Expression Pattern of Tenascin-C, Matrilin-2, and Aggrecan in Diseases Affecting the Corneal Endothelium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Corneal Specimens
2.2. Preparation of Histological Sections
2.3. Immunohistochemistry (IHC) and Chromogenic Immunohistochemistry (CHR-IHC)
2.4. Evaluation and CHR-IHC Scoring
2.5. Statistical Analysis
3. Results
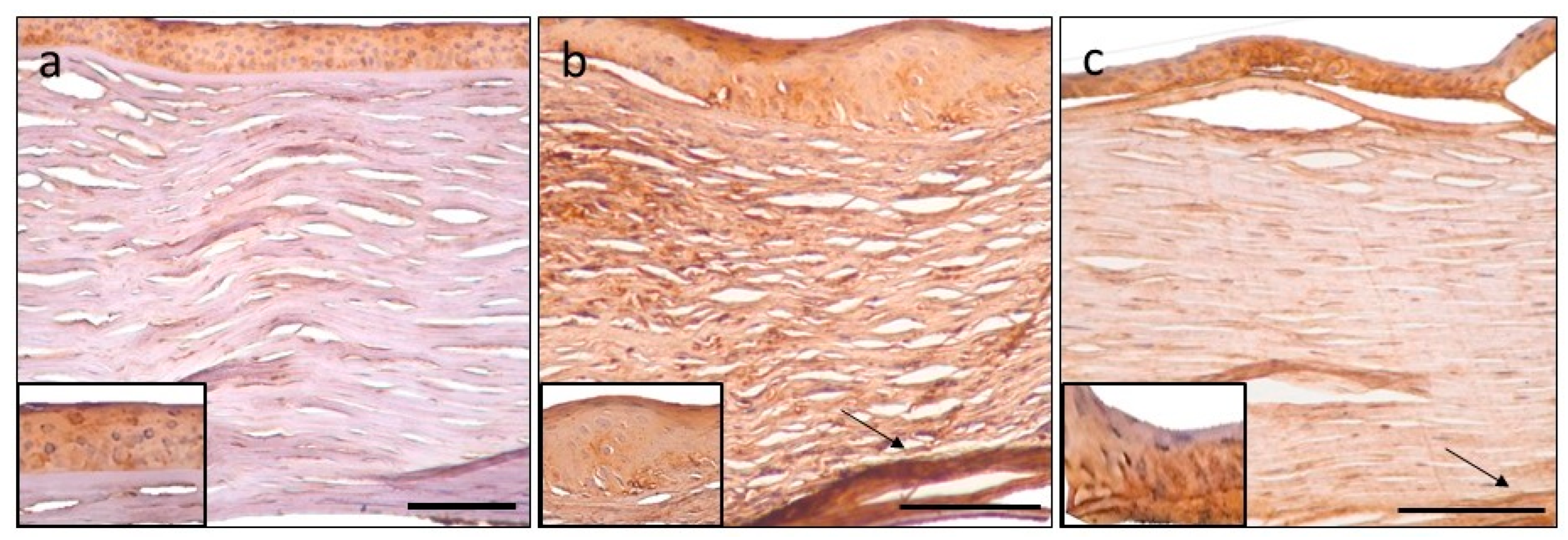
3.1. Tenascin-C CHR-IHC
3.2. Matrilin-2 CHR-IHC
3.3. Aggrecan CHR-IHC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michelacci, Y.M. Collagens and proteoglycans of the corneal extracellular matrix. Braz. J. Med. Biol. Res. 2003, 36, 1037–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meek, K.M.; Fullwood, N.J. Corneal and scleral collagens—A microscopist’s perspective. Micron 2001, 32, 261–272. [Google Scholar] [CrossRef]
- Puri, S.; Coulson-Thomas, Y.M.; Gesteira, T.F.; Coulson-Thomas, V.J. Distribution and function of glycosaminoglycans and proteoglycans in the development, homeostasis and pathology of the ocular surface. Front. Cell Dev. Biol. 2020, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E. Extracellular matrix, supramolecular organisation and shape. J. Anat. 1995, 187, 259–269. [Google Scholar] [PubMed]
- Esko, J.D.; Kimata, K.; Lindahl, U. Proteoglycans and sulfated glycosaminoglycans. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esco, J.D., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009; p. 800. [Google Scholar]
- Maurice, D.M. The structure and transparency of the cornea. J. Physiol. 1957, 136, 263–286. [Google Scholar] [CrossRef]
- Módis, L. Structure of the Corneal Stroma. In Organization of the Extracellular Matrix: A Polarization Microscopic Approach, 1st ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 124–130. [Google Scholar]
- Goldberg, M.F.; Ferguson, T.A.; Pepose, J.S. Detection of cellular adhesion molecules in inflamed human corneas. Ophthalmology 1994, 101, 161–168. [Google Scholar] [CrossRef]
- Nemeth, G.; Felszeghy, S.; Kenyeres, A.; Szentmary, N.; Berta, A.; Süveges, I.; Módis, L. Cell adhesion molecules in stromal corneal dystrophies. Histol. Histopathol. 2008, 23, 945–952. [Google Scholar]
- McKay, T.B.; Schlötzer-Schrehardt, U.; Pal-Ghosh, S.; Stepp, M.A. Integrin: Basement membrane adhesion by corneal epithelial and endothelial cells. Exp. Eye Res. 2020, 198, 108–138. [Google Scholar] [CrossRef]
- Pouw, A.E.; Greiner, M.A.; Coussa, R.G.; Jiao, C.; Han, I.C.; Skeie, J.M.; Fingert, J.H.; Mullins, R.F.; Sohn, E.H. Cell–matrix interactions in the eye: From cornea to choroid. Cells 2021, 10, 687. [Google Scholar] [CrossRef]
