Admission Lactate Concentration, Base Excess, and Alactic Base Excess Predict the 28-Day Inward Mortality in Shock Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
Data Collection
2.2. Data Coding and Statistical Analysis
- category 0—if BE was above (less severe or no acidaemia) and lactate concentration was below (non-severe or no hyperlactataemia) their respective cut-off values, i.e., no patient had severe acidaemia and hyperlactataemia;
- category 1—if either BE was below or lactate concentration was above their respective cut-off values, i.e., patients with either severe acidaemia or severe hyperlactataemia;
- category 2—if both BE was below and lactate concentration was above their respective cut-off values, i.e., patients with coexisting severe acidosis and hyperlactataemia.
3. Results
4. Discussion
Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vincent, J.-L.; De Mendonca, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Crit. Care Med. 1988, 26, 1793–1800. [Google Scholar] [CrossRef] [Green Version]
- Masyuk, M.; Wernly, B.; Lichtenauer, M.; Franz, M.; Kabisch, B.; Muessig, J.M.; Zimmermann, G.; Lauten, A.; Schulze, P.C.; Hoppe, U.C.; et al. Prognostic relevance of serum lactate kinetics in critically ill patients. Intensiv. Care Med. 2019, 45, 55–61. [Google Scholar] [CrossRef]
- Davis, J.W.; Kaups, K.L. Base deficit in the elderly: A marker of severe injury and death. J. Trauma 1998, 45, 873–877. [Google Scholar] [CrossRef]
- Zante, B.; Reichenspurner, H.; Kubik, M.; Kluge, S.; Schefold, J.C.; Pfortmueller, C.A. Base excess is superior to lactate-levels in prediction of ICU mortality after cardiac surgery. PLoS ONE 2018, 5, e0205309. [Google Scholar] [CrossRef] [Green Version]
- Vitek, V.; Cowley, R.A. Blood lactate in the prognosis of various forms of shock. Ann Surg. 1971, 173, 308–313. [Google Scholar] [CrossRef]
- Bakker, J.; Coffernils, M.; Leon, M.; Gris, P.; Vincent, J.L. Blood lactate levels are superior to oxygen-derived variables in predicting outcome in human septic shock. Chest 1991, 99, 956–962. [Google Scholar] [CrossRef]
- Jansen, T.C.; van Bommel, J.; Woodward, R.; Mulder, P.G.; Bakker, J. Association between blood lactate levels, Sequential Organ Failure Assessment subscores, and 28-day mortality during early and late intensive care unit stay: A retrospective observational study. Crit. Care Med. 2009, 37, 2369–2374. [Google Scholar] [CrossRef]
- Vanni, S.; Viviani, G.; Baioni, M.; Pepe, G.; Nazerian, P.; Socci, F.; Bartolucci, M.; Bartolini, M.; Grifoni, S. Prognostic value of plasma lactate levels among patients with acute pulmonary embolism: The thrombo-embolism lactate outcome study. Ann. Emerg. Med. 2013, 61, 330–338. [Google Scholar] [CrossRef]
