Is Less Always More? A Prospective Two-Centre Study Addressing Clinical Outcomes in Leadless versus Transvenous Single-Chamber Pacemaker Recipients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
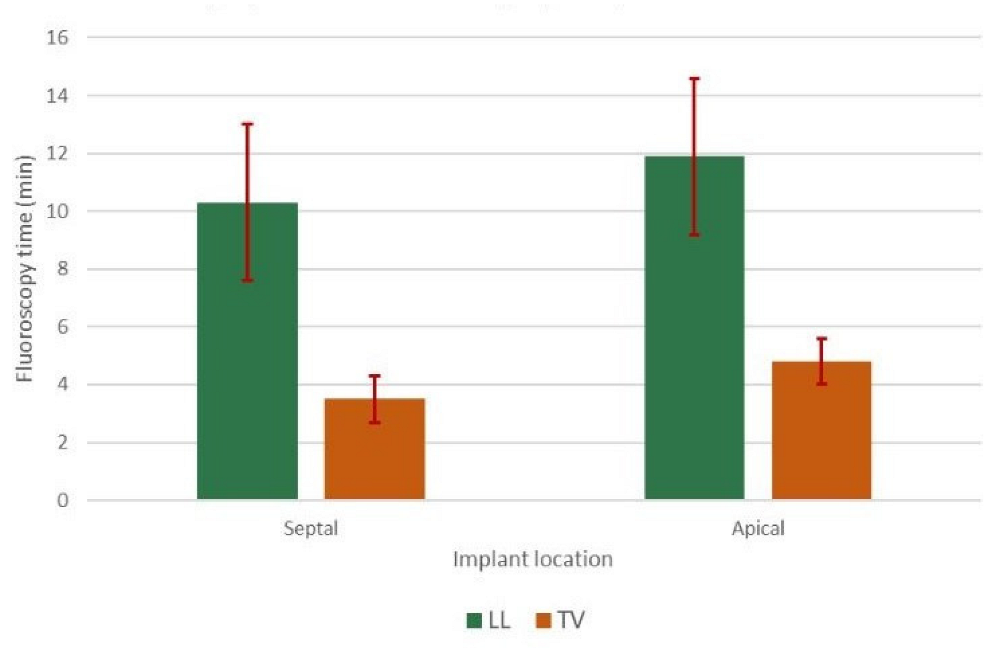
3.2. Procedural Data
3.3. Data at Follow-Up
3.4. Complication Analysis
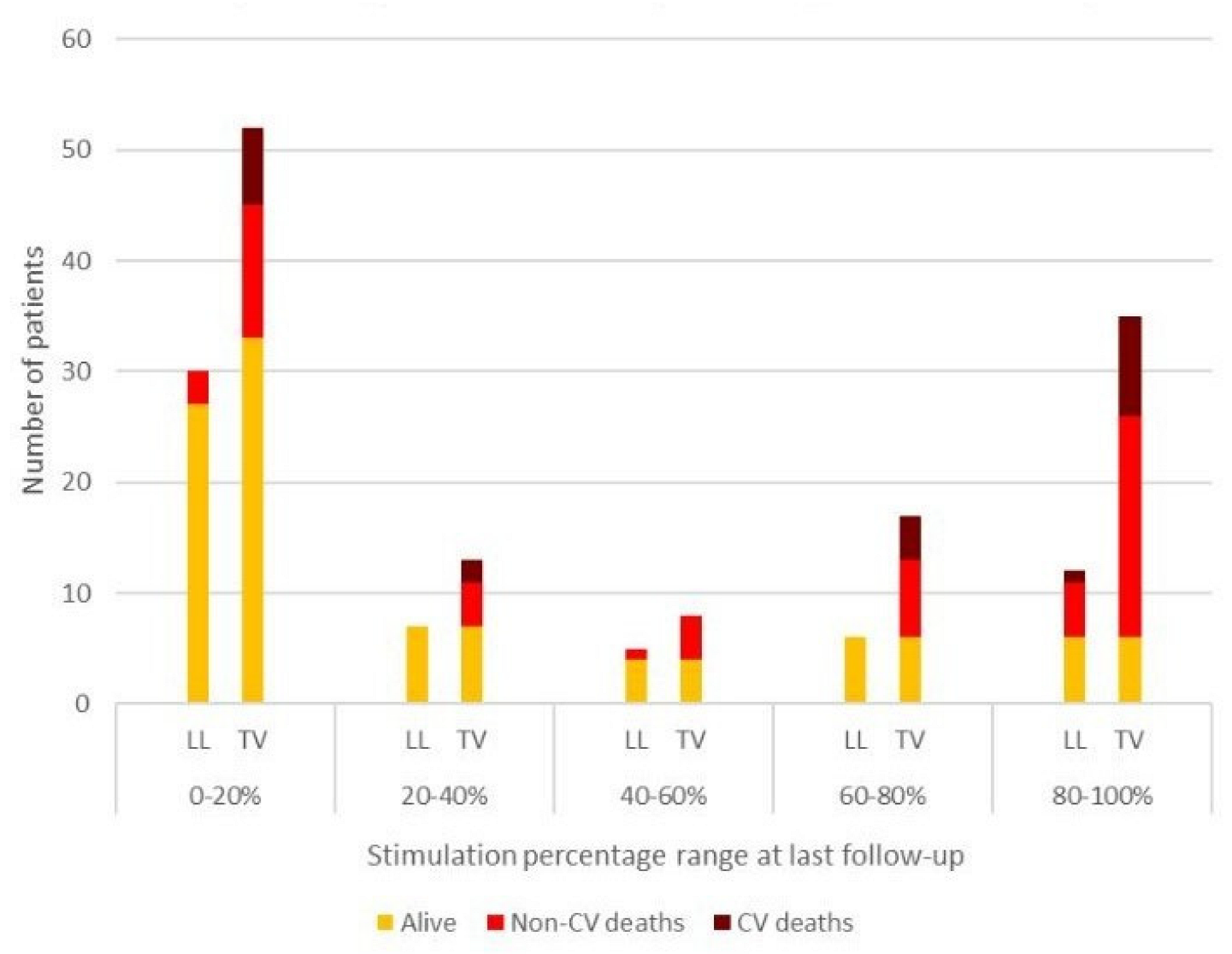
3.5. Mortality and Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Proclemer, A.; Zecchin, M.; D’Onofrio, A.; Boriani, G.; Ricci, R.P.; Rebellato, L.; Ghidina, M.; Bianco, G.; Bernardelli, E.; Miconi, A.; et al. Registro Italiano Pacemaker e Defibrillatori—Bollettino Periodico 2018. Associazione Italiana di Aritmologia e Cardios-timolazione [The Pacemaker and Implantable Cardioverter-Defibrillator Registry of the Italian Association of Arrhythmology and Cardiac Pacing—2018 Annual report]. G. Ital. Cardiol. (Rome) 2020, 21, 157–169. [Google Scholar]
- Biffi, M.; Capobianco, C.; Spadotto, A.; Bartoli, L.; Sorrentino, S.; Minguzzi, A.; Piemontese, G.P.; Angeletti, A.; Toniolo, S.; Statuto, G. Pacing devices to treat bradycardia: Current status and future perspectives. Expert Rev. Med. Devices 2021, 18, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Krahn, A.D.; Longtin, Y.; Philippon, F.; Birnie, D.H.; Manlucu, J.; Angaran, P.; Rinne, C.; Coutu, B.; Low, R.A.; Essebag, V.; et al. Prevention of Arrhythmia Device Infection Trial: The PADIT Trial. J. Am. Coll. Cardiol. 2018, 72, 3098–3109. [Google Scholar] [CrossRef]
- Ritter, P.; Duray, G.Z.; Steinwender, C.; Soejima, K.; Omar, R.; Mont, L.; Boersma, L.V.; Knops, R.E.; Chinitz, L.; Zhang, S.; et al. Early performance of a miniaturized leadless cardiac pacemaker: The Micra Transcatheter Pacing Study. Eur. Heart J. 2015, 36, 2510–2519. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Duray, G.Z.; Omar, R.; Soejima, K.; Neuzil, P.; Zhang, S.; Narasimhan, C.; Steinwender, C.; Brugada, J.; Lloyd, M.; et al. A Leadless Intracardiac Transcatheter Pacing System. N. Engl. J. Med. 2016, 374, 533–541. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Al-Samadi, F.; Clementy, N.; Garweg, C.; Martinez-Sande, J.L.; Piccini, J.P.; Iacopino, S.; Lloyd, M.; Viñolas Prat, X.; Jacobsen, M.D.; et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: A comparison to the investigational study and a transvenous historical control. Heart Rhythm. 2018, 15, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sande, J.L.; Garcia-Seara, J.; Gonzalez-Melchor, L.; Rodriguez-Mañero, M.; Baluja, A.; Fernandez-Lopez, X.A.; Gonzalez Juanatey, J.R. Conventional single-chamber pacemakers versus transcatheter pacing systems in a «real world» cohort of patients: A comparative prospective single-center study. Indian Pacing Electrophysiol. J. 2021, 21, 89–94. [Google Scholar] [CrossRef]