- Mobaraki, M.; Abbasi, R.; Vandchali, S.O.; Ghaffari, M.; Moztarzadeh, F.; Mozafari, M. Corneal repair and regeneration: Current concepts and future directions. Front. Bioeng. Biotechnol. 2019, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Udalova, I.A.; Ruhmann, M.; Thomson, S.J.; Midwood, K.S. Expression and immune function of tenascin-C. Crit. Rev. Immunol. 2011, 31, 115–145. [Google Scholar] [CrossRef] [PubMed]
- Orend, G.; Chiquet-Ehrismann, R. Tenascin-C induced signaling in cancer. Cancer Lett. 2006, 244, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, S.; Reinhard, J.; Faissner, A. Immunomodulatory role of the extracellular matrix protein tenascin-C in neuroinflammation. Biochem. Soc. Trans. 2019, 47, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Chiquet-Ehrismann, R.; Tannheimer, M.; Koch, M.; Brunner, A.; Spring, J.; Martin, D.; Baumgartner, S.; Chiquet, M. Tenascin-C expression by fibroblasts is elevated in stressed collagen gels. J. Cell Biol. 1994, 127, 2093–2101. [Google Scholar] [CrossRef]
- Deák, F.; Piecha, D.; Bachrati, C.; Paulsson, M.; Kiss, I. Primary structure and expression of matrilin-2, the closest relative of cartilage matrix protein within the von Willebrand factor type A-like module superfamily. J. Biol. Chem. 1997, 272, 9268–9274. [Google Scholar] [CrossRef] [Green Version]
- Wagener, R.; Ehlen, H.W.; Ko, Y.P.; Kobbe, B.; Mann, H.H.; Sengle, G.; Paulsson, M. The matrilins–adaptor proteins in the extracellular matrix. FEBS Lett. 2005, 579, 3323–3329. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.E.; Torricelli, A.A.; Marino, G.K. Corneal epithelial basement membrane: Structure, function and regeneration. Exp. Eye Res. 2020, 194, 108002. [Google Scholar] [CrossRef]
- Kiani, C.; Liwen, C.; Wu, Y.J.; Albert, J.Y.; Burton, B.Y. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Ghatak, S.; Maytin, E.V.; Mack, J.A.; Hascall, V.C.; Atanelishvili, I.; Rodriguez, R.M.; Markwald, R.R.; Misra, S. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int. J. Cell Biol. 2015, 2015, 834893. [Google Scholar] [CrossRef] [PubMed]
- Bencze, J.; Szarka, M.; Bencs, V.; Szabó, R.N.; Módis, L.V.; Aarsland, D.; Hortobágyi, T. Lemur tyrosine kinase 2 (LMTK2) level inversely correlates with phospho-tau in neuropathological stages of Alzheimer’s disease. Brain Sci. 2020, 10, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csonka, T.; Murnyák, B.; Szepesi, R.; Bencze, J.; Bognár, L.; Klekner, Á.; Hortobágyi, T. Assessment of Candidate Immunohistochemical Prognostic Markers of Meningioma Recurrence. Folia Neuropathol. 2016, 54, 114–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremble, P.; Chiquet-Ehrismann, R.; Werb, Z. The extracellular matrix ligands fibronectin and tenascin collaborate in regulating collagenase gene expression in fibroblasts. Mol. Biol. Cell. 1994, 5, 439–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljubimov, A.V.; Saghizadeh, M.; Spirin, K.S.; Khin, H.L.; Lewin, S.L.; Zardi, L.; Bourdon, M.A.; Kenney, M.C. Expression of tenascin-C splice variants in normal and bullous keratopathy human corneas. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1135–1142. [Google Scholar]