- Shapiro, N.I.; Howell, M.D.; Talmor, D.; Nathanson, L.A.; Lisbon, A.; Wolfe, R.E.; Weiss, J.W. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 2005, 45, 524–548. [Google Scholar] [CrossRef]
- Ferreruela, M.; Raurich, J.M.; Ayestarán, I.; Llompart-Pou, J.A. Hyperlactataemia in ICU patients: Incidence, causes and associated mortality. J. Crit. Care 2017, 42, 200–205. [Google Scholar] [CrossRef]
- Chertoff, J.; Chisum, M.; Simmons, L.; King, B.; Walker, M.; Lascano, J. Prognostic utility of plasma lactate measured between 24 and 48 h after initiation of early goal-directed therapy in the management of sepsis, severe sepsis, and septic shock. J. Intensiv. Care 2016, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.G.; Song, J.; Park, D.W.; Moon, S.; Cho, H.J.; Kim, J.Y.; Park, J.; Cha, J.H. Prognostic value of lactate levels and lactate clearance in sepsis and septic shock with initial hyperlactataemia: A retrospective cohort study according to the Sepsis-3 definitions. Medicine 2021, 100, e24835. [Google Scholar] [CrossRef]
- Burstein, B.; Vallabhajosyula, S.; Ternus, B.; Barsness, G.W.; Kashani, K.; Jentzer, J.C. The Prognostic Value of Lactate in Cardiac Intensive Care Unit Patients with Cardiac Arrest and Shock. Shock 2021, 55, 613–619. [Google Scholar] [CrossRef]
- Jobin, S.P.; Maitra, S.; Baidya, D.K.; Subramaniam, R.; Prasad, G.; Seenu, V. Role of serial lactate measurement to predict 28-day mortality in patients undergoing emergency laparotomy for perforation peritonitis: Prospective observational study. J. Intensiv. Care 2019, 7, 58. [Google Scholar] [CrossRef]
- Rypulak, E.; Szczukocka, M.; Zyzak, K.; Piwowarczyk, P.; Borys, M.; Czuczwar, M. Transportation of patients with severe respiratory failure on ECMO support. Four-year experience of a single ECMO center. Anaesthesiol. Intensiv. Ther. 2020, 52, 91–96. [Google Scholar] [CrossRef]
- Rimachi, R.; Bruzzi de Carvahlo, F.; Orellano-Jimenez, C.; Cotton, F.; Vincent, J.L.; De Backer, D. Lactate/pyruvate ratio as a marker of tissue hypoxia in circulatory and septic shock. Anaesth. Intensiv. Care 2012, 40, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Nakane, M. Biological effects of the oxygen molecule in critically ill patients. J. Intensiv. Care 2020, 8, 95. [Google Scholar] [CrossRef]
- Russel, A.; Rivers, E.; Giri, P.; Jaehne, A.; Nguyen, B. A physiologic Approach to Hemodynamic Monitoring and Optimizing Oxygen Delivery in Shock Resuscitation. J. Clin. Med. 2020, 9, 2052. [Google Scholar] [CrossRef]