- Burri, H.; Starck, C.; Auricchio, A.; Biffi, M.; Burri, M.; D’Avila, A.; Deharo, J.C.; Glikson, M.; Israel, C.; Lau, C.P.; et al. EHRA expert consensus statement and practical guide on optimal implantation technique for conventional pacemakers and implantable cardioverter-defibrillators: Endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin-American Heart Rhythm Society (LAHRS). Europace 2021, 23, 983–1008. [Google Scholar]
- Mascheroni, J.; Mont, L.; Stockburger, M.; Patwala, A.; Retzlaff, H.; Gallagher, A.G. A validation study of intraoperative performance metrics for training novice cardiac resynchronization therapy implanters. Int. J. Cardiol. 2020, 307, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.Y.; Saberwal, B.; Lambiase, P.D.; Koo, C.Y.; Lee, S.; Gopalamurugan, A.B.; Rogers, D.P.; Lowe, M.D.; Chow, A.W. A simple infection- control protocol to reduce serious cardiac device infections. Europace 2014, 16, 1482–1489. [Google Scholar] [CrossRef]
- Piccini, J.P.; El-Chami, M.; Wherry, K.; Crossley, G.H.; Kowal, R.C.; Stromberg, K.; Longacre, C.; Hinnenthal, J.; Bockstedt, L. Contemporaneous Comparison of Outcomes Among Patients Implanted with a Leadless vs. Transvenous Single-Chamber Ventricular Pacemaker. JAMA Cardiol. 2021, 6, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Bockstedt, L.; Longacre, C.; Higuera, L.; Stromberg, K.; Crossley, G.; Kowal, R.C.; Piccini, J.P. Leadless vs. transvenous single-chamber ventricular pacing in the Micra CED study: 2-year follow-up. Eur. Heart J. 2022, 43, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Burri, H. Leadless pacing: Is this the end of the road for transvenous pacemakers? Eur. Heart J. 2022, 43, 1216–1218. [Google Scholar] [CrossRef] [PubMed]
- Fortescue, E.B.; Berul, C.I.; Cecchin, F.; Walsh, E.P.; Triedman, J.K.; Alexander, M.E. Patient, procedural, and hardware factors associated with pacemaker lead failures in pediatrics and congenital heart disease. Heart Rhythm. 2004, 1, 150–159. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Clementy, N.; Garweg, C.; Omar, R.; Duray, G.Z.; Gornick, C.C.; Leyva, F.; Sagi, V.; Piccini, J.P.; Soejima, K.; et al. Leadless Pacemaker Implantation in Hemodialysis Patients: Experience with the Micra Transcatheter Pacemaker. JACC Clin. Electrophysiol. 