The Feasibility of Whole-Liver Drainage with a Novel 8 mm Fully Covered Self-Expandable Metal Stent Possessing an Ultra-Slim Introducer for Malignant Hilar Biliary Obstructions
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
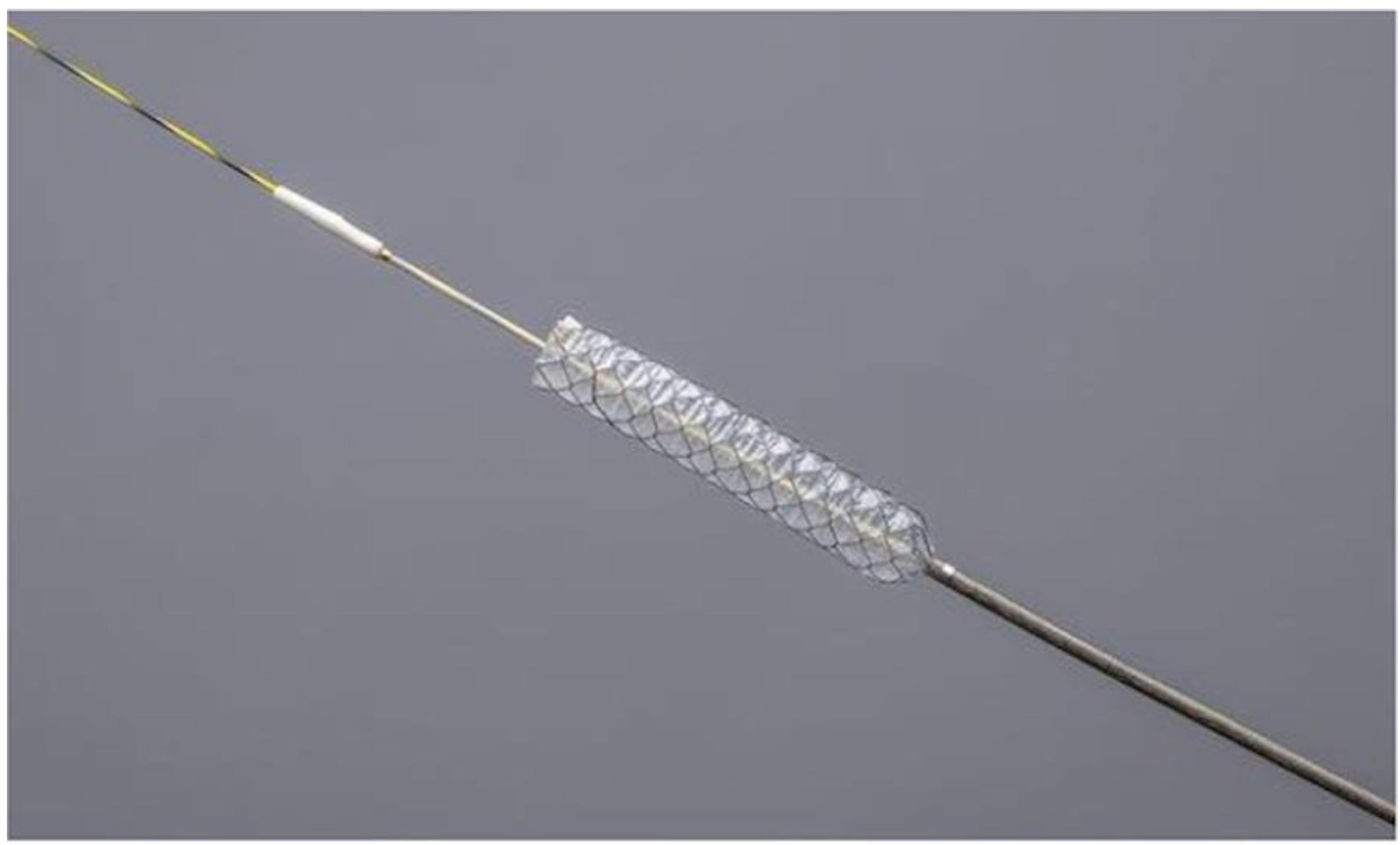
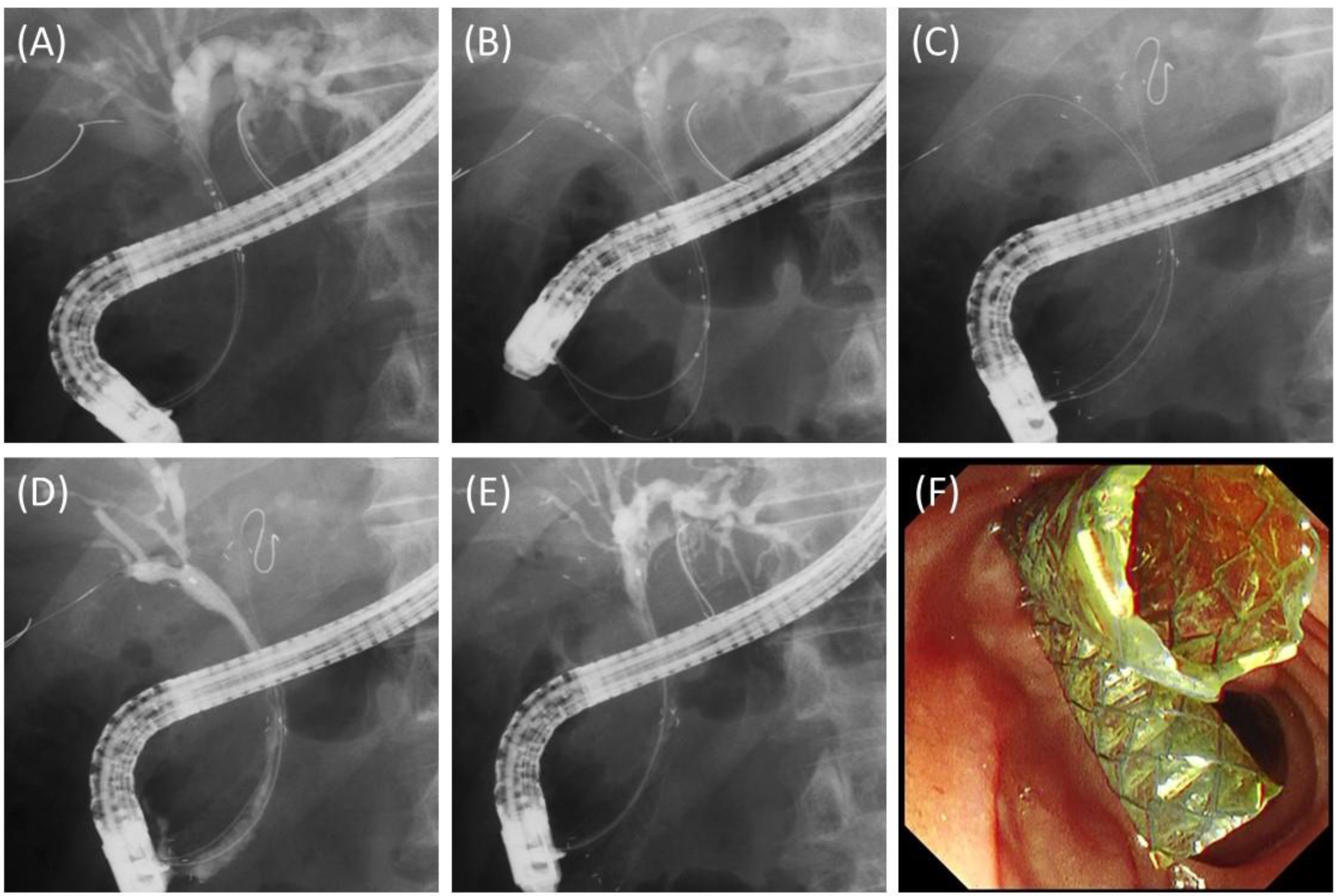
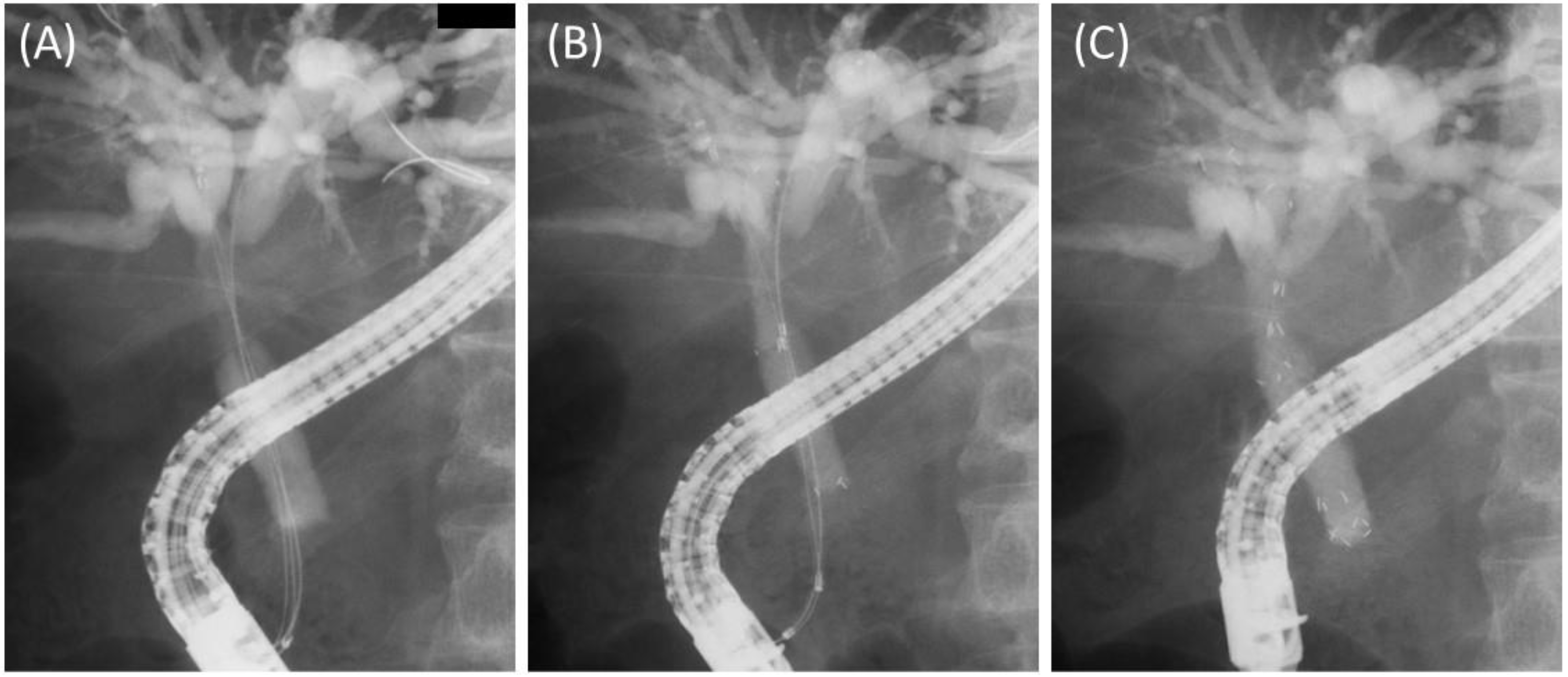
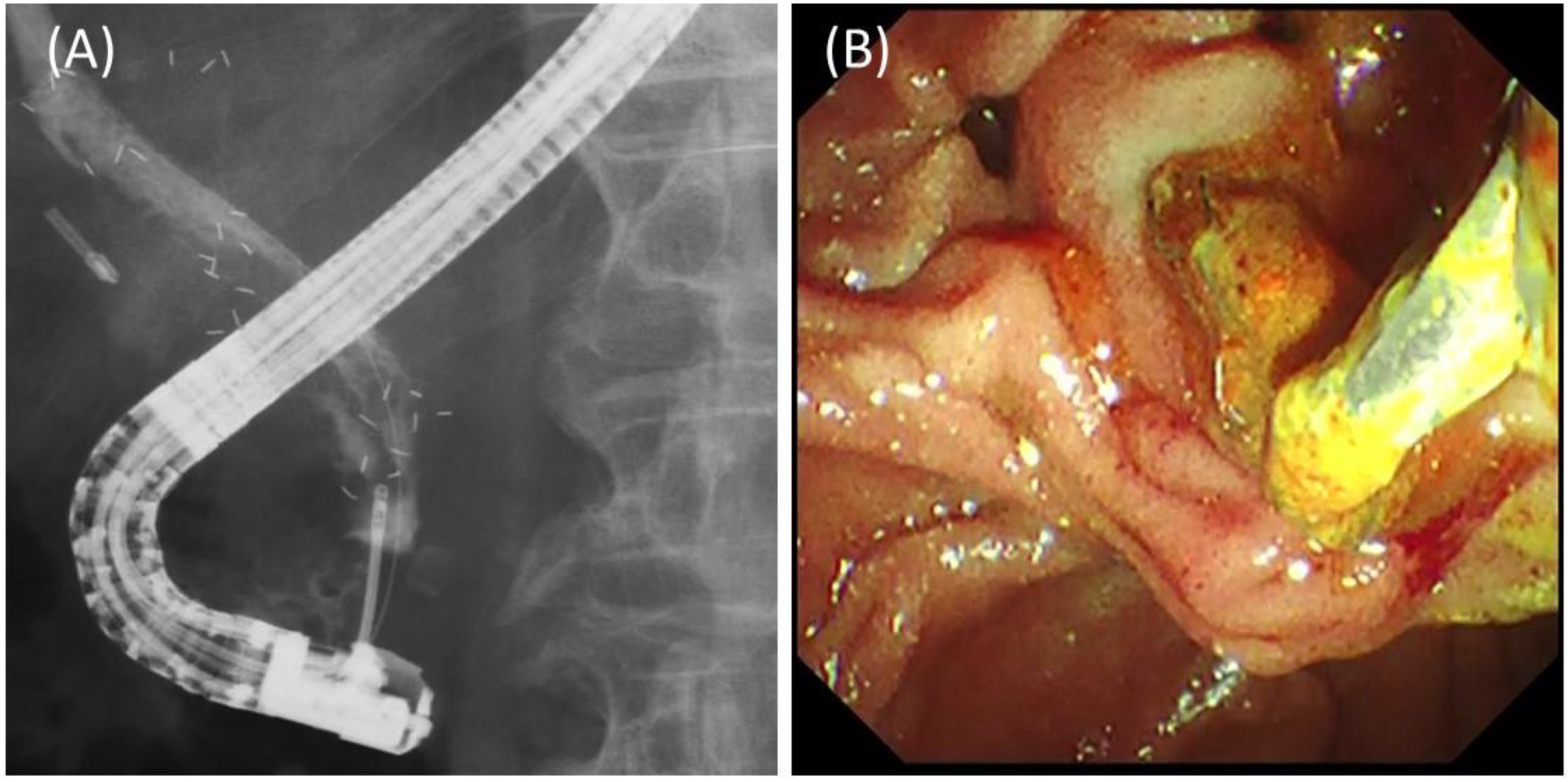
2.2. Procedures
2.3. Outcome Measures and Definitions
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Details and Outcomes of the Procedures
3.3. Long-Term Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Larghi, A.; Tringali, A.; Lecca, P.G.; Giordano, M.; Costamagna, G. Management of Hilar Biliary Strictures. Am. J. Gastroenterol. 2008, 103, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Rerknimitr, R.; Angsuwatcharakon, P.; Ratanachu-Ek, T.; Khor, C.J.L.; Ponnudurai, R.; Moon, J.H.; Seo, D.W.; Pantongrag-Brown, L.; Sangchan, A.; Pisespongsa, P.; et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J. Gastroenterol. Hepatol. 2013, 28, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Dumonceau, J.M.; Tringali, A.; Papanikolaou, I.S.; Blero, D.; Mangiavillano, B.; Schmidt, A.; Vanbiervliet, G.; Costamagna, G.; Devière, J.; García-Cano, J.; et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline–Updated October 2017. Endoscopy 2018, 50, 910–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.H.; Kim, T.H.; Moon, J.H.; Lee, S.H.; Choi, H.J.; Hwangbo, Y.; Hyun, J.J.; Choi, J.