- Kenney, M.C.; Nesburn, A.B.; Burgeson, R.E.; Butkowski, R.J.; Ljubimov, A.V. Abnormalities of the extracellular matrix in keratoconus corneas. Cornea. 1997, 16, 345–351. [Google Scholar] [CrossRef]
- Maseruka, H.; Bonshek, R.E.; Tullo, A.B. Tenascin-C expression in normal, inflamed, and scarred human corneas. Br. J. Ophthalmol. 1997, 81, 677–682. [Google Scholar] [CrossRef] [Green Version]
- Sumioka, T.; Kitano, A.; Flanders, K.C.; Okada, Y.; Yamanaka, O.; Fujita, N.; Iwanishi, H.; Kao, W.W.; Saika, S. Impaired cornea wound healing in a tenascin-C deficient mouse model. Lab. Investig. 2013, 93, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Szalai, E.; Felszeghy, S.; Hegyi, Z.; Módis, L., Jr.; Berta, A.; Kaarniranta, K. Fibrillin-2, tenascin-C, matrilin-2, and matrilin-4 are strongly expressed in the epithelium of human granular and lattice type I corneal dystrophies. Mol. Vis. 2012, 18, 1927–1936. [Google Scholar]
- Akhtar, S.; Bron, A.J.; Hawksworth, N.R.; Bonshek, R.E.; Meek, K.M. Ultrastructural morphology and expression of proteoglycans, big-H3, tenascin-C, fibrillin-1, and fibronectin in bullous keratopathy. Br. J. Ophthalmol. 2001, 85, 720–731. [Google Scholar] [CrossRef] [Green Version]
- Korpos, É.; Deák, F.; Kiss, I. Matrilin-2, an extracellular adaptor protein, is needed for the regeneration of muscle, nerve and other tissues. Neural Regen. Res. 2015, 10, 866–869. [Google Scholar] [PubMed]
- Malin, D.; Sonnenberg-Riethmacher, E.; Guseva, D.; Wagener, R.; Aszódi, A.; Irintchev, A.; Riethmacher, D. The extracellular-matrix protein matrilin 2 participates in peripheral nerve regeneration. J. Cell Sci. 2009, 122, 995–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichikawa, T.; Suenaga, Y.; Koda, T.; Ozaki, T.; Nakagawara, A. ΔNp63/BMP-7-dependent expression of matrilin-2 is involved in keratinocyte migration in response to wounding. Biochem. Biophys. Res. Commun. 2008, 369, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Watson, M.A.; Lyman, M.; Perry, A.; Aldape, K.D.; Deák, F.; Gutmann, D.H. Matrilin-2 expression distinguishes clinically relevant subsets of pilocytic astrocytoma. Neurology 2006, 66, 127–130. [Google Scholar] [CrossRef]
- Torricelli, A.A.; Singh, V.; Santhiago, M.R.; Wilson, S.E. The corneal epithelial basement membrane: Structure, function, and disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6390–6400. [Google Scholar] [CrossRef]