2019, 5, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Darlington, D.; Brown, P.; Carvalho, V.; Bourne, H.; Mayer, J.; Jones, N.; Walker, V.; Siddiqui, S.; Patwala, A.; Kwok, C.S. Efficacy and safety of leadless pacemaker: A systematic review, pooled analysis and meta-analysis. Indian Pacing Electrophysiol. J. 2022, 22, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Kirkfeldt, R.E.; Johansen, J.B.; Nohr, E.A.; Jørgensen, O.D.; Nielsen, J.C. Complications after cardiac implantable electronic device implantations: An analysis of a complete, nationwide cohort in Denmark. Eur. Heart J. 2014, 35, 1186–1194. [Google Scholar] [CrossRef]
- Ngo, L.; Nour, D.; Denman, R.A.; Walters, T.E.; Haqqani, H.M.; Woodman, R.J.; Ranasinghe, I. Safety and Efficacy of Leadless Pacemakers: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e019212. [Google Scholar] [CrossRef]
- Biffi, M.; Bertini, M.; Mazzotti, A.; Gardini, B.; Mantovani, V.; Ziacchi, M.; Valzania, C.; Martignani, C.; Diemberger, I.; Boriani, G. Long-term RV threshold behavior by automated measurements: Safety is the standpoint of pacemaker longevity! J. Pacing Clin. Electrophysiol. 2011, 34, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.; Wallace, K.; Stromberg, K.; Piccini, J.P.; Roberts, P.R.; El-Chami, M.F.; Soejima, K.; Garweg, C.; Fagan, D.H.; Lloyd, M.S. A Predictive Model for the Long-Term Electrical Performance of a Leadless Transcatheter Pacemaker. JACC Clin. Electrophysiol. 2021, 7, 502–512. [Google Scholar] [CrossRef] [PubMed]

| Leadless (n = 72) |
Transvenous
(n = 272) |
Significance
(p < 0.05) | |
|---|---|---|---|
| Baseline characteristics | |||
| Age; median [SEM *] | 79.5 [2.5] | 85.0 [1.0] | p < 0.01 |
| Sex (female) | 26/72 (36%) | 111/272 (41%) | p = 0.47 |
| Diabetes mellitus | 19/72 (26.4%) | 49/272 (18.0%) | p < 0.01 |
| Hypertension | 51/72 (70.8%) | 212/272 (77.9%) | p = 0.21 |
| Ejection fraction; median [SEM] | 57 [3]% | 59 [2]% | p = 0.49 |
| Permanent atrial fibrillation | 58/72 (80.6%) | 262/272 (96.3%) | p < 0.01 |
| Ischaemic heart disease | 14/72 (19.4%) | 83/272 (30.5%) | p = 0.06 |
| Previous CIED extraction | 7/72 (9.7%) | 2/272 (0.7%) | p < 0.01 |
| Surgical or percutaneous treatment of valvular disease | 20/72 (27.8%) | 82/272 (30.1%) | p = 0.70 |
| Chronic kidney disease ** (GFR < 60 mL/min/1.73 m2) | 26/72 (36.1%) | 157/272 (57.7%) | p < 0.01 |