-H.; Jeong, S.; Kim, J.H.; et al. Bilateral versus unilateral placement of metal stents for inoperable high-grade malignant hilar biliary strictures: A multicenter, prospective, randomized study (with video). Gastrointest. Endosc. 2017, 86, 817–827. [Google Scholar] [CrossRef]
- Hakuta, R.; Kogure, H.; Nakai, Y.; Kawakami, H.; Maguchi, H.; Mukai, T.; Iwashita, T.; Saito, T.; Togawa, O.; Matsubara, S.; et al. Unilateral versus Bilateral Endoscopic Nasobiliary Drainage and Subsequent Metal Stent Placement for Unresectable Malignant Hilar Obstruction: A Multicenter Randomized Controlled Trial. J. Clin. Med. 2021, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Meybodi, M.A.; Shakoor, D.; Nanavati, J.; Ichkhanian, Y.; Vosoughi, K.; Brewer Gutierrez, O.I.; Kalloo, A.N.; Singh, V.; Kumbhari, V.; Ngamruengphong, S.; et al. Unilateral versus bilateral endoscopic stenting in patients with unresectable malignant hilar obstruction: A systematic review and meta-analysis. Endosc. Int. Open 2020, 8, E281–E290. [Google Scholar] [CrossRef]
- Mukai, T.; Yasuda, I.; Nakashima, M.; Doi, S.; Iwashita, T.; Iwata, K.; Kato, T.; Tomita, E.; Moriwaki, H. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: A randomized controlled trial. J. Hepato-Biliary-Pancreatic Sci. 2012, 20, 214–222. [Google Scholar] [CrossRef]
