Management of Renal Angiomyolipomas in Tuberous Sclerosis Complex: A Case Report and Literature Review
Abstract
1. Introduction
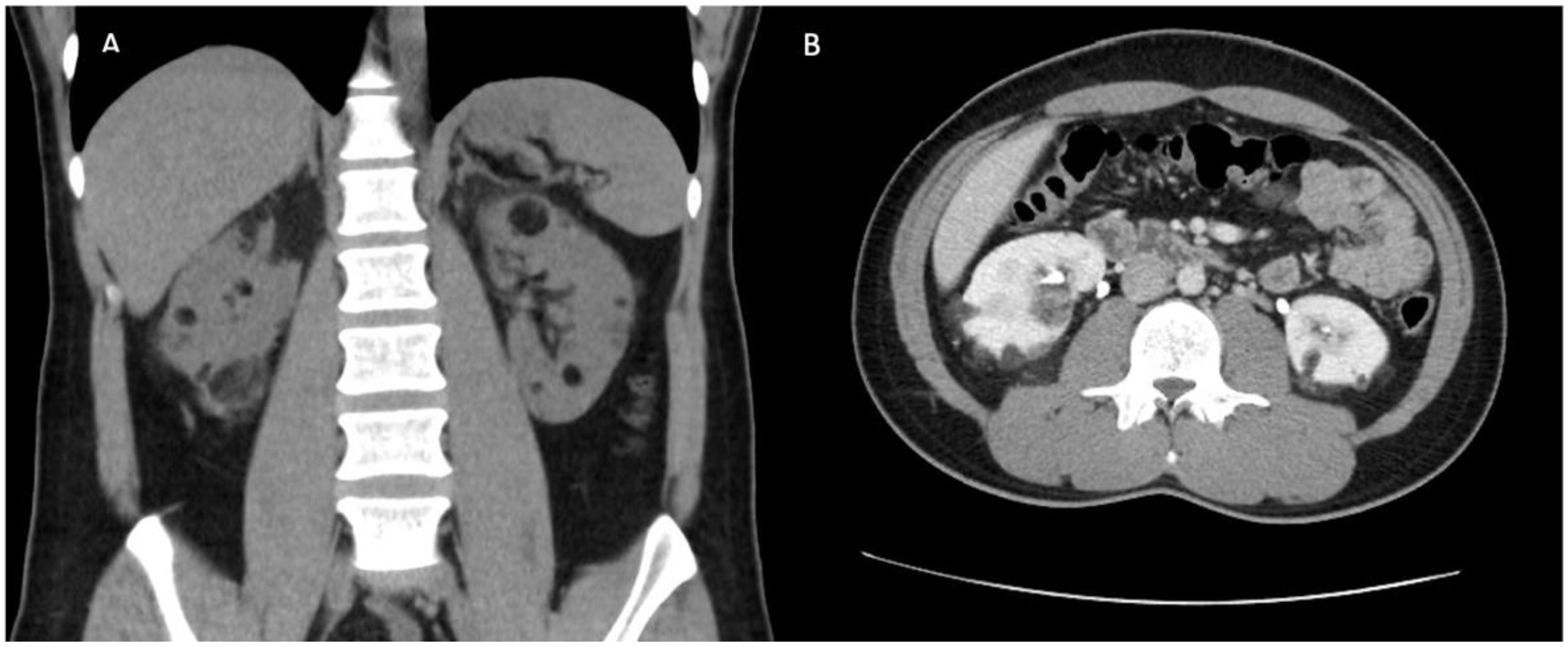
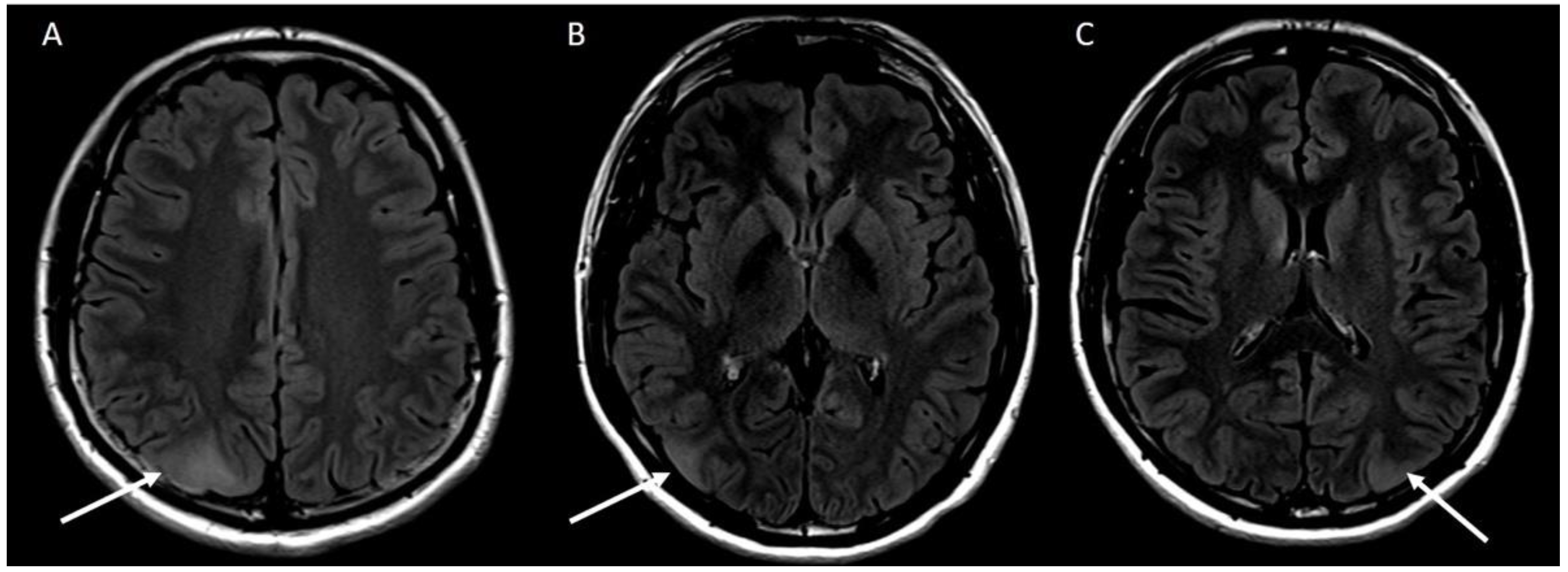
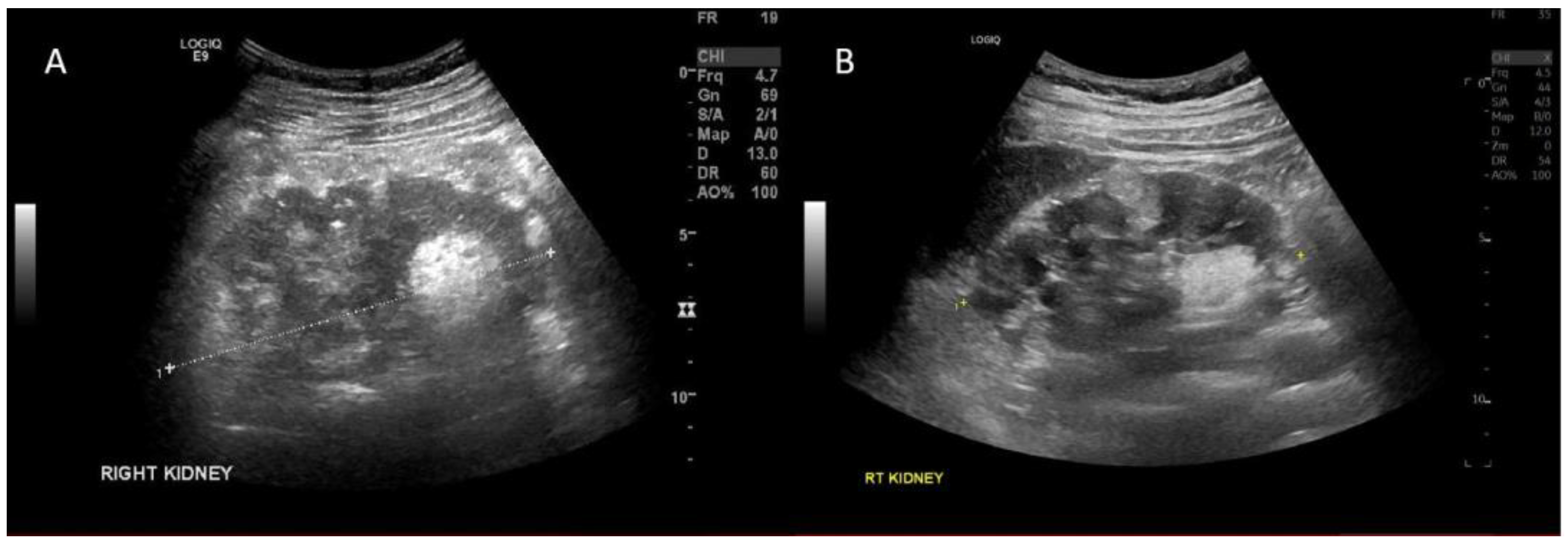
2. Case Report
3. Discussion and Literature Review
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Henske, E.P.; Jozwiak, S.; Kingswood, J.C.; Sampson, J.R.; Thiele, E.A. Tuberous sclerosis complex. Nat. Rev. Diease Prim. 2016, 2, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Borkowska, J.; Schwatz, R.; Kotulska, K.; Jozwiak, S. Tuberous sclerosis complex tumors and tumorigenesis. Int. J. Dermatol. 2011, 50, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Crino, P.; Nathanson, K.; Henske, E.P. The Tuberous Sclerosis Complex. N. Engl. J. Med. 2006, 355, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Bissler, J.J.; Kingswood, J.C.; Radzikowska, E.; Zonnenberg, B.A.; Frost, M.; Belousova, E.; Sauter, M.; Nonomura, N.; Brakemeier, S.; de Vries, P.J.; et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013, 381, 817–824. [Google Scholar] [CrossRef]
- Tyburczy, M.E.; Dies, K.A.; Glass, J.; Camposano, S.; Chekaluk, Y.; Thorner, A.R.; Lin, L.; Krueger, D.; Franz, D.N.; Thiele, E.A.; et al. Mosaic and Intronic Mutations in TSC1/TSC2 Explain the Majority of TSC Patients with No Mutation Identified by Conventional Testing. PLoS Genet. 2015, 11, e1005637. [Google Scholar] [CrossRef] [PubMed]
- Northrup, H.; Aronow, M.E.; Bebin, E.M.; Bissler, J.; Darling, T.N.; de Vries, P.J.; Frost, M.D.; Fuchs, Z.; Gosnell, E.S.; Gupta, N.; et al. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr. Neurol. 2021, 123, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Dillman, J.; Trout, A.; Smith, E.; Towbin, A.J. Hereditary Renal Cystic Disorders: Imaging of the Kidneys and Beyond. Pediatr. Imaging 2017, 37, 924–946. [Google Scholar] [CrossRef] [PubMed]
