Adjunctive hemoadsorption in critically ill COVID-19 patients requiring extracorporeal membrane oxygenation (ECMO): The CytoSorb Therapy in COVID-19 patients (CTC) multicenter registry. In Proceedings of the European Society of Intensive Care Medicine (ESICM) Conference, Paris, France, 22–26 October 2022. [Google Scholar]
- Akil, A.; Ziegeler, S.; Reichelt, J.; Rehers, S.; Abdalla, O.; Semik, M.; Fischer, S. Combined Use of CytoSorb and ECMO in Patients with Severe Pneumogenic Sepsis. Thorac. Cardiovasc. Surg. 2021, 69, 246–251. [Google Scholar] [CrossRef]
- Stockmann, H.; Thelen, P.; Stroben, F.; Pigorsch, M.; Keller, T.; Krannich, A.; Spies, C.; Treskatsch, S.; Ocken, M.; Kunz, J.V.; et al. CytoSorb Rescue for COVID-19 Patients With Vasoplegic Shock and Multiple Organ Failure: A Prospective, Open-Label, Randomized Controlled Pilot Study. Crit. Care Med. 2022, 50, 964–976. [Google Scholar] [CrossRef]
- Rieder, M.; Duerschmied, D.; Zahn, T.; Lang, C.; Benk, C.; Lother, A.; Biever, P.; Bode, C.; Wengenmayer, T.; Staudacher, D.; et al. Cytokine Adsorption in Severe Acute Respiratory Failure Requiring Veno-Venous Extracorporeal Membrane Oxygenation. ASAIO J. 2021, 67, 332–338. [Google Scholar] [CrossRef]
- Pieri, M.; Fominskiy, E.; Nardelli, P.; Bonizzoni, M.A.; Scandroglio, A.M. CytoSorb purification in critically ill SARS-CoV-2 patients. Int. J. Artif. Organs. 2022, 45, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Geraci, T.C.; Kon, Z.N.; Moazami, N.; Chang, S.H.; Carillo, J.; Chen, S.; Fargnoli, A.; Alimi, M.; Pass, H.; Galloway, A.; et al. Hemoadsorption for management of patients on veno-venous ECMO support for severe COVID-19 acute respiratory distress syndrome. J. Card. Surg. 2021, 36, 4256–4264. [Google Scholar] [CrossRef] [PubMed]
- Paisey, C.; Patvardhan, C.; Mackay, M.; Vuylsteke, A.; Bhagra, S.K. Continuous hemadsorption with cytokine adsorber for severe COVID-19: A case series of 15 patients. Int. J. Artif. Organs. 2021, 44, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Rieder, M.; Zahn, T.; Benk, C.; Lother, A.; Bode, C.; Staudacher, D.; Duerschmied, D.; Supady, A. Cytokine adsorption in a patient with severe coronavirus disease 2019 related acute respiratory distress syndrome requiring extracorporeal membrane oxygenation therapy: A case report. Artif. Organs. 2021, 45, 191–194. [Google Scholar] [CrossRef]
- Rodeia, S.C.; Martins, F.L.; Fortuna, P.; Bento, L. Cytokine Adsorption Therapy during Extracorporeal Membrane Oxygenation in Adult Patients with COVID-19. Blood. Purif. 2022, 51, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Akil, A.; Ziegeler, S.; Rehers, S.; Ernst, E.C.; Fischer, S. Blood purification therapy in patients with severe COVID-19 requiring veno-venous ECMO therapy: A retrospective study. Int. J. Artif. Organs. 2022, 45, 615–622. [Google Scholar] [CrossRef]
- Song, T.; Hayanga, J.; Durham, L.; Garrison, L.; McCarthy, P.; Barksdale, A.; Smith, D.; Bartlett, R.; Jaros, M.; Nelson, P.; et al. CytoSorb Therapy in COVID-19 (CTC) Patients Requiring Extracorporeal Membrane Oxygenation: A Multicenter, Retrospective Registry. Front. Med. 2021, 8, 773461. [Google Scholar] [CrossRef]
- Song, T.; Hayanga, J.; Durham, L.; Garrison, L.; Smith, D.; Molnar, Z.; Scheier, J.; Wendt, D.; Deliargyris, E.; Moazami, N. Early initiation of hemoadsorption reduces the need for organ support in critically ill COVID-19 patients on ECMO: A post-hoc analysis from the CytoSorb Therapy in COVID-19 (CTC) Registry. In Proceedings of the 33rd Annual Elso Conference, Boston, MA, USA, 14–17 September 2022. [Google Scholar]
- Extracorporeal Life Support Organization (ELSO). COVID-19 Registry Dashboard. Available online: https://www.elso.org/Registry/FullCOVID-19RegistryDashboard.aspx (accessed on 14 March 2022).