| Chronic haemodialysis | 5/72 (6.9%) | 2/272 (0.7%) | p < 0.01 |
| Bedridden/cognitive impairment | 0/72 (0%) | 3/272 (1.1%) | p = 0.37 |
| 30-day perioperative complications | |||
| Pericardial effusion | 0/72 (0%) | 4/272 (1.5%) | p = 0.30 |
| Tamponade | 0/72 (0%) | 1/272 (0.4%) | p = 0.61 |
| Lead/device dislocation | 1/72 (1.4%) | 0/272 (0%) | p = 0.06 |
| Pneumothorax [requiring drainage] | / | 6/272 (2.2%) [1/272 (0.4%)] | / |
| Haematoma [requiring surgical revision] | 3/72 (4.2%) [0/72 (0%)] | 3/272 (1.1%) [1/272 (0.4%)] | p = 0.08 |
| Overall | 4/72 (5.6%) | 14/272 (5.1%) | p = 0.33 |
|
Leadless
(n = 72) |
Transvenous
(n = 272) |
Significance
(p < 0.05) | |
|---|---|---|---|
| Electrical parameters after implantation | |||
| Mean capture threshold (V × 0.4 ms) | 0.75 ± 0.08 | 0.69 ± 0.04 | p = 0.79 |
| Mean impedance (Ohm) | 748 ± 28 | 698 ± 15 | p = 0.06 |
| Mean intrinsic R wave amplitude (mV) | 9.8 ± 0.6 | 10.8 ± 0.4 | p = 0.58 |
| Electrical parameters at follow-up | |||
| Mean capture threshold (V × 0.4 ms) | 0.62 ± 0.04 | 0.79 ± 0.03 | p = 0.005 |
| Mean capture threshold increase > 1 V | 2/72 (2.7%) | 9/262 (3.4%) | p = 0.12 |
| Mean impedance (Ohm) | 636 ± 18 | 606 ± 14 | p = 0.009 |
| Mean intrinsic R wave amplitude (mV) | 11.6 ± 0.5 | 12.0 ± 0.4 | p = 0.86 |
| Patient data at follow-up | |||
| Mean follow-up time (months) | 22.8 ± 2.6 | 23.7 ± 1.1 | p = 0.31 |
| Superficial Suture Infection [requiring surgical revision] | 0/72 (0%) | 3/272 (1.1%) [2/272 (0.8%)] | p = 0.37 |
| Haematoma | 0/72 (0%) | 1/272 (0.4%) | p = 0.61 |
| Skin sore | 0/72 (0%) | 1/272 (0.4%) | p = 0.61 |
| Overall long-term complications | 0/72 (0%) | 5/272 (1.9%) | p = 0.25 |
| Pacing system revisions [Repositioning] [Lead addition] | 0/72 (0%) | 6/272 (2.3%) [5/272 (1.9%)] [1/272 (0.4%)] | p = 0.20 |
| Multivariate Analysis of All-Cause Mortality in LL vs. TV | ||||
|---|---|---|---|---|
| Variable | Leadless (n = 72) | Transvenous (n = 272) | ||
| Hazard Ratio [95% CI] | p Value | Hazard Ratio [95% CI] | p Value | |
| Age | 1.019 [0.909–1.142] | 0.74 | 1.073 [1.026–1.122] | 0.002 |
| Female sex | 1.381 [0.227–8.394] | 0.73 | 1.507 [0.881–2.576] | 0.13 |
| Diabetes mellitus | 1.352 [0.1666–11.037] | 0.78 | 1.864 [1.020–3.406] | 0.05 |
| Chronic kidney disease * | 0.564 [0.078–4.069] | 0.57 | 1.774 [0.922–3.413] | 0.09 |
| Ischaemic heart disease | 2.109 [0.421–10.550] | 0.36 | 1.156 [0.666–2.005] | 0.61 |
