The Association between Elevated Hematocrit and Retinal Artery Occlusion in Adult Patients
Abstract
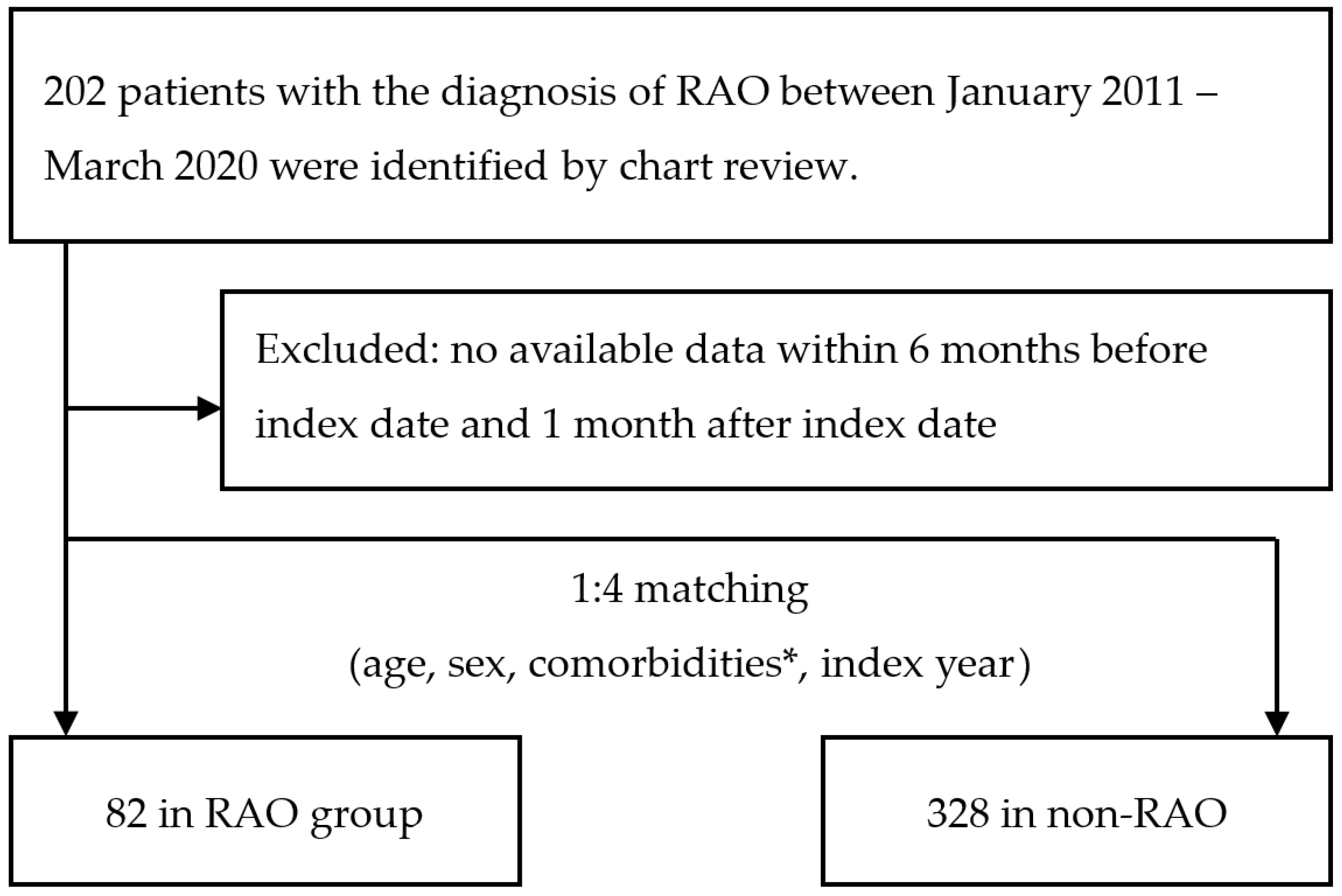
:1. Introduction
2. Materials and Methods
2.1. Case Enrollment
2.2. Data Collection
2.3. Statistical Analysis
2.4. Narrative Reviews on the Association between Hct Levels and Embolism
3. Results
3.1. Comparison of RAO and Non-RAO Patients in Terms of Demographic Data and Available Laboratory Data
3.2. Factors Associated with RAO
3.3. Narrative Review of the Association between Hct and Embolism
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schrag, M.; Youn, T.; Schindler, J.; Kirshner, H.; Greer, D. Intravenous Fibrinolytic Therapy in Central Retinal Artery Occlusion: A Patient-Level Meta-analysis. JAMA Neurol. 2015, 72, 1148–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.S.; Ho, C.H.; Chu, C.C.; Wang, J.J.; Tseng, S.H.; Jan, R.L. Risk of retinal artery occlusion in patients with diabetes mellitus: A retrospective large-scale cohort study. PLoS ONE 2018, 13, e0201627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, R.M.; Chaturvedi, S.; Eliott, D.; Joshi, N.; Puklin, J.E.; Abrams, G.W. Mechanisms of retinal arterial occlusive disease in African American and Caucasian patients. Stroke 1999, 30, 1506–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayreh, S.S.; Podhajsky, P.A.; Zimmerman, M.B. Retinal artery occlusion: Associated systemic and ophthalmic abnormalities. Ophthalmology 2009, 116, 1928–1936. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.; Wang, J.J.; Smith, W. Risk factors and significance of finding asymptomatic retinal emboli. Clin. Exp. Ophthalmol. 2000, 28, 13–17. [Google Scholar] [CrossRef]
- Sharma, R.A.; Dattilo, M.; Newman, N.J.; Biousse, V. Treatment of Nonarteritic Acute Central Retinal Artery Occlusion. Asia. Pac. J. Ophthalmol. 2018, 7, 235–241. [Google Scholar]
- Recchia, F.M.; Brown, G.C. Systemic disorders associated with retinal vascular occlusion. Curr. Opin. Ophthalmol. 2000, 11, 462–467. [Google Scholar] [CrossRef]
- Chen, S.N.; Chao, C.C.; Hwang, J.F.; Yang, C.M. Clinical manifestations of central retinal artery occlusion in eyes of proliferative diabetic retinopathy with previous vitrectomy and panretinal photocoagulation. Retina 2014, 34, 1861–1866. [Google Scholar] [CrossRef]