- Xia, M.-X.; Cai, X.-B.; Pan, Y.-L.; Wu, J.; Gao, D.-J.; Ye, X.; Wang, T.-T.; Hu, B. Optimal stent placement strategy for malignant hilar biliary obstruction: A large multicenter parallel study. Gastrointest. Endosc. 2019, 91, 1117–1128.e9. [Google Scholar] [CrossRef]
- Costamagna, G.; Tringali, A. Can we insert a covered stent, partially or not, in case of hilar biliary stenosis? Endosc. Int. Open 2017, 5, E1218–E1219. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Okumura, F.; Naitoh, I.; Fukusada, S.; Kachi, K.; Ozeki, T.; Anbe, K.; Iwasaki, H.; Mizushima, T.; Kobayashi, Y.; et al. Feasibility of the placement of a novel 6-mm diameter threaded fully covered self-expandable metal stent for malignant hilar biliary obstructions (with videos). Gastrointest. Endosc. 2016, 84, 352–357. [Google Scholar] [CrossRef]
- Yoshida, T.; Hara, K.; Imaoka, H.; Hijioka, S.; Mizuno, N.; Ishihara, M.; Tanaka, T.; Tajika, M.; Niwa, Y.; Yamao, K. Benefits of side-by-side deployment of 6-mm covered self-expandable metal stents for hilar malignant biliary obstructions. J. Hepato-Biliary-Pancreatic Sci. 2016, 23, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, H.; Nakache, R.; Diamond, T. Management Strategies in Resection for Hilar Cholangiocarcinoma. Ann. Surg. 1992, 215, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Isayama, H.; Hamada, T.; Yasuda, I.; Itoi, T.; Ryozawa, S.; Nakai, Y.; Kogure, H.; Koike, K. Tokyo criteria 2014 for transpapillary biliary stenting. Dig. Endosc. 