- Franz, D.N.; Belousova, E.; Sparagana, S.; Bebin, E.M.; Frost, M.; Kuperman, R.; Witt, O.; Kohrman, M.H.; Flamini, J.R.; Wu, J.Y.; et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013, 381, 125–132. [Google Scholar] [CrossRef]
- French, J.A.; Lawson, J.A.; Yapici, Z.; Ikeda, H.; Polster, T.; Nabbout, R.; Curatolo, P.; de Vries, P.J.; Dlugos, D.J.; Berkowitz, N.; et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): A phase 3, randomised, double-blind, placebo-controlled study. Lancet 2016, 388, 2153–2163. [Google Scholar] [CrossRef]
- Franz, D.N.; Belousova, E.; Sparagana, S.; Bebin, E.M.; Frost, M.D.; Kuperman, R.; Witt, O.; Kohrman, M.H.; Flamini, J.R.; Wu, J.Y.; et al. Long-Term Use of Everolimus in Patients with Tuberous Sclerosis Complex: Final Results from the EXIST-1 Study. PLoS ONE 2016, 11, e0158476. [Google Scholar] [CrossRef] [PubMed]
- Bissler, J.J.; Kingswood, J.C.; Radzikowska, E.; Zonnenberg, B.A.; Frost, M.; Belousova, E.; Sauter, M.; Nonomura, N.; Brakemeier, S.; de Vries, P.J.; et al. Everolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: Extension of a randomized controlled trial. Nephrol. Dial. Transpl. 2016, 31, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Guo, H.; Wang, W.; Li, H.; Sun, H.; Shi, B.; Zhang, Y. Assessing the outcomes of everolimus on renal angiomyolipoma associated with tuberous sclerosis complex in China: A two years trial. Orphanet J. Rare Dis. 2018, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Saxena, A.; Kingswood, J.C. Management of everolimus-associated adverse events in patients with tuberous sclerosis complex: A practical