- Hernandez, G.; Boerma, E.C.; Dubin, A.; Bruhn, A.; Koopmans, M.; Edul, V.K.; Ruiz, C.; Castro, R.; Pozo, M.O.; Pedreros, C.; et al. Severe abnormalities in microvascular perfused vessel density are associated to organ dysfunctions and mortality and can be predicted by hyperlactataemia and norepinephrine requirements in septic shock patients. J. Crit. Care 2013, 28, e9–e14. [Google Scholar] [CrossRef]
- Hernandez, G.; Bellomo, R.; Bakker, J. The ten pitfalls of lactate clearance in sepsis. Intensiv. Care Med. 2019, 45, 82–85. [Google Scholar] [CrossRef]
- Suetrong, B.; Walley, K.R. Lactic Acidosis in Sepsis: It’s Not All Anaerobic: Implications for Diagnosis and Management. Chest 2016, 149, 252–261. [Google Scholar] [CrossRef]
- Garcia-Alvarez, M.; Marik, P.; Bellomo, R. Sepsis-associated hyperlactataemia. Crit. Care 2014, 9, 503. [Google Scholar] [CrossRef] [Green Version]
- Marik, P.E.; Bellomo, R. Lactate clearance as a target of therapy in sepsis: A flawed paradigm. OA Crit. Care 2013, 1, 3. [Google Scholar] [CrossRef]
- Ince, C.; Mik, E.G. Microcirculatory and mitochondrial hypoxia in sepsis, shock, and resuscitation. J. Appl. Physiol. 2016, 120, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Sterling, S.A.; Puskarich, M.A.; Jones, A.E. The effect of liver disease on lactate normalisation in severe sepsis and septic shock: A cohort study. Clin. Exp. Emerg. Med. 2015, 2, 197–202. [Google Scholar] [CrossRef]
- Donnino, M.W.; Andersen, L.W.; Chase, M.; Berg, K.M.; Tidswell, M.; Giberson, T.; Wolfe, R.; Moskowitz, A.; Smithline, H.; Ngo, L.; et al. Randomized, Double-Blind, Placebo-Controlled Trial of Thiamine as a Metabolic Resuscitator in Septic Shock: A Pilot Study. Crit. Care Med. 2016, 44, 360–367. [Google Scholar] [CrossRef]
- Gattinoni, L.; Vasques, F.; Camporota, L.; Meessen, J.; Romitti, F.; Pasticci, I.; Duscio, E.; Vassalli, F.; Forni, L.G.; Payen, D.; et al. Understanding Lactataemia in Human Sepsis. Potential Impact for Early Management. Am. J. Respir. Crit. Care Med. 2019, 200, 582–589. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensiv. Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Kashani, K.B.; Wiley, B.M.; Patel, P.C.; Baran, D.A.; Barsness, G.W.; Henry, T.D.; Van Diepen, S. Laboratory Markers of Acidosis and Mortality in Cardiogenic Shock: Developing a Definition of Hemometabolic Shock. Shock 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Coghill, J.E.; Guy, A.; Bradbury, A.W.; Adam, D.J.; Scriven, J.M. Serum lactate and base deficit as predictors of mortality after ruptured abdominal aortic aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Caputo, N.D.; Kanter, M.; Fraser, R.; Simon, R. Comparing biomarkers of traumatic shock: The utility of anion gap, base excess, and serum lactate in the ED. Am. J. Emerg. Med. 2015, 33, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.W.; Shapiro, N.I.; Donnino, M.W.; Baker, C.; Rosen, C.L. Serum lactate and base deficit as predictors of mortality in normotensive elderly blunt trauma patients. J. Trauma 2009, 66, 1040–1044. [Google Scholar] [CrossRef]