2014, 27, 259–264. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Vienne, A.; Hobeika, E.; Gouya, H.; Lapidus, N.; Fritsch, J.; Choury, A.D.; Chryssostalis, A.; Gaudric, M.; Pelletier, G.; Buffet, C.; et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: The role of liver volume assessment. Gastrointest. Endosc. 2010, 72, 728–735. [Google Scholar] [CrossRef]
- Tringali, A.; Boskoski, I.; Costamagna, G. Endoscopic Stenting in Hilar Cholangiocarcinoma: When, How, and How Much to Drain? Gastroenterol. Res. Pract. 2019, 2019, 5161350. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Moon, J.H.; Park, S. Biliary stenting for hilar malignant biliary obstruction. Dig. Endosc. 2019, 32, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Nagino, M.; Hirano, S.; Yoshitomi, H.; Aoki, T.; Uesaka, K.; Unno, M.; Ebata, T.; Konishi, M.; Sano, K.; Shimada, K.; et al. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J. Hepato-Biliary-Pancreatic Sci. 2020, 28, 26–54. [Google Scholar] [CrossRef]
- Qumseya, B.J.; Jamil, L.H.; Elmunzer, B.J.; Riaz, A.; Ceppa, E.P.; Thosani, N.C.; Buxbaum, J.L.; Storm, A.C.; Sawhney, M.S.; Pawa, S.; et al. ASGE guideline on the role of endoscopy in the management of malignant hilar obstruction. Gastrointest. Endosc. 2021, 94, 222–234.e22. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, S.H.; You, M.S.; Shin, B.