A Machine Learning Model to Predict Cardiovascular Events during Exercise Evaluation in Patients with Coronary Heart Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Characteristics
2.2. Cardiopulmonary Exercise Testing
2.3. Statistical Methods
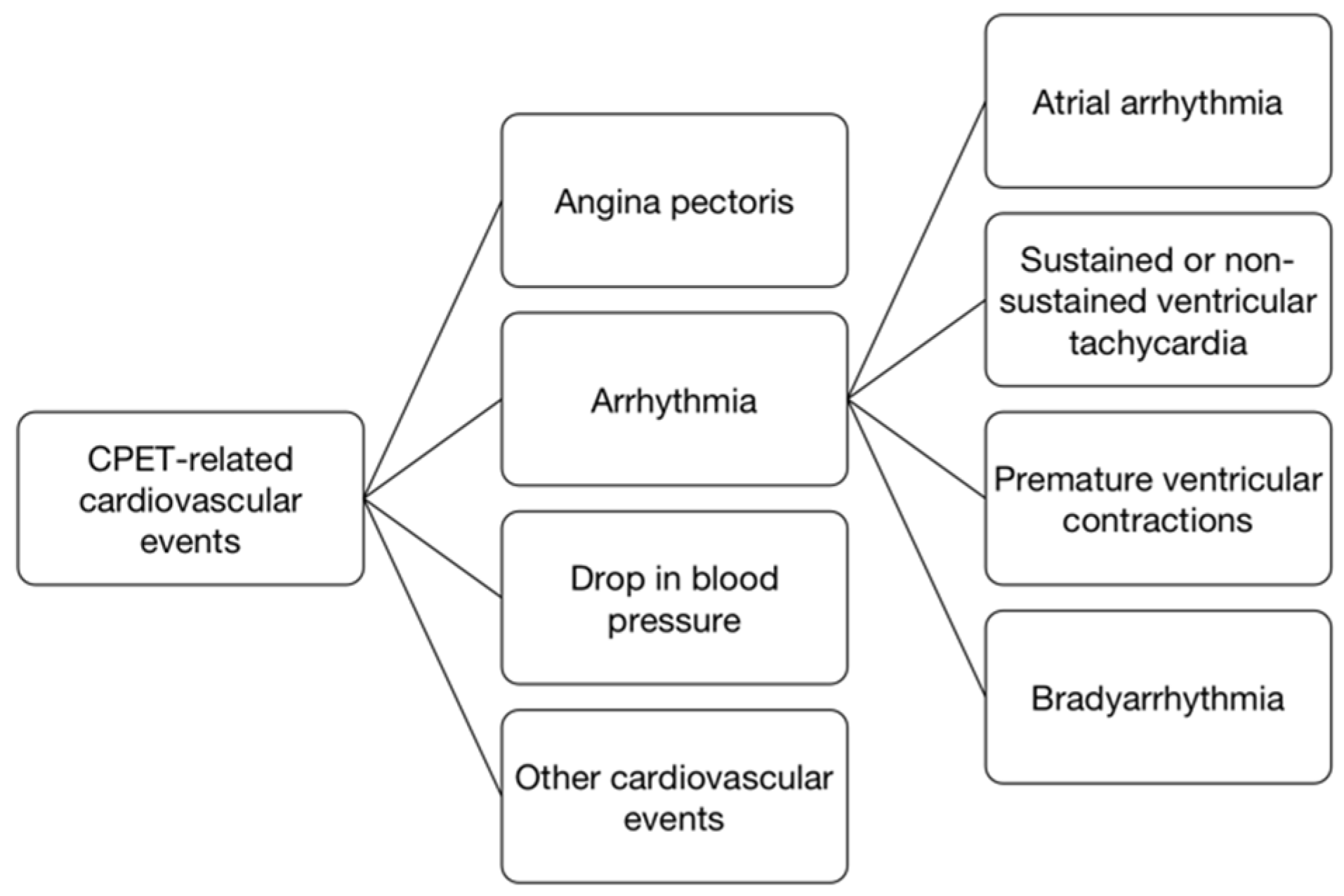
3. Results
3.1. General Information of Patients
3.2. Development of Prediction Models for Cardiovascular Events during CPET in CHD Patients
4. Discussion
4.1. Advantages of Machine Learning Methodology Based on Feature Selection
4.2. Important Predictive Value of Multi-Dimensional Clinical Information Based on CPET
4.3. Application of the Prediction Model
4.4. Research Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Varghese, T.; Schultz, W.M.; McCue, A.A.; Lambert, C.T.; Sandesara, P.B.; Eapen, D.J.; Gordon, N.F.; A Franklin, B.; Sperling, L.S. Physical activity in the prevention of coronary heart disease: Implications for the clinician. Heart 2016, 102, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 Focused Update: Clinical Recommendations for Cardiopulmonary Exercise Testing Data Evaluation in Specific Patient Populations. Circulation 2016, 133, e694–e711. [Google Scholar] [CrossRef] [Green Version]
- Saito, M.; Ueshima, K.; Saito, M.; Iwasaka, T.; Daida, H.; Kohzuki, M.; Makita, S.; Adachi, H.; Yokoi, H.; Omiya, K.; et al. Safety of exercise-based cardiac rehabilitation and exercise testing for cardiac patients in Japan: A nationwide survey. Circ. J. 2014, 78, 1646–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skalski, J.; Allison, T.G.; Miller, T.D. The safety of cardiopulmonary exercise testing in a population with high-risk cardiovascular diseases. Circulation 2012, 126, 2465–2472. [Google Scholar] [CrossRef] [Green Version]
- Hermes, I.L.; Marianna, G.S.; Jessica, R.C.; Carlos, B.-R.; Rafael, C.-D.; Dolores, R.-S.M.; Pedro, I. Development and validation of a risk calculator predicting exercise-induced ventricular arrhythmia in patients with cardiovascular disease. Int. J. Cardiol. 2016, 220, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Fletcher, G.F.; Balady, G.J.; Amsterdam, E.A.; Chaitman, B.; Eckel, R.; Fleg, J.; Froelicher, V.F.; Leon, A.S.; Piña, I.L.; Rodney, R.; et al. Exercise standards for testing and training: A statement for healthcare professionals from the American Heart Association. Circulation 2001, 104, 1694–1740. [Google Scholar] [CrossRef] [Green Version]