- Gale, S.C.; Kocik, J.F.; Creath, R.; Crystal, J.S.; Dombrovskiy, V.Y. A comparison of initial lactate and initial base deficit as predictors of mortality after severe blunt trauma. J. Surg. Res. 2016, 205, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.W.; Dirks, R.C.; Kaups, K.L.; Tran, P. Base deficit is superior to lactate in trauma. Am. J. Surg. 2018, 215, 682–685. [Google Scholar] [CrossRef]
- Mutschler, M.; Nienaber, U.; Brockamp, T.; Wafaisade, A.; Fabian, T.; Paffrath, T.; Bouillon, B.; Maegele, M.; Trauma Register DGU. Renaissance of base deficit for the initial assessment of trauma patients: A base deficit-based classification for hypovolemic shock developed on data from 16,305 patients derived from the TraumaRegister DGU®. Crit. Care 2013, 17, R42. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, I.; Chor, W.P.; Chue, K.M.; Tan, C.S.; Tan, H.L.; Siddiqui, F.J.; Hartman, M. Is arterial base deficit still a useful prognostic marker in trauma? A systematic review. Am. J. Emerg. Med. 2016, 34, 626–635. [Google Scholar] [CrossRef]
- Jouffroy, R.; Léguillier, T.; Gilbert, B.; Tourtier, J.P.; Bloch-Laine, E.; Ecollan, P.; Bounes, V.; Boularan, J.; Gueye-Ngalgou, P.; Nivet-Antoine, V.; et al. Pre-Hospital Lactatemia Predicts 30-Day Mortality in Patients with Septic Shock—Preliminary Results from the LAPHSUS Study. J. Clin. Med. 2020, 9, 3290. [Google Scholar] [CrossRef]
- Schork, A.; Moll, K.; Haap, M.; Riessen, R.; Wagner, R. Course of lactate, pH and base excess for prediction of mortality in medical intensive care patients. PLoS ONE 2021, 16, e0261564. [Google Scholar] [CrossRef]
- Hsu, J.-C.; Lee, I.-K.; Huang, W.-C.; Chen, Y.-C.; Tsai, C.-Y. Clinical Characteristics and Predictors of Mortality in Critically Ill Influenza Adult Patients. J. Clin. Med. 2020, 9, 1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, I.; Kumar, P.; Molloy, S.; Rhodes, A.; Newman, P.J.; Grounds, R.M.; Bennett, E.D. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensiv. Care Med. 2001, 27, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Wernly, B.; Heramvand, N.; Masyuk, M.; Rezar, R.; Bruno, R.R.; Kelm, M.; Niederseer, D.; Lichtenauer, M.; Hoppe, U.C.; Bakker, J.; et al. Acidosis predicts mortality independently from hyperlactataemia in patients with sepsis. Eur. J. Intern. Med. 2020, 76, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Lang, W.; Zander, R. The accuracy of calculated base excess in blood. Clin. Chem. Lab. Med. 2002, 40, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.M.; Lan, N.S.H.; Williams, T.A.; Harahsheh, Y.; Chapman, A.R.; Dobb, G.J.; Magder, S. A comparison of prognostic significance of strong ion gap (SIG) with other acid-base markers in the critically ill: A cohort study. J. Intensiv. Care 2016, 4, 43. [Google Scholar] [CrossRef]