- Jurkunas, U.V.; Bitar, M.; Rawe, I. Colocalization of increased transforming growth factor-β-induced protein (TGFBIp) and clusterin in Fuchs endothelial corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1129–1136. [Google Scholar] [CrossRef] [Green Version]
- Rada, J.A.; Thoft, R.A.; Hassell, J.R. Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Dev. Biol. 1991, 147, 303–312. [Google Scholar] [CrossRef]
- Bouhenni, R.; Hart, M.; Al-Jastaneiah, S.; AlKatan, H.; Edward, D.P. Immunohistochemical expression and distribution of proteoglycans and collagens in sclerocornea. Int. Ophthalmol. 2013, 33, 691–700. [Google Scholar] [CrossRef]
- Wilson, S.E. Corneal wound healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef]
- Kenney, M.C.; Chwa, M.; Alba, A.; Saghizadeh, M.; Huang, Z.S.; Brown, D.J. Localization of TIMP-1, TIMP-2, TIMP-3, gelatinase A and gelatinase B in pathological human corneas. Curr. Eye Res. 1998, 17, 238–246. [Google Scholar] [CrossRef]
- Nishida, T.; Inui, M.; Nomizu, M. Peptide therapies for ocular surface disturbances based on fibronectin–integrin interactions. Prog. Retin. Eye Res. 2015, 47, 38–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Wang, T.; Wang, Y.; Meng, F.; Ying, M.; Han, R.; Hao, P.; Wang, L.; Li, X. Blockade of extracellular high-mobility group box 1 attenuates inflammation-mediated damage and haze grade in mice with corneal wounds. Int. Immunopharmacol. 2020, 83, 106468. [Google Scholar] [CrossRef] [PubMed]
- Dhaouadi, S.; Benabderrazek, R.; Erne, W.; Loustau, T.; Fayçal, C.A.; Ksouri, A.; Murdamoothoo, D.; Mörgelin, M.; Kungl, A.J.; Jung, A. Novel human tenascin-C function-blocking camel single domain nanobodies. Front. Immunol. 2021, 12, 635166. [Google Scholar] [CrossRef]
- Gupta, S.; Fink, M.K.; Ghosh, A.; Tripathi, R.; Sinha, P.R.; Sharma, A.; Hesemann, N.P.; Chaurasia, S.S.; Giuliano, E.A.; Mohan, R.R. Novel combination BMP7 and HGF gene therapy instigates selective myofibroblast apoptosis and reduces corneal haze in vivo. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1045–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poleni, P.E.; Bianchi, A.; Etienne, S.; Koufany, M.; Sebillaud, S.; Netter, P.; Terlain, B.; Jouzeau, J.Y. Agonists of peroxisome proliferators-activated receptors (PPAR) α, β/δ or γ reduce transforming growth factor (TGF)-β-induced proteoglycans’ production in chondrocytes. Osteoarthr. Cartil. 2007, 15, 493–505. [Google Scholar] [CrossRef]








| Patient | Age | Sex | Clinical Findings | Systemic Disease |
|---|---|---|---|---|
| 1 | 84 | female | stromal decompensation, AC IOL | IDDM |
| 2 | 67 | female | stromal decompensation, PC IOL, glaucoma | NIDDM |
| 3 | 75 | female | stromal decompensation, glaucoma | - |
| 4 | 79 | female | stromal decompensation, aphakia | ischaemic heart disease, hypertension |
| 5 | 84 | female | stromal opacity, AC IOL | NIDDM |