- Goodacre, S.; Wilson, R.; Shephard, N.; Nicholl, J. Derivation and validation of a risk adjustment model for predicting seven day mortality in emergency medical admissions: Mixed prospective and retrospective cohort study. BMJ 2012, 1, e2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, N.; Li, M.; He, L.; Xie, B.; Wang, L.; Zhang, R.; Yu, Y.; Sun, X.; Pan, Z.; Wang, K. Predicting 30-days mortality for MIMIC-III patients with sepsis-3: A machine learning approach using XGboost. J. Transl. Med. 2020, 18, 462. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.T.; Salinas, J. Machine Learning for Predicting Outcomes in Trauma. Shock 2017, 48, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Moore, C.L.; Cheung, K.H.; Brandt, C. Predicting urinary tract infections in the emergency department with machine learning. PLoS ONE 2018, 13, e0194085. [Google Scholar] [CrossRef]
- Xiao, J.; Ding, R.; Xu, X.; Guan, H.; Feng, X.; Sun, T.; Zhu, S.; Ye, Z. Comparison and development of machine learning tools in the prediction of chronic kidney disease progression. J. Transl. Med. 2019, 17, 119. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gribskov, M. IRESpy: An XGBoost model for prediction of internal ribosome entry sites. BMC Bioinform. 2019, 20, 409. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Patra, A.; Khasawneh, H.; Korfiatis, P.; Rajamohan, N.; Suman, G.; Majumder, S.; Panda, A.; Johnson, M.P.; Larson, N.B.; et al. Radiomics-Based Machine-Learning Models Can Detect Pancreatic Cancer on Prediagnostic CTs at a Substantial Lead Time Prior to Clinical Diagnosis. Gastroenterology 2022, 1, S0016-5085(22)00728-4. [Google Scholar] [CrossRef]
- Franklin, B.A.; Thompson, P.D.; Al-Zaiti, S.S.; Albert, C.M.; Hivert, M.-F.; Levine, B.D.; Lobelo, F.; Madan, K.; Sharrief, A.Z.; Eijsvogels, T.M.; et al. Exercise-Related Acute Cardiovascular Events and Potential Deleterious Adaptations Following Long-Term Exercise Training: Placing the Risks into Perspective-An Update: A Scientific Statement from the American Heart Association. Circulation 2020, 141, e705–e736. [Google Scholar] [CrossRef]
- Keytsman, C.; Dendale, P.; Hansen, D. Chronotropic Incompetence during Exercise in Type 2 Diabetes: Aetiology, Evaluation Methodology, Prognostic Impact and Therapy. Sports Med. 2015, 45, 985–995. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Ramos, R.P.; Alencar, M.C.; Treptow, E.; Arbex, F.; Ferreira, E.M.V.; Neder, J.A. Clinical usefulness of response profiles to rapidly incremental cardiopulmonary exercise testing. Pulm. Med. 2013, 2013, 359021. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Bandera, F.; Ozemek, C.; Systrom, D.; Arena, R. Cardiopulmonary Exercise Testing: What Is its Value? J. Am. Coll. Cardiol. 2017, 70, 1618–1636. [Google Scholar] [CrossRef]