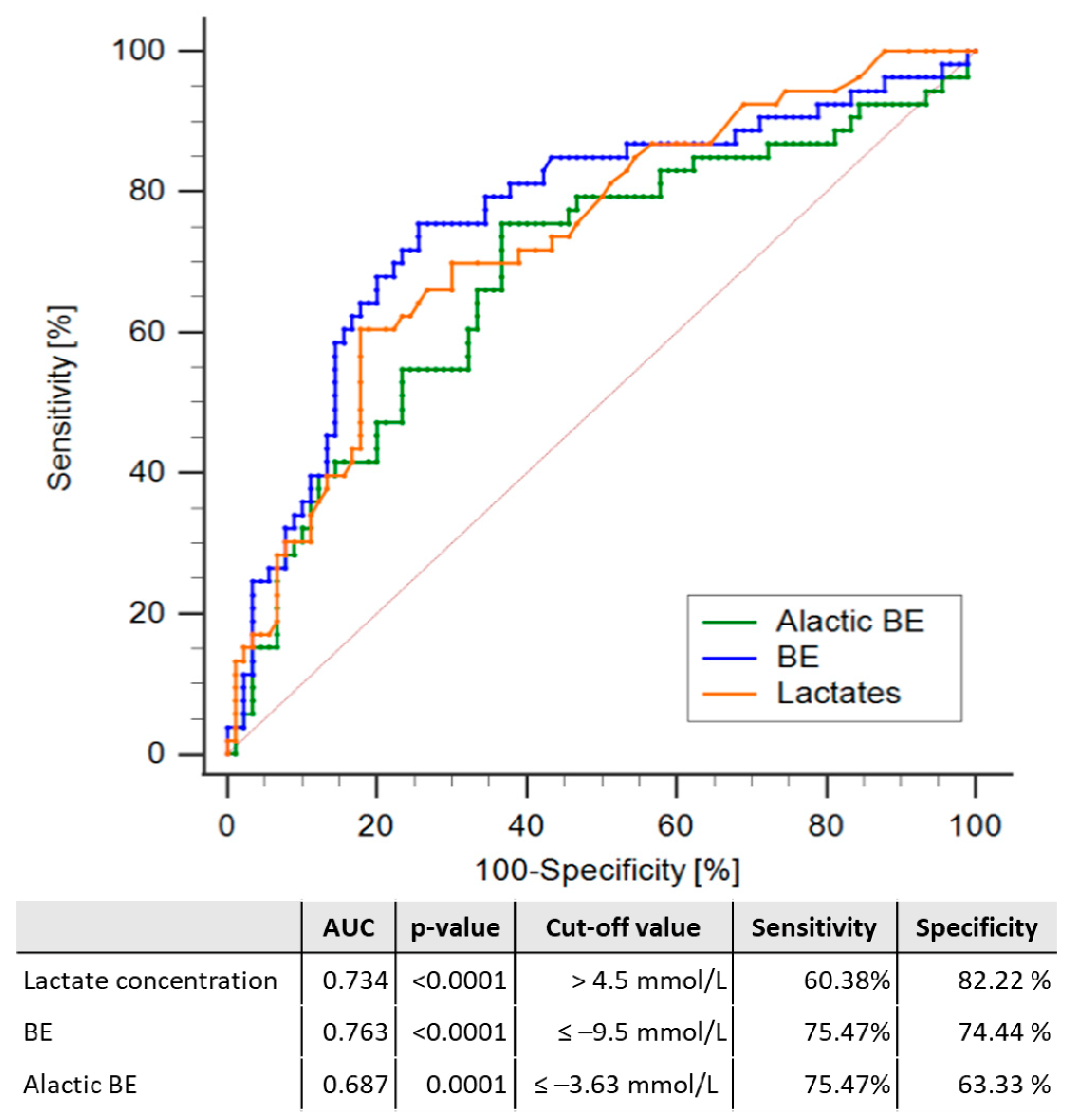


| Qualitative Data | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Survivors | Non-Survivors | # p Value | |||||||||
| Parameter | N | % | N | % | |||||||
| Number of patients | 90 | 63 | 53 | 37 | |||||||
| Men | 65 | 72.2 | 30 | 56.6 | 0.0677 | ||||||
| Cardiogenic shock | 18 | 20.0 | 18 | 34.0 | 0.0743 | ||||||
| Hypovolemic shock | 23 | 25.6 | 5 | 9.4 | 0.0278 | ||||||
| Septic shock | 49 | 54.4 | 30 | 56.6 | 0.8626 | ||||||
| Other shocks | 4 | 4.4 | 3 | 5.7 | 0.7102 | ||||||
| Additional catecholamine | 23 | 25.6 | 27 | 50.9 | 0.0034 | ||||||
| eGFR < 30 mL/min/1.73 m2 | 29 | 32.2 | 20 | 37.7 | 0.5849 | ||||||
| SaO2 < 94% | 28 | 31.1 | 27 | 50.9 | 0.0216 | ||||||
| standard HCO3− < 22 mmol/L | 49 | 54.4 | 46 | 86.8 | <0.0001 | ||||||
| pH < 7.35 | 61 | 67.8 | 45 | 84.9 | 0.0295 | ||||||
| BE < −3 mmol/L | 60 | 66.7 | 46 | 86.8 | 0.0098 | ||||||
| Lactate concentration > 2 mmol/L | 51 | 56.7 | 46 | 86.8 | 0.0002 | ||||||
| Continuous and Discrete Data | |||||||||||
| Survivors | Non-Survivors | ||||||||||
| Parameter | Mean | SD | Median | 25 P. | 75 P. | Mean | SD | Median | 25 P. | 75 P. | p Value |
| Age (years) | 58.42 | 14.54 | 61 | 47 | 69 | 65.51 | 11.85 | 67 | 59 | 72 | 0.0031 |
| APACHE II | 21.91 | 7.49 | 22 | 16.25 | 27 | 31.55 | 7.38 | 32 | 27 | 38 | <0.0001 * |
| SOFA | 11.67 | 3.09 | 12 | 10 | 14 | 14.45 | 2.76 | 15 | 12.75 | 16 | <0.0001 * |
| Length of ICU stay (days) | 9.57 | 8 | 6 | 4 | 14 | 5.78 | 6.41 | 3 | 1 | 10 | 0.6427 * |
| HR (beats/min) | 108.73 | 17.99 | 110 | 95 | 120 | 111.04 | 22.94 | 115 | 100 | 120 | 0.3364 * |
| Systolic BP (mmHg) | 123.69 | 29.60 | 120.00 | 105.00 | 145.00 | 105.111 | 31.8764 | 109.000 | 82.75 | 122.00 | 0.0236 * |
| Diastolic BP (mmHg) | 66.79 | 15.64 | 67.00 | 58.00 | 80.00 | 53.556 | 17.3700 | 56.000 | 40.50 | 64.25 | 0.0026 * |
| Creatinine (mg/dL) | 2.05 | 1.66 | 1.44 | 0.98 | 2.7 | 2.3 | 1.64 | 1.68 | 1.14 | 2.82 | 0.162 * |
| pH | 7.29 | 0.13 | 7.3 | 7.23 | 7.38 | 7.17 | 0.16 | 7.2 | 7.12 | 7.29 | <0.0001 |
| Standard HCO3 (mmol/L) | 21 | 5.46 | 20.95 | 17.7 | 24.2 | 16.1 | 5.79 | 15.7 | 13.28 | 18.25 | <0.0001 |
| Standard BE (mmol/L) | −5.65 | 7.17 | −5.46 | −9.53 | −0.93 | −12.37 | 8.07 | −12.26 | −16.98 | −9.15 | <0.0001 |