| 6 | 71 | female | stromal decompensation, PC IOL, myopia | - |
| 7 | 74 | female | stromal decompensation, aphakia | thrombocythaemia |
| 8 | 73 | female | stromal decompensation, PC IOL | hypertension |
| 9 | 68 | female | stromal decompensation, PC IOL, glaucoma, macular degeneration | Sjögren syndrome |
| 10 | 66 | female | stromal decompensation, aphakia, glaucoma | hypertension |
| 11 | 77 | male | stromal decompensation, PC IOL, macular degeneration | - |
| 12 | 64 | female | stromal opacity | - |
| 13 | 79 | female | stromal decompensation, glaucoma, PC IOL | - |
| 14 | 63 | male | stromal decompensation, PC IOL | RA, cardiomyopathy, hypertension |
| 15 | 82 | female | stromal decompensation, PC IOL, glaucoma | hypertension |
| 16 | 67 | male | stromal opacity, PC IOL, glaucoma | - |
| 17 | 68 | female | stromal decompensation, PC IOL | - |
| 18 | 70 | male | stromal decompensation. AC IOL, glaucoma | - |
| 19 | 68 | male | stromal decompensation, PC IOL, myopia | - |
| 20 | 55 | male | stromal opacity, PC IOL, glaucoma | - |
| Patient | Age | Sex | Clinical Findings | Systemic Disease |
|---|---|---|---|---|
| 1 | 70 | female | stromal opacity, shallow AC | RA |
| 2 | 70 | female | stromal opacity, glaucoma | - |
| 3 | 70 | female | stromal edema, PC IOL, glaucoma | NIDDM, hypertension |
| 4 | 66 | female | stromal edema, glaucoma, PC IOL | - |
| 5 | 80 | female | stromal opacity, PC IOL, amblyopia | NIDDM |
| 6 | 62 | female | stromal opacity, glaucoma, amblyopia | RA, hypertension |
| 7 | 84 | female | stromal edema, cataract | hypertension |
| 8 | 44 | female | stromal edema, shallow AC | - |
| 9 | 70 | male | stomal opacity, PC IOL, glaucoma | hypertension |
| PBK | FECD | Control | |
|---|---|---|---|
| Epithelium | 2.00 * ±0.79 | 1.67 ±0.58 | 1.15 ±0.63 |
| Bowman’s membrane | 0.80 ±0.77 | 0.88 ±0.83 | 0.22 ±0.36 |
| Anterior stroma | 1.50 **** ±0.51 | 0.88 * ±0.35 | 0.30 ±0.42 |
| Middle stroma | 1.40 **** ±0.60 | 1.00 ** ±0.00 | 0.25 ±0.42 |
| Posterior stroma | 1.85 **** ±0.37 | 1.67 *** ±0.50 | 0.35 ±0.47 |
| Descemet’s membrane | 0.15 ±0.37 | 0.00 | 0.15 ±0.34 |
| Endothelium | 1.06 ±0.46 | 1.11 ±0.60 | 0.70 ±0.63 |
| PBK vs. Control | FECD vs. Control | PBK vs. FECD | |
|---|---|---|---|
| Epithelium | 0.0105 | 0.3706 | 0.6945 |
| Bowman’s membrane | 0.0579 | 0.0883 | 0.9685 |
| Anterior stroma | <0.0001 | 0.0109 | 0.0162 |
| Middle stroma | <0.0001 | 0.0013 | 0.0622 |
| Posterior stroma | <0.0001 | 0.0004 | 0.3391 |
| Descemet’s membrane | 0.9999 | 0.4771 | 0.5360 |
| Endothelium | 0.0713 | 0.1500 | 0.8661 |
| PBK | FECD | Control | |
|---|---|---|---|
| Epithelium | 2.29 ** ±0.61 | 1.00 ±1.00 | 1.30 ±0.50 |
| Bowman’s membrane | 0.30 ±0.57 | 0.78 ±0.83 | 0.20 ±0.40 |
| Anterior stroma | 0.10 ±0.31 | 0.56 * ±0.73 | 0.00 |