-S.; Choi, Y.H.; Kang, J.; Jang, S.; Paik, W.H.; Ryu, J.K.; Kim, Y.-T. Step-wise endoscopic approach to palliative bilateral biliary drainage for unresectable advanced malignant hilar obstruction. Sci. Rep. 2019, 9, 13207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.H.; Moon, J.H.; Choi, J.-H.; Lee, S.H.; Lee, Y.N.; Paik, W.H.; Jang, D.K.; Cho, B.W.; Yang, J.K.; Hwangbo, Y.; et al. Prospective comparison of endoscopic bilateral stent-in-stent versus stent-by-stent deployment for inoperable advanced malignant hilar biliary stricture. Gastrointest. Endosc. 2019, 90, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Isayama, H.; Komatsu, Y.; Tsujino, T.; Sasahira, N.; Hirano, K.; Toda, N.; Nakai, Y.; Yamamoto, N.; Tada, M.; Yoshida, H.; et al. A prospective randomised study of "covered" versus "uncovered" diamond stents for the management of distal malignant biliary obstruction. Gut 2004, 53, 729–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tringali, A.; Hassan, C.; Rota, M.; Rossi, M.; Mutignani, M.; Aabakken, L. Covered vs. uncovered self-expandable metal stents for malignant distal biliary strictures: A systematic review and meta-analysis. Laryngo-Rhino-Otologie 2018, 50, 631–641. [Google Scholar] [CrossRef]
- Miura, S.; Kanno, A.; Masamune, A.; Hamada, S.; Hongou, S.; Yoshida, N.; Nakano, E.; Takikawa, T.; Kume, K.; Kikuta, K.; et al. Risk factors for recurrent biliary obstruction following placement of self-expandable metallic stents in patients with malignant perihilar biliary stricture. Laryngo-Rhino-Otologie 2016, 48, 536–545. [Google Scholar] [CrossRef]