- Chang, Y.S.; Weng, S.F.; Chang, C.; Wang, J.J.; Tseng, S.H.; Ko, S.Y.; Su, S.B.; Huang, C.C.; Wang, Y.J.; Jan, R.L. Risk of Retinal Artery Occlusion in Patients with End-Stage Renal Disease: A Retrospective Large-Scale Cohort Study. Medicine 2016, 95, e3281. [Google Scholar] [CrossRef]
- Braekkan, S.K.; Mathiesen, E.B.; Njølstad, I.; Wilsgaard, T.; Hansen, J.-B. Hematocrit and risk of venous thromboembolism in a general population. The Tromso study. Haematologica 2010, 95, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Eischer, L.; Tscholl, V.; Heinze, G.; Traby, L.; Kyrle, P.A.; Eichinger, S. Hematocrit and the risk of recurrent venous thrombosis: A prospective cohort study. PLoS ONE 2012, 7, e38705. [Google Scholar] [CrossRef]
- Ahn, B.Y.; Choi, K.D.; Choi, Y.J.; Jea, S.Y.; Lee, J.E. Isolated monocular visual loss as an initial manifestation of polycythemia vera. J. Neurol. Sci. 2007, 258, 151–153. [Google Scholar] [CrossRef]
- Rao, K.; Shenoy, S.B.; Kamath, Y.; Kapoor, S. Central retinal artery occlusion as a presenting manifestation of polycythaemia vera. BMJ Case Rep. 2016, 2016, bcr2016216417. [Google Scholar] [CrossRef]
- Blackford, J.U. Propensity scores: Method for matching on multiple variables in down syndrome research. Intellect. Dev. Disabil. 2009, 47, 348–357. [Google Scholar] [CrossRef] [Green Version]
- Kolodgie, F.D.; Virmani, R.; Finn, A.V.; Romero, M.E. Embolic Myocardial Infarction as a Consequence of Atrial Fibrillation: A Prevailing Disease of the Future. Circulation 2015, 132, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Camaro, C.; Aengevaeren, W.R. Acute myocardial infarction due to coronary artery embolism in a patient with atrial fibrillation. Neth. Heart J. 2009, 17, 297–299. [Google Scholar] [CrossRef]
- Kamel, H.; Healey, J.S. Cardioembolic Stroke. Circ. Res. 2017, 120, 514–526. [Google Scholar] [CrossRef]
- Ntaios, G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 333–340. [Google Scholar] [CrossRef]
- Chang, Y.S.; Jan, R.L.; Weng, S.F.; Wang, J.J.; Chio, C.C.; Wei, F.T.; Chu, C.C. Retinal artery occlusion and the 3-year risk of stroke in Taiwan: A nationwide population-based study. Am. J. Ophthalmol. 2012, 154, 645–652.e641. [Google Scholar] [CrossRef]
- Zimmerman, L.E. Embolism of central retinal artery; secondary to myocardial infarction with mural thrombosis. Arch. Ophthalmol. 1965, 73, 822–826. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Moss, S.E.; Meuer, S.M. Retinal emboli and cardiovascular disease: The Beaver Dam Eye Study. Arch. Ophthalmol. 2003, 121, 1446–1451. [Google Scholar] [CrossRef]
