A Case Series of Deep Transcranial Magnetic Stimulation Treatment for Patients with Obsessive-Compulsive Disorder in the Tokyo Metropolitan Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
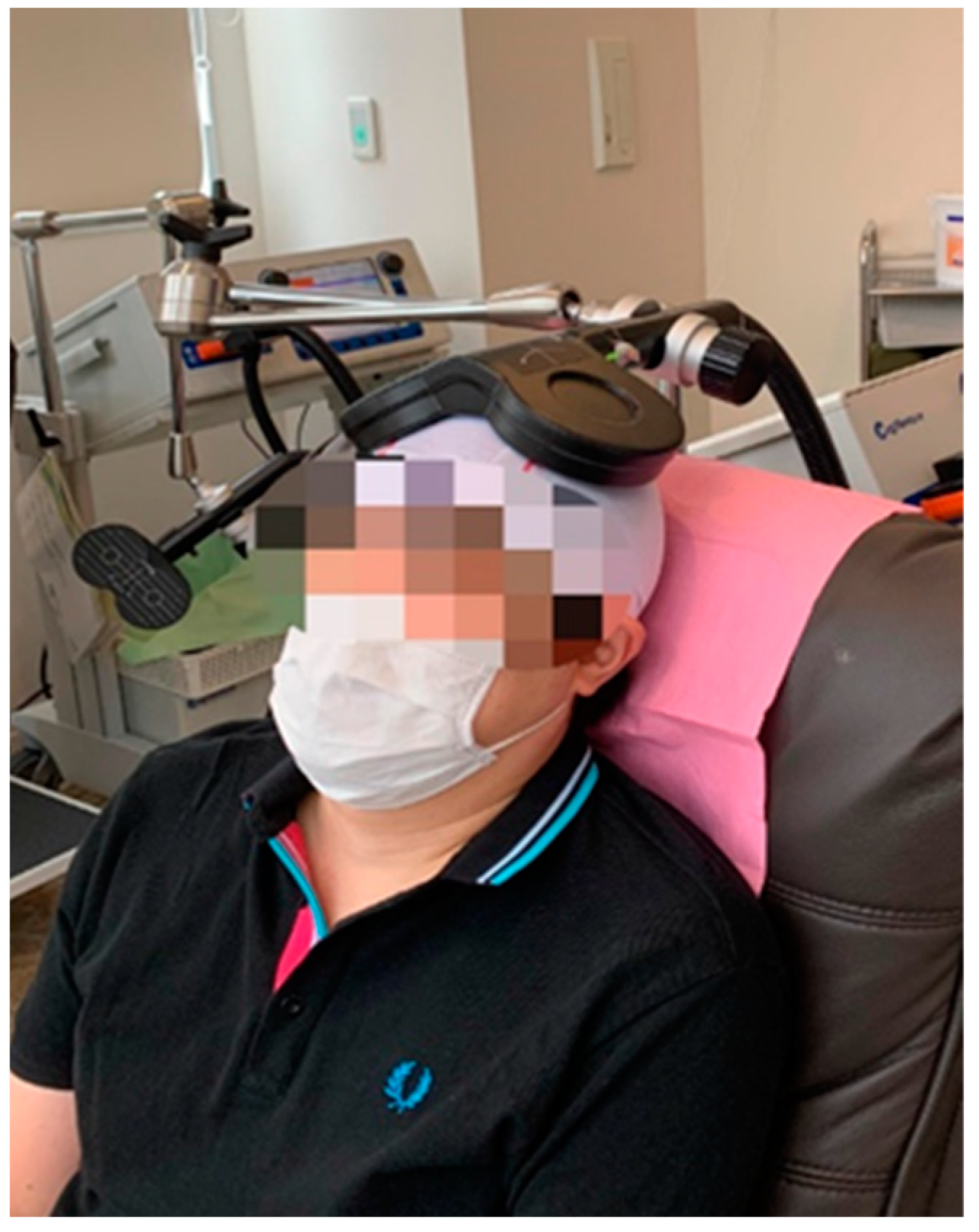
2.2. dTMS Protocol
2.3. Resting Motor Threshold (RMT) Measurement
2.4. Individualized Exposure Stimulation
2.5. Clinical Assessment
2.6. Sub-Analysis Based on Clinical Epidemiological Information
2.7. Statistical Analysis
2.8. Assessment of Adverse Events
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruscio, A.M.; Stein, D.; Chiu, W.T.; Kessler, R.C. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry 2010, 15, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlingon, VA, USA, 2013. [Google Scholar]
- Simpson, H.B.; Huppert, J.; Petkova, E.; Foa, E.B.; Liebowitz, M.R. Response Versus Remission in Obsessive-Compulsive Disorder. J. Clin. Psychiatry 2006, 67, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.D.; Welsh, R.C.; Gehring, W.J.; Abelson, J.L.; Himle, J.A.; Liberzon, I.; Taylor, S.F. Error-related hyperactivity of the anterior cingulate cortex in obses-sive-compulsive disorder. Biol. Psychiatry 2005, 57, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.J.; Römmler, J.; Ehlis, A.-C.; Heidrich, A.; Fallgatter, A.J. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe). Cogn. Brain Res. 2004, 20, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Kammen, A.; Cavaleri, J.; Lam, J.; Frank, A.C.; Mason, X.; Choi, W.; Penn, M.; Brasfield, K.; Van Noppen, B.; Murray, S.B.; et al. Neuromodulation of OCD: A review of invasive and non-invasive methods. Front. Neurol. 2022, 13, 909264. [Google Scholar] [CrossRef] [PubMed]
- Gremel, C.M.; Costa, R.M. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat. Commun. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmi, L.; Alyagon, U.; Barnea-Ygael, N.; Zohar, J.; Dar, R.; Zangen, A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018, 11, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmi, L.; Tendler, A.; Bystritsky, A.; Hollander, E.; Blumberger, D.M.; Daskalakis, J.; Ward, H.; Lapidus, K.; Goodman, W.; Casuto, L.; et al. Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obses-sive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. Am. J. Psychiatry 2019, 176, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Kizaki, J.; Takahashi, S.; Mimura, M. TMS Database Registry Consortium Research Project in Japan (TReC-J) for Future Personalized Psychiatry. J. Pers. Med. 2022, 12, 844. [Google Scholar] [CrossRef] [PubMed]