- Kurita, A.; Uza, N.; Asada, M.; Yoshimura, K.; Takemura, T.; Yazumi, S.; Kodama, Y.; Seno, H. Stent placement above the sphincter of Oddi is a useful option for patients with inoperable malignant hilar biliary obstruction. Surg. Endosc. 2021, 36, 2869–2878. [Google Scholar] [CrossRef]
- Cosgrove, N.; Siddiqui, A.A.; Adler, D.G.; Shahid, H.; Sarkar, A.; Sharma, A.; Kowalski, T.E.; Loren, D.; Warndorf, M.; Chennat, J.; et al. A Comparison of Bilateral Side-by-Side Metal Stents Deployed Above and Across the Sphincter of Oddi in the Management of Malignant Hilar Biliary Obstruction. J. Clin. Gastroenterol. 2017, 51, 528–533. [Google Scholar] [CrossRef]
- Kitamura, K.; Yamamiya, A.; Ishii, Y.; Mitsui, Y.; Nomoto, T.; Yoshida, H. Side-by-side partially covered self-expandable metal stent placement for malignant hilar biliary obstruction. Endosc. Int. Open 2017, 05, E1211–E1217. [Google Scholar] [CrossRef] [Green Version]
- Ishigaki, K.; Hamada, T.; Nakai, Y.; Isayama, H.; Sato, T.; Hakuta, R.; Saito, K.; Saito, T.; Takahara, N.; Mizuno, S.; et al. Retrospective Comparative Study of Side-by-Side and Stent-in-Stent Metal Stent Placement for Hilar Malignant Biliary Obstruction. Am. J. Dig. Dis. 2020, 65, 3710–3718. [Google Scholar] [CrossRef]