- Brown, D.W.; Giles, W.H.; Croft, J.B. Hematocrit and the risk of coronary heart disease mortality. Am. Heart J. 2001, 142, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Tong, P.C.; Kong, A.P.; So, W.Y.; Ng, M.H.; Yang, X.; Ng, M.C.; Ma, R.C.; Ho, C.S.; Lam, C.W.; Chow, C.C.; et al. Hematocrit, independent of chronic kidney disease, predicts adverse cardiovascular outcomes in chinese patients with type 2 diabetes. Diabetes Care 2006, 29, 2439–2444. [Google Scholar] [CrossRef] [Green Version]
- Paul, L.; Jeemon, P.; Hewitt, J.; McCallum, L.; Higgins, P.; Walters, M.; McClure, J.; Dawson, J.; Meredith, P.; Jones, G.C.; et al. Hematocrit predicts long-term mortality in a nonlinear and sex-specific manner in hypertensive adults. Hypertension 2012, 60, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Toss, F.; Nordström, A.; Nordström, P. Association between hematocrit in late adolescence and subsequent myocardial infarction in Swedish men. Int. J. Cardiol. 2013, 168, 3588–3593. [Google Scholar] [CrossRef]
- Gotoh, S.; Hata, J.; Ninomiya, T.; Hirakawa, Y.; Nagata, M.; Mukai, N.; Fukuhara, M.; Ikeda, F.; Ago, T.; Kitazono, T.; et al. Hematocrit and the risk of cardiovascular disease in a Japanese community: The Hisayama Study. Atherosclerosis 2015, 242, 199–204. [Google Scholar] [CrossRef]
- Yang, R.; Wang, A.; Ma, L.; Su, Z.; Chen, S.; Wang, Y.; Wu, S.; Wang, C. Hematocrit and the incidence of stroke: A prospective, population-based cohort study. Ther. Clin. Risk Manag. 2018, 14, 2081–2088. [Google Scholar] [CrossRef] [Green Version]
- Takaoka, N.; Sairenchi, T.; Irie, F.; Matsushita, M.; Nagao, M.; Umesawa, M.; Haruyama, Y.; Watanabe, H.; Yamagishi, H.; Iso, H.; et al. High Hematocrit Levels Are Associated with Risk of Cardiovascular Mortality among Middle-Aged Japanese Women: The Ibaraki Prefectural Health Study (IPHS). Tohoku J. Exp. Med. 2019, 249, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warny, M.; Helby, J.; Birgens, H.S.; Bojesen, S.E.; Nordestgaard, B.G. Arterial and venous thrombosis by high platelet count and high hematocrit: 108 521 individuals from the Copenhagen General Population Study. J. Thromb. Haemost. 2019, 17, 1898–1911. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, L.; Wang, H.; Qi, H.; Zhang, J.; Wang, Y. Branch retinal artery occlusion secondary to high-altitude exposure and diabetic retinopathy: A case report. BMC Ophthalmol. 2020, 20, 281. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Raman, R.; Sharma, T. Polycythemia causing posterior segment vascular occlusions. Oman J. Ophthalmol. 2017, 10, 33–35. [Google Scholar] [CrossRef]