guide. Orphanet J. Rare Dis. 2017, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Overwater, I.E.; Rietman, A.B.; Van Eeghen, A.M.; De Wit, M.C.Y. Everolimus for the treatment of refractory seizures associated with tuberous sclerosis complex (TSC): Current perspectives. Ther. Clin. Risk Manag. 2019, 15, 951–955. [Google Scholar] [CrossRef] [PubMed]
- De Wit, D.; Schneider, T.C.; Moes, D.J.A.R.; Roozen, C.F.M.; Den Hartigh, J.; Gelderblom, H.; Guchelaar, H.J.; van der Hoeven, J.J.; Links, T.P.; Kapiteijn, E.; et al. Everolimus pharmacokinetics and its exposure-toxicity relationship in patients with thyroid cancer. Cancer Chemother. Pharmacol. 2016, 78, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, V.; Bajwa, S.J.; Bajaj, A. New orally active anticoagulants in critical care and anesthesia practice: The good, the bad and the ugly. Ann. Card. Anaesth. 2013, 16, 193–200. [Google Scholar] [CrossRef] [PubMed]
| Study | Patients | Age (Everolimus Group) | Randomisation | Inclusion Criteria | Primary Outcome | Adverse Events |
|---|---|---|---|---|---|---|
| EXIST-1 [8] (SEGA) | 117 | 1–24 Median 9.5 | 2:1 everolimus versus placebo, target trough concentration 5–15 ng/mL | At least one SEGA with diameter ≥1 cm on MRI that was enlarging, or new lesion or hydrocephalus | >50% reduction in total volume of all SEGAs Reached by 42% at 24 weeks, 3% in placebo | Mouth ulceration 32% Stomatitis 31% Convulsions 23% Pyrexia 22% |
| EXIST-2 [4] (AML) | 118 | 18–61 Median 32 | 2:1 everolimus 10 mg daily versus placebo | Age >18 with at least one AML 3 cm or larger in longest diameter Definite diagnosis of TSC or sporadic LAM Excluded if had had complication or intervention on AML in prior 6 months | ≥ 50% reduction in AML volume (sum of volume of all target AMLs identified at baseline) By week 24 Reached by 55% for everolimus, 0% for placebo | Stomatitis 48% Nasopharyngitis 24% Acne-like skin lesions 22% Headache 22% Cough 20% Hypercholesterolaemia 20% |