| Lactate level (mmol/L) | 3.36 | 3.03 | 2.45 | 1.3 | 3.9 | 6.43 | 4.85 | 5 | 2.58 | 8.3 | <0.0001 * |
| aBE (mmol/L) | −2.29 | 6.13 | −1.93 | −6.24 | 1.44 | −5.93 | 6.39 | −6.46 | −10.19 | −3.36 | 0.001 |
| Stratifying Variable | Non-Survivors | Mortality Rate (%) |
|---|---|---|
| BE > −9.5 mmol/L | 13 | 16.25 |
| BE < −9.5 mmol/L | 40 | 63.49 |
| Lactate concentration < 4.5 mmol/L | 21 | 22.11 |
| Lactate concentration > 4.5 mmol/L | 32 | 66.67 |
| aBE > −3.63 mmol/L | 13 | 18.57 |
| aBE < −3.63 mmol/L | 40 | 54.79 |
| Category 0 (BE > −9.5 mmol/L and lactates < 4.5 mmol/L) | 11 | 15.71 |
| Category 1 (either BE < −9.5 mmol/L or lactates > 4.5 mmol/L) | 12 | 21.81 |
| Category 2 (both BE > −9.5 mmol/L and lactates < 4.5 mmol/L) | 30 | 78.94 |
| Unadjusted Model | Adjusted Model | |||||
|---|---|---|---|---|---|---|
| Stratifying Variable | HR | 95% CI | p Value | HR | 95% CI | p Value |
| BE < −9.5 mmol/L | 4.26 | 2.27–7.98 | <0.0001 | 4.22 | 2.21–8.05 | <0.0001 |
| Lactate concentration > 4.5 mmol/L | 3.58 | 2.05–6.23 | <0.0001 | 4.62 | 2.56–8.33 | <0.0001 |
| aBE < −3.63 mmol/L | 3.09 | 1.65–5.78 | 0.0004 | 3.19 | 1.62–6.27 | 0.0008 |
| Change of one category | 2.68 | 1.88–3.84 | <0.0001 | 2.78 | 1.94–4.01 | <0.0001 |
| Creatinine Concentration | eGFR | |||
|---|---|---|---|---|
| Parameters | Rho | p Value | rho | p Value |
| BE | −0.29 | 0.0004 | 0.31 | 0.0002 |
| Lactate concentration | 0.02 | 0.7952 | −0.03 | 0.7216 |
| aBE | −0.36 | 0.0000 | 0.37 | 0.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smuszkiewicz, P.; Jawień, N.; Szrama, J.; Lubarska, M.; Kusza, K.; Guzik, P. Admission Lactate Concentration, Base Excess, and Alactic Base Excess Predict the 28-Day Inward Mortality in Shock Patients. J. Clin. Med. 2022, 11, 6125. https://doi.org/10.3390/jcm11206125
Smuszkiewicz P, Jawień N, Szrama J, Lubarska M, Kusza K, Guzik P. Admission Lactate Concentration, Base Excess, and Alactic Base Excess Predict the 28-Day Inward Mortality in Shock Patients. Journal of Clinical Medicine. 2022; 11(20):6125. https://doi.org/10.3390/jcm11206125
Chicago/Turabian StyleSmuszkiewicz, Piotr, Natalia Jawień, Jakub Szrama, Marta Lubarska, Krzysztof Kusza, and Przemysław Guzik. 2022. "Admission Lactate Concentration, Base Excess, and Alactic Base Excess Predict the 28-Day Inward Mortality in Shock Patients" Journal of Clinical Medicine 11, no. 20: 6125. https://doi.org/10.3390/jcm11206125
APA StyleSmuszkiewicz, P., Jawień, N., Szrama, J., Lubarska, M., Kusza, K., & Guzik, P. (2022). Admission Lactate Concentration, Base Excess, and Alactic Base Excess Predict the 28-Day Inward Mortality in Shock Patients. Journal of Clinical Medicine, 11(20), 6125. https://doi.org/10.3390/jcm11206125