- Kawakubo, K.; Isayama, H.; Nakai, Y.; Togawa, O.; Sasahira, N.; Kogure, H.; Sasaki, T.; Matsubara, S.; Yamamoto, N.; Hirano, K.; et al. Risk factors for pancreatitis following transpapillary self-expandable metal stent placement. Surg. Endosc. 2011, 26, 771–776. [Google Scholar] [CrossRef]
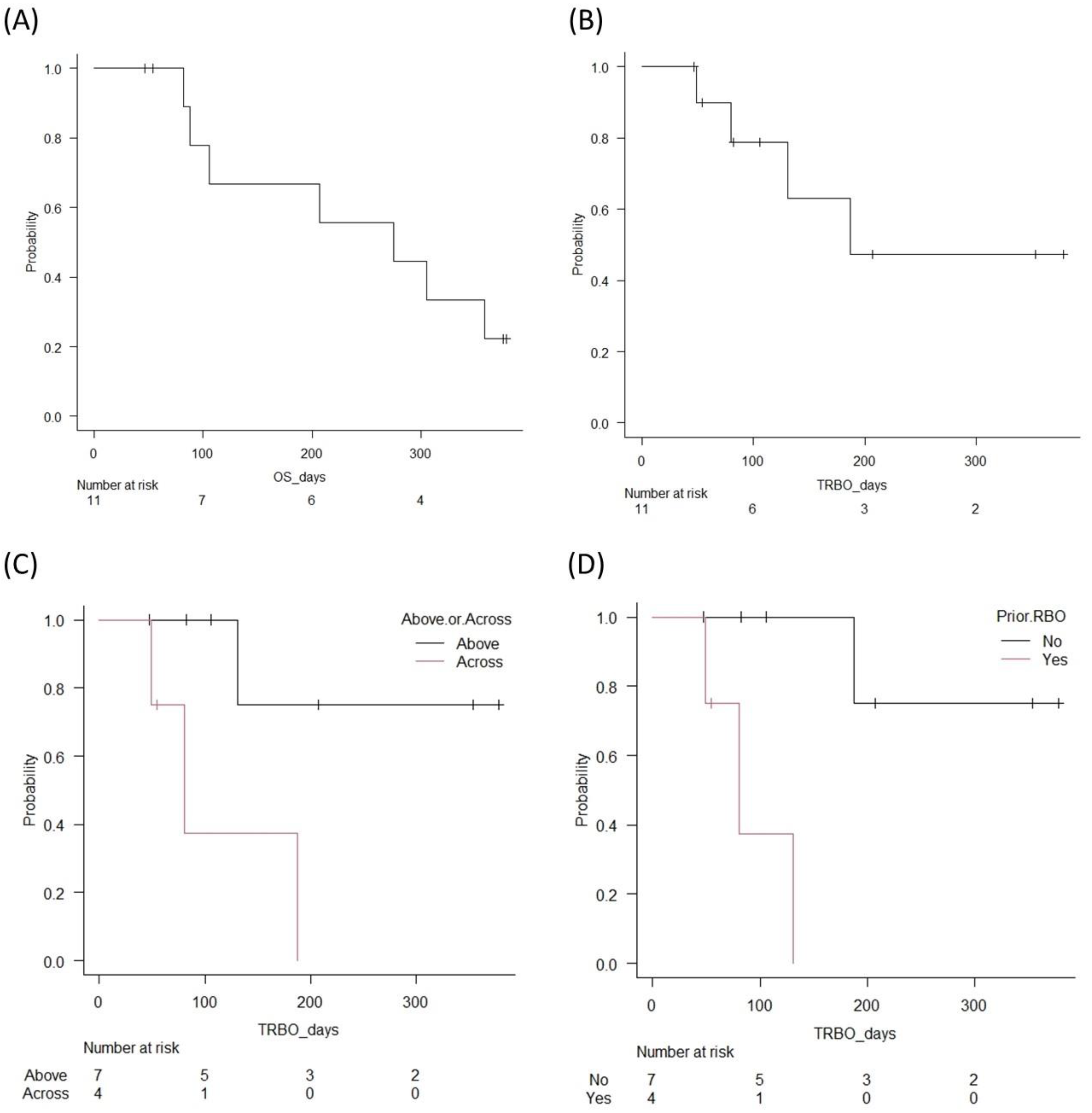
| Age, years | 81 (60–85) |
| Sex, male | 9 (81.8) |
| Primary cancer | |
| Gallbladder | 4 (36.4) |
| Bile duct | 4 (36.4) |
| Pancreas | 1 (9.1) |
| Colon | 1 (9.1) |
| Malignant lymphoma | 1 (9.1) |
| Bismuth classification | |
| I | 1 (9.1) |
| II | 7 (63.6) |
| IIIa | 3 (27.3) |
| Prior drainage | 7 (63.6) |
| Prior RBO | 4 (36.4) |
| Chemotherapy | 6 (54.5) |
| Technical success | 11 (100) |
| Functional success | 11 (100) |
| CBD diameter, mm | 8 (6–12) |
| Number of stents | |
| 2 | 9 (81.8) |
| 3 | 2 (18.2) |
| Stent length | |
| 60 mm | 12 |
| 80 mm | 11 |
| 100 mm | 1 |
| Stent position | |
| Above the papilla | 7 (63.6) |
| Across the papilla | 4 (36.4) |
| Dilation of the stricture | 1 (9.1) |
| Procedure time, mins | 62 (30–84) |
| Early Aes | |
| Pancreatitis (mild) | 2 (18.2) |
| Follow-up period, days | 207 (47–378) |
| RBO | |
| All pts (n = 11) | 4 (36.4) |
| Pts with stents above the papilla (n = 7)/across the papilla (n = 4) | 1 (14.3)/3 (75) |
| Pts without prior RBO (n = 7)/with RBO (n = 4) | 1 (14.3)/3 (75) |
| Causes of RBO | |
| Sludge | 4 |
| TRBO, days (95% CI) | |