| EXIST-3 [9] (Seizures) | 366 | 2–56 Median 10.1 | 1:1:1 to: Low exposure (LE) target: 3–7 ng/mL High exposure (HE) target: 9–15 ng/mL Placebo | TSC and treatment resistant epilepsy 16 or more seizures in 8 week baseline phase, receiving 1–3 antiepileptic drugs | 25% reduction in seizure frequency 37% in placebo arm 52.1% in LE group (median 29.3% reduction seizure frequency) 70% in HE group (median 39.6% reduction) | LE: Stomatitis 55% (0% grade 3 or 4) Diarrhoea 17% HE: Stomatitis 64% (4% grade 3 or 4) Diarrhoea 22% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunter-Dickson, M.; Wu, P.; Athavale, A.; Wang, A.Y. Management of Renal Angiomyolipomas in Tuberous Sclerosis Complex: A Case Report and Literature Review. J. Clin. Med. 2022, 11, 6084. https://doi.org/10.3390/jcm11206084
Hunter-Dickson M, Wu P, Athavale A, Wang AY. Management of Renal Angiomyolipomas in Tuberous Sclerosis Complex: A Case Report and Literature Review. Journal of Clinical Medicine. 2022; 11(20):6084. https://doi.org/10.3390/jcm11206084
Chicago/Turabian StyleHunter-Dickson, Mitchell, Patrick Wu, Akshay Athavale, and Amanda Ying Wang. 2022. "Management of Renal Angiomyolipomas in Tuberous Sclerosis Complex: A Case Report and Literature Review" Journal of Clinical Medicine 11, no. 20: 6084. https://doi.org/10.3390/jcm11206084
APA StyleHunter-Dickson, M., Wu, P., Athavale, A., & Wang, A. Y. (2022). Management of Renal Angiomyolipomas in Tuberous Sclerosis Complex: A Case Report and Literature Review. Journal of Clinical Medicine, 11(20), 6084. https://doi.org/10.3390/jcm11206084