| Middle stroma | 0.00 | 0.33 ±0.50 | 0.00 |
| Posterior stroma | 0.30 ±0.47 | 0.56 ** ±0.53 | 0.00 |
| Descemet’s membrane | 0.05 ±0.22 | 0.56 * ±0.73 | 0.00 |
| Endothelium | 0.71 *** ±0.69 | 1.29 ±0.49 | 1.70 ±0.50 |
| PBK vs. Control | FECD vs. Control | PBK vs. FECD | |
|---|---|---|---|
| Epithelium | 0.0012 | 0.4415 | 0.0021 |
| Bowman’s membrane | 0.7336 | 0.0975 | 0.1333 |
| Anterior stroma | 0.5269 | 0.0260 | 0.0391 |
| Middle stroma | 0.9999 | 0.0737 | 0.0230 |
| Posterior stroma | 0.0658 | 0.0081 | 0.2371 |
| Descemet’s membrane | 0.9999 | 0.0260 | 0.0180 |
| Endothelium | 0.0009 | 0.3348 | 0.0340 |
| PBK | FECD | Control | |
|---|---|---|---|
| Epithelium | 1.47 ±0.62 | 1.33 ±0.58 | 1.3 ±0.82 |
| Bowman’s membrane | 1.25 ±1.02 | 1.66 ±1.22 | 1.00 ±0.94 |
| Anterior stroma | 0.55 * ±0.76 | 0.44 ±0.73 | 0.00 |
| Middle stroma | 0.8 ±0.95 | 0.55 ±0.88 | 0.00 |
| Posterior stroma | 1.2 * ±1.32 | 1.55 ** ±1.33 | 0.00 |
| Descemet’s membrane | 0.00 | 0.00 | 0.00 |
| Endothelium | 1.14 ±0.36 | 1.00 ±0.00 | 0.88 ±0.33 |
| PBK vs. Control | FECD vs. Control | PBK vs. FECD | |
|---|---|---|---|
| Epithelium | 0.7146 | 0.9999 | 0.9421 |
| Bowman’s membrane | 0.5963 | 0.2199 | 0.3767 |
| Anterior stroma | 0.0296 | 0.0867 | 0.6397 |
| Middle stroma | 0.0637 | 0.2105 | 0.6749 |
| Posterior stroma | 0.0113 | 0.0031 | 0.5033 |
| Descemet’s membrane | - | - | - |
| Endothelium | 0.2490 | 0.9999 | 0.5579 |
| PBK vs. Control | FECD vs. Control | PBK vs. FECD | |
|---|---|---|---|
| tenascinC | <0.0001 | <0.0001 | 0.0491 |
| matrilin-2 | 0.7426 | 0.0196 | 0.0028 |
| mggrecan | 0.0024 | 0.0324 | 0.7530 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varkoly, G.; Hortobágyi, T.G.; Gebri, E.; Bencze, J.; Hortobágyi, T.; Módis, L., Jr. Expression Pattern of Tenascin-C, Matrilin-2, and Aggrecan in Diseases Affecting the Corneal Endothelium. J. Clin. Med. 2022, 11, 5991. https://doi.org/10.3390/jcm11205991
Varkoly G, Hortobágyi TG, Gebri E, Bencze J, Hortobágyi T, Módis L Jr. Expression Pattern of Tenascin-C, Matrilin-2, and Aggrecan in Diseases Affecting the Corneal Endothelium. Journal of Clinical Medicine. 2022; 11(20):5991. https://doi.org/10.3390/jcm11205991
Chicago/Turabian StyleVarkoly, Gréta, Tibor G. Hortobágyi, Enikő Gebri, János Bencze, Tibor Hortobágyi, and László Módis, Jr. 2022. "Expression Pattern of Tenascin-C, Matrilin-2, and Aggrecan in Diseases Affecting the Corneal Endothelium" Journal of Clinical Medicine 11, no. 20: 5991. https://doi.org/10.3390/jcm11205991
APA StyleVarkoly, G., Hortobágyi, T. G., Gebri, E., Bencze, J., Hortobágyi, T., & Módis, L., Jr. (2022). Expression Pattern of Tenascin-C, Matrilin-2, and Aggrecan in Diseases Affecting the Corneal Endothelium. Journal of Clinical Medicine, 11(20), 5991. https://doi.org/10.3390/jcm11205991