- Hayanga, J. 100 patient CTC results. Manuscr. Prep. 2022. [Google Scholar]
- Friedrichson, B.; Kloka, J.A.; Neef, V.; Mutlak, H.; Old, O.; Zacharowski, K.; Piekarski, F. Extracorporeal membrane oxygenation in coronavirus disease 2019: A nationwide cohort analysis of 4279 runs from Germany. Eur. J. Anaesthesiol. 2022, 39, 445–451. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Slutsky, A.S.; Bein, T.; Windisch, W.; Weber-Carstens, S.; Brodie, D. Complete countrywide mortality in COVID patients receiving ECMO in Germany throughout the first three waves of the pandemic. Crit. Care 2021, 25, 413. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Strassmann, S.; Merten, M.; Bein, T.; Windisch, W.; Meybohm, P.; Weber-Carstens, S. High In-Hospital Mortality Rate in Patients with COVID-19 Receiving Extracorporeal Membrane Oxygenation in Germany: A Critical Analysis. Am. J. Respir. Crit. Care Med. 2021, 204, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.H.; Gazzaniga, A.B.; Jefferies, M.R.; Huxtable, R.F.; Haiduc, N.J.; Fong, S.W. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans. Am. Soc. Artif. Intern. Organs. 1976, 22, 80–93. [Google Scholar] [PubMed]
- Ramanathan, K.; Antognini, D.; Combes, A.; Paden, M.; Zakhary, B.; Ogino, M.; MacLaren, G.; Brodie, D.; Shekar, K. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet. Respir. Med. 2020, 8, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napp, L.C.; Ziegeler, S.; Kindgen-Milles, D. Rationale of Hemoadsorption during Extracorporeal Membrane Oxygenation Support. Blood. Purif. 2019, 48, 203–214. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, J.C.; Chiscano-Camon, L.; Ruiz-Sanmartin, A.; Palmada, C.; Paola Plata-Menchaca, E.; Franco-Jarava, C.; Perez-Carrasco, M.; Hernandez-Gonzalez, M.; Ferrer, R. Cytokine Hemoadsorption as Rescue Therapy for Critically Ill Patients With SARS-CoV-2 Pneumonia With Severe Respiratory Failure and Hypercytokinemia. Front. Med. 2022, 8, 779038. [Google Scholar] [CrossRef]
- Connelly, K.G.; Moss, M.; Parsons, P.E.; Moore, E.E.; Moore, F.A.; Giclas, P.C.; Seligman, P.A.; Repine, J.E. Serum ferritin as a predictor of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1997, 155, 21–25. [Google Scholar] [CrossRef]
- Gandini, O.; Criniti, A.; Ballesio, L.; Giglio, S.; Galardo, G.; Gianni, W.; Santoro, L.; Angeloni, A.; Lubrano, C. Serum Ferritin is an independent risk factor for Acute Respiratory Distress Syndrome in COVID-19. J. Infect. 2020, 81, 986–989. [Google Scholar] [CrossRef]
- He, X.; Yao, F.; Chen, J.; Wang, Y.; Fang, X.; Lin, X.; Long, H.; Wang, Q.; Wu, Q. The poor prognosis and influencing factors of high D-dimer levels for COVID-19 patients. Sci. Rep. 2021, 11, 1830. [Google Scholar] [CrossRef]
- Putzu, A.; Schorer, R. Hemoadsorption in critically ill patients with or without COVID-19: A word of caution. J. Crit. Care 2021, 65, 140–141. [Google Scholar] [CrossRef]
- Köhler, T.; Schwier, E.; Henzler, D.; Eickmeyer, C. Does adjunctive hemoadsorption with CytoSorb affect survival of COVID-19 patients on ECMO? A critical statement. J. Crit. Care 2021, 66, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Hayanga, J.; Durham, L.; Garrison, L.; Smith, D.; Bartlett, R.; Scheier, J.; Molnar, Z.; Wendt, D.; Deliargyris, E.; et al. Adjunctive hemoadsorption in critically ill COVID-19 patients requiring extracorporeal membrane oxygenation (ECMO): Results of the CytoSorb Therapy in COVID-19 patients (CTC) multicenter registry. Crit. Care Med. 2022; Submitted. [Google Scholar]


| Before CytoSorb Mean ± SD | After CytoSorb Mean ± SD | p-Value | Patients n | |
|---|---|---|---|---|
| CRP, mg/dL [80,83,84,85,86,87,89] | 39.35 ± 36.2 | 20.39 ± 20.24 | 0.039 | 74 |
| PCT, ng/mL [80,84,85,87] | 6.90 ± 7.01 | 2.98 ± 4.10 | 0.299 | 36 |