- Tolin, D.F.; Abramowitz, J.S.; Diefenbach, G.J. Defining response in clinical trials for obsessive-compulsive disorder: A signal detection analysis of the Yale-Brown obsessive compulsive scale. J. Clin. Psychiatry 2005, 66, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Roth, Y.; Tendler, A.; Arikan, M.K.; Vidrine, R.; Kent, D.; Muir, O.; MacMillan, C.; Casuto, L.; Grammer, G.; Sauve, W.; et al. Real-world efficacy of deep TMS for obsessive-compulsive disorder: Post-marketing data collected from twenty-two clinical sites. J. Psychiatr. Res. 2020, 137, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Jonas, W.B. The Myth of the Placebo Response. Front. Psychiatry 2019, 10, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Dysken, M.W.; Pheley, A.M.; Hoover, K.M. The Yale-Brown Obsessive-Compulsive Scale: Measures of internal con-sistency. Psychiatry Res. 1994, 51, 203–211. [Google Scholar] [CrossRef]
- Laposa, J.M.; Hawley, L.L.; Grimm, K.J.; Katz, D.E.; Rector, N.A. What Drives OCD Symptom Change During CBT Treatment? Temporal Relationships Among Obsessions and Compulsions. Behav. Ther. 2019, 50, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Rostami, R.; Kazemi, R.; Jabbari, A.; Madani, A.S.; Rostami, H.; Taherpour, M.A.; Molavi, P.; Jaafari, N.; Kuo, M.-F.; Vicario, C.M.; et al. Efficacy and clinical predictors of response to rTMS treatment in pharmacoresistant obsessive-compulsive disorder (OCD): A retrospective study. BMC Psychiatry 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, S.M.D.D.; van der Werf, Y.D.; van Campen, A.D.; Arns, M.; Sack, A.T.; Hoogendoorn, A.W.; van Balkom, A.J.L.M.; Batelaan, N.M.; Eijndhoven, P.; Hendriks, G.-J.; et al. Repetitive transcranial magnetic stimulation for obsessive-compulsive disorder: A systematic review and pairwise/network meta-analysis. J. Affect. Disord. 2022, 302, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Li, H.; Bu, X.; Li, X.; Cao, L.