- Arévalo Simental, D.E.; Melo-Granados, E.A.R.; Quezada, S.C.; Escamilla, M.A.P.; Orozco, C.L.S.; Buenrostro, J.E.J. Hemiretinal Artery Occlusion in an 11-Year-Old Child with Dextrocardia. Case Rep. Ophthalmol. Med. 2016, 2016, 5104789. [Google Scholar] [CrossRef]
- Arıkan, G.; Saatci, A.O.; Kahraman, S.; Pişkin, Ö.; Men, S.; Ündar, B. Central retinal artery occlusion as the presenting sign of essential thrombocythemia. Turk. J. Haematol. 2011, 28, 146–148. [Google Scholar] [CrossRef]
- Imai, E.; Kunikata, H.; Udono, T.; Nakagawa, Y.; Abe, T.; Tamai, M. Branch retinal artery occlusion: A complication of iron-deficiency anemia in a young adult with a rectal carcinoid. Tohoku J. Exp. Med. 2004, 203, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, D.; Hetzel, A.; Geibel-Zehender, A.; Schulte-Mönting, J. Systemic diseases in non-inflammatory branch and central retinal artery occlusion—An overview of 416 patients. Eur. J. Med. Res. 2007, 12, 595–603. [Google Scholar]
- Hoki, S.L.; Varma, R.; Lai, M.Y.; Azen, S.P.; Klein, R. Prevalence and associations of asymptomatic retinal emboli in Latinos: The Los Angeles Latino Eye Study (LALES). Am. J. Ophthalmol. 2008, 145, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Callizo, J.; Feltgen, N.; Pantenburg, S.; Wolf, A.; Neubauer, A.S.; Jurklies, B.; Wachter, R.; Schmoor, C.; Schumacher, M.; Junker, B.; et al. Cardiovascular Risk Factors in Central Retinal Artery Occlusion: Results of a Prospective and Standardized Medical Examination. Ophthalmology 2015, 122, 1881–1888. [Google Scholar] [CrossRef]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]


| Total (n = 410) | RAO (n = 82) | Non-RAO (n = 328) | p Value | |
|---|---|---|---|---|
| Age, years (mean ± SD) | 68.9 ± 15.5 | 65.1 ± 16.6 | 69.8 ± 15.1 | 0.013 |
| Gender (%) | 0.497 | |||
| Male | 272 (66) | 57 (70) | 215 (66) | |
| Female | 138 (34) | 25 (31) | 113 (35) | |
| Comorbidities (%) | ||||
| Hypertension | 247 (60) | 47 (57) | 200 (61) | 0.545 |
| Diabetes mellitus | 101 (25) | 23 (28) | 78 (24) | 0.422 |
| Dyslipidemia | 154 (38) | 32 (39) | 122 (37) | 0.760 |
| Chronic kidney disease | 31 (8) | 4 (5) | 27 (8) | 0.304 |
| Stroke | 151 (37) | 27 (33) | 124 (38) | 0.413 |
| Atrial fibrillation | 41 (10) | 8 (10) | 33 (10) | 0.934 |
| Hematology parameters * | ||||
| WBC (1000/µL) | 8.0 ± 6.7 | 7.5 ± 2.2 | 8.2 ± 7.5 | 0.384 |
| Hb (g/dL) | 12.9 ± 2.0 | 13.4 ± 1.9 | 12.8 ± 2.0 | 0.015 |
| Hct (%) | 38.6 ± 5.7 | 39.9 ± 5.3 | 38.2 ± 5.8 | 0.021 |
| Platelet (1000/µL) | 222.6 ± 71.6 | 223.4 ± 64.6 | 222.5 ± 73.4 | 0.911 |
| eGFR (ml/min/1.73 m2) | 70.7 ± 24.1 | 69.0 ± 25.2 | 71.2 ± 23.8 | 0.480 |
| GPT (U/L) | 32.2 ± 48.1 | 25.2 ± 19.4 | 34.0 ± 52.8 | 0.136 |
| Fundus Change (%) | CRAO/BRAO (n =82) | |||
| Retinal opacification | 78 (95) | |||
| Cherry-red spot | 58 (71) | |||
| Artery attenuation | 40 (49) | |||
| Disc edema | 15 (18) | |||
| Box carring | 15 (18) | |||
| Emboli | 6 (7) | |||
| Variables | Beta | OR (95% CI) | p Value |
|---|---|---|---|
| Age | |||
| <40 | 0.889 | 2.44 (0.79–7.48) | 0.120 |
| 40–55 | 0.506 | 1.66 (0.77–3.60) | 0.200 |
| 55–65 | 0.214 | 1.24 (0.60–2.56) | 0.563 |
| >65 | 1 | 1 | 1 |
| Hematology parameters | |||
| WBC < 9 (1000/µL) | 0.161 | 1.17 (0.64–2.15) | 0.603 |
| eGFR ≤ 60 (ml/min/1.73 m2) | 0.400 | 1.49 (0.84–2.64) | 0.170 |
| Platelet <215 (1000/µL) | 0.158 | 1.17 (0.71–1.94) | 0.539 |
| GPT ≤ 30 (U/L) | 0.507 | 1.66 (0.94–2.92) | 0.079 |
| Hct ≥ 40 (%) | 0.556 | 1.74 (1.01–3.02) | 0.046 |
| Hb ≥ 13 (g/dL) | 0.389 | 1.48 (0.84–2.58) | 0.172 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, W.-Y.; Lin, P.-C.; Yin, C.-H.; Yang, K.-T.; Shih, E.-J.; Chen, J.-S. The Association between Elevated Hematocrit and Retinal Artery Occlusion in Adult Patients. J. Clin. Med. 2022, 11, 6116. https://doi.org/10.3390/jcm11206116
Lai W-Y, Lin P-C, Yin C-H, Yang K-T, Shih E-J, Chen J-S. The Association between Elevated Hematocrit and Retinal Artery Occlusion in Adult Patients. Journal of Clinical Medicine. 2022; 11(20):6116. https://doi.org/10.3390/jcm11206116
Chicago/Turabian StyleLai, Wei-Yu, Pei-Chin Lin, Chun-Hao Yin, Kuang-Tsu Yang, En-Jie Shih, and Jin-Shuen Chen. 2022. "The Association between Elevated Hematocrit and Retinal Artery Occlusion in Adult Patients" Journal of Clinical Medicine 11, no. 20: 6116. https://doi.org/10.3390/jcm11206116
APA StyleLai, W. -Y., Lin, P. -C., Yin, C. -H., Yang, K. -T., Shih, E. -J., & Chen, J. -S. (2022). The Association between Elevated Hematocrit and Retinal Artery Occlusion in Adult Patients. Journal of Clinical Medicine, 11(20), 6116. https://doi.org/10.3390/jcm11206116