| IL-6, pg/mL [20,86,87,88] | 439.50 ± 194.45 | 120.65 ± 19.72 | 0.049 | 39 |
| D-dimer, mg/L [20,83,84,85,86,89] | 12.07 ± 11.69 | 11.07 ± 11.94 | 0.292 | 70 |
| Ferritin, ng/mL [84,85,86,87,89] | 1860 ± 492.50 | 1249.12 ± 511.32 | 0.15 | 41 |
| Norepinephrine, µg/kg BW/min [20,80,82,86,88] | 0.391 ± 0.319 | 0.036 ± 0.035 | 0.067 | 56 |
| PaO2/FiO2, mmHg [13,83,88,89] | 96.55 ± 10.62 | 166.08 ± 24.66 | 0.028 | 59 |
| Study | Indication | Mortality Reported at | Control Group |
|---|---|---|---|
| Akil et al., 2021 [80] | ARDS; sepsis | 30 days | Reported cohort |
| Supady et al., 2021 [20] | COVID-19 | 30 days | Reported cohort |
| Akil et al., 2022 [88] | COVID-19 | 90 days | Reported cohort |
| Stockmann et al., 2022 [81] | COVID-19 | 30 days | Reported cohort |
| Rieder et al., 2021 [82] | ARDS | ICU | Reported cohort |
| Hayanga et al., 2022 [79] | COVID-19 | 90 days | ELSO registry for COVID-19 in the US |
| Pieri et al., 2021 [83] | COVID-19 | 30 days | ELSO registry for COVID-19 in the EU |
| Geraci et al., 2021 [84] | COVID-19 | 90 days | ELSO registry for COVID-19 in the US |
| Paisey et al., 2021 [85] | COVID-19 | 90 days | ELSO registry for COVID-19 in the EU |
| Kogelmann et al., 2020 [13] | ARDS; sepsis | 30-day; hospital | APACHE II |
| Study | Study Design | CytoSorb© Patients, n | Mortality % | Source of Control/Predicted Mortality | Control Patients n | Mortality % | ∂ Mortality ARR |
|---|---|---|---|---|---|---|---|
| Akil et al., 2021 [80] | Retrospective, observational | 13 | 0% | Control group | 7 | 57% | −57% |
| Supady et al., 2021 [20] * | RCT | 17 | 82% | Control group | 17 | 24% | +58% |
| Akil et al., 2022 [88] * | Retrospective, observational | 16 | 38% | Control group | 10 | 30% | +8% |
| Stockmann et al., 2022 [81] * | RCT | 9 | 78% | Control group | 7 | 100% | −22% |
| Rieder et al., 2021 [82] | Retrospective, observational | 9 | 44.4% | Control group | 9 | 78% | −33% |
| Hayanga et al., 2022 [79] * | Retrospective, observational | 100 | 26% | ELSO US registry | 100 | 49% | −23% |
| Pieri et al., 2021 [83] * | Retrospective, observational | 15 | 54% | ELSO EU registry | 15 | 30% | +24% |
| Geraci et al., 2021 [84] * | Retrospective, observational | 10 | 10% | ELSO US registry | 10 | 49% | −39% |
| Paisey et al., 2021 [85] | Retrospective, observational | 10 | 20% | ELSO EU registry | 10 | 42% | −22% |
| Kogelmann et al., 2020 [13] | Retrospective, observational | 7 | 43% | APACHE II (39) | 7 | 91% | −48% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akil, A.; Napp, L.C.; Rao, C.; Klaus, T.; Scheier, J.; Pappalardo, F. Use of CytoSorb© Hemoadsorption in Patients on Veno-Venous ECMO Support for Severe Acute Respiratory Distress Syndrome: A Systematic Review. J. Clin. Med. 2022, 11, 5990. https://doi.org/10.3390/jcm11205990
Akil A, Napp LC, Rao C, Klaus T, Scheier J, Pappalardo F. Use of CytoSorb© Hemoadsorption in Patients on Veno-Venous ECMO Support for Severe Acute Respiratory Distress Syndrome: A Systematic Review. Journal of Clinical Medicine. 2022; 11(20):5990. https://doi.org/10.3390/jcm11205990
Chicago/Turabian StyleAkil, Ali, L. Christian Napp, Cristina Rao, Teresa Klaus, Joerg Scheier, and Federico Pappalardo. 2022. "Use of CytoSorb© Hemoadsorption in Patients on Veno-Venous ECMO Support for Severe Acute Respiratory Distress Syndrome: A Systematic Review" Journal of Clinical Medicine 11, no. 20: 5990. https://doi.org/10.3390/jcm11205990
APA StyleAkil, A., Napp, L. C., Rao, C., Klaus, T., Scheier, J., & Pappalardo, F. (2022). Use of CytoSorb© Hemoadsorption in Patients on Veno-Venous ECMO Support for Severe Acute Respiratory Distress Syndrome: A Systematic Review. Journal of Clinical Medicine, 11(20), 5990. https://doi.org/10.3390/jcm11205990