- Wagner, J.; Agostoni, P.; Arena, R.; Belardinelli, R.; Dumitrescu, D.; Hager, A.; Myers, J.; Rauramaa, R.; Riley, M.; Takken, T.; et al. The Role of Gas Exchange Variables in Cardiopulmonary Exercise Testing for Risk Stratification and Management of Heart Failure with Reduced Ejection Fraction. Am. Heart J. 2018, 202, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Ren, C.; Zhao, W.; Tao, L.; Xu, S.; Zhang, C.; Gao, W. Development and Validation of a Prediction Model for Cardiovascular Events in Exercise Evaluation of Coronary Heart Disease Patients After Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 2022, 26, 798446. [Google Scholar] [CrossRef]



| Parameters(%), Mean ± SD | Value |
|---|---|
| Age (years) | 57.5 ± 12.9 |
| Male, N (%) | 11,647 (69.9) |
| Baseline BMI (kg/m2) | 25.5 ± 3.4 |
| Hypertension, N (%) | 8786 (52.7) |
| Hyperlipidemia, N (%) | 8637 (51.8) |
| Diabetes, N (%) | 3999 (24.0) |
| Smoking history, N (%) | 7071 (42.1) |
| Family history of CHD, N (%) | 4562 (27.4) |
| Exercise habit, N (%) | 10,454 (62.8) |
| Parameters | Value |
|---|---|
| VO2peak (mL·kg−1·min−1) | 21.4 ± 6.3 |
| VO2peak/Pred (%) | 69.8 ± 10.4 |
| RERpeak | 1.12 ± 0.12 |
| HRpeak (bpm) | 136 ± 23 |
| O2-pulse peak (mL/beat) | 11.4 ± 3.2 |
| SBPpeak (mmHg) | 171 ± 21 |
| VO2@AT (mL·kg−1·min−1) | 15.9 ± 5.8 |
| HR@AT (bpm) | 109 ± 15 |
| O2-pulse@AT (mL/beat) | 8.4 ± 2.6 |
| Resting HR | 73.9 ± 15.4 |
| Resting SBP (mmHg) | 127 ± 19 |
| VE/VCO2slope | 28.7 ± 4.2 |
| Feature Name | Total Score | |
|---|---|---|
| 1 | Age | 730 |
| 2 | Duration of diabetes | 662 |
| 3 | Diabetes history | 662 |
| 4 | Myocardial infarction history | 658 |
| 5 | Male | 642 |
| 6 | VE/VCO2slope | 630 |
| 7 | Smoking history | 616 |
| 9 | Hyperlipidemia history | 552 |
| 8 | VO2@AT | 564 |
| 10 | Hypertension history | 528 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, T.; Liu, D.; Lin, Z.; Ren, C.; Zhao, W.; Gao, W. A Machine Learning Model to Predict Cardiovascular Events during Exercise Evaluation in Patients with Coronary Heart Disease. J. Clin. Med. 2022, 11, 6061. https://doi.org/10.3390/jcm11206061
Shen T, Liu D, Lin Z, Ren C, Zhao W, Gao W. A Machine Learning Model to Predict Cardiovascular Events during Exercise Evaluation in Patients with Coronary Heart Disease. Journal of Clinical Medicine. 2022; 11(20):6061. https://doi.org/10.3390/jcm11206061
Chicago/Turabian StyleShen, Tao, Dan Liu, Zi Lin, Chuan Ren, Wei Zhao, and Wei Gao. 2022. "A Machine Learning Model to Predict Cardiovascular Events during Exercise Evaluation in Patients with Coronary Heart Disease" Journal of Clinical Medicine 11, no. 20: 6061. https://doi.org/10.3390/jcm11206061