| Left ventricular ejection fraction | 0.958 [0.878–1.045] | 0.33 | 1.000 [0.974–1.027] | 1.00 |
| Ventricular stimulation percentage | 5.856 [0.994–34.484] | 0.05 | 1.128 [0.655–1.943] | 0.66 |
| Multivariate Analysis of All-Cause Mortality in Both Cohorts | ||||
| Variable | Hazard Ratio [95% CI] | p Value | ||
| Leadless vs. transvenous | 0.929 [0.422–2.043] | 0.85 | ||
| Age | 1.071 [1.027–1.117] | 0.001 | ||
| Female sex | 1.473 [0.888–2.444] | 0.13 | ||
| Diabetes mellitus | 1.617 [0.911–2.869] | 0.10 | ||
| Chronic kidney disease * | 1.704 [0.953–3.047] | 0.07 | ||
| Ischaemic heart disease | 1.226 [0.741–2.031] | 0.43 | ||
| Left ventricular ejection fraction | 0.995 [0.971–1.019] | 0.67 | ||
| Percentage ventricular stimulation | 1.347 [0.805–2.253] | 0.26 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertelli, M.; Toniolo, S.; Ziacchi, M.; Gasperetti, A.; Schiavone, M.; Arosio, R.; Capobianco, C.; Mitacchione, G.; Statuto, G.; Angeletti, A.; et al. Is Less Always More? A Prospective Two-Centre Study Addressing Clinical Outcomes in Leadless versus Transvenous Single-Chamber Pacemaker Recipients. J. Clin. Med. 2022, 11, 6071. https://doi.org/10.3390/jcm11206071
Bertelli M, Toniolo S, Ziacchi M, Gasperetti A, Schiavone M, Arosio R, Capobianco C, Mitacchione G, Statuto G, Angeletti A, et al. Is Less Always More? A Prospective Two-Centre Study Addressing Clinical Outcomes in Leadless versus Transvenous Single-Chamber Pacemaker Recipients. Journal of Clinical Medicine. 2022; 11(20):6071. https://doi.org/10.3390/jcm11206071
Chicago/Turabian StyleBertelli, Michele, Sebastiano Toniolo, Matteo Ziacchi, Alessio Gasperetti, Marco Schiavone, Roberto Arosio, Claudio Capobianco, Gianfranco Mitacchione, Giovanni Statuto, Andrea Angeletti, and et al. 2022. "Is Less Always More? A Prospective Two-Centre Study Addressing Clinical Outcomes in Leadless versus Transvenous Single-Chamber Pacemaker Recipients" Journal of Clinical Medicine 11, no. 20: 6071. https://doi.org/10.3390/jcm11206071
APA StyleBertelli, M., Toniolo, S., Ziacchi, M., Gasperetti, A., Schiavone, M., Arosio, R., Capobianco, C., Mitacchione, G., Statuto, G., Angeletti, A., Martignani, C., Diemberger, I., Forleo, G. B., & Biffi, M. (2022). Is Less Always More? A Prospective Two-Centre Study Addressing Clinical Outcomes in Leadless versus Transvenous Single-Chamber Pacemaker Recipients. Journal of Clinical Medicine, 11(20), 6071. https://doi.org/10.3390/jcm11206071