| All pts (n = 11) | 187 (49—NA) |
| Pts with stents above the papilla (n = 7)/across the papilla (n = 4) | NA (131—NA)/80 (49—NA) |
| Pts without prior RBO (n = 7)/with RBO (n = 4) | NA (187—NA)/80 (49—NA) |
| Revision for RBO | 3 (27.3) |
| Exchange for 8 mm FCSEMSs | 2 |
| Exchange for NBTs | 1 |
| Late AEs other than RBO | 0 |
| OS, days (95% CI) | 275 (82—NA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsubara, S.; Nakagawa, K.; Suda, K.; Otsuka, T.; Oka, M.; Nagoshi, S. The Feasibility of Whole-Liver Drainage with a Novel 8 mm Fully Covered Self-Expandable Metal Stent Possessing an Ultra-Slim Introducer for Malignant Hilar Biliary Obstructions. J. Clin. Med. 2022, 11, 6110. https://doi.org/10.3390/jcm11206110
Matsubara S, Nakagawa K, Suda K, Otsuka T, Oka M, Nagoshi S. The Feasibility of Whole-Liver Drainage with a Novel 8 mm Fully Covered Self-Expandable Metal Stent Possessing an Ultra-Slim Introducer for Malignant Hilar Biliary Obstructions. Journal of Clinical Medicine. 2022; 11(20):6110. https://doi.org/10.3390/jcm11206110
Chicago/Turabian StyleMatsubara, Saburo, Keito Nakagawa, Kentaro Suda, Takeshi Otsuka, Masashi Oka, and Sumiko Nagoshi. 2022. "The Feasibility of Whole-Liver Drainage with a Novel 8 mm Fully Covered Self-Expandable Metal Stent Possessing an Ultra-Slim Introducer for Malignant Hilar Biliary Obstructions" Journal of Clinical Medicine 11, no. 20: 6110. https://doi.org/10.3390/jcm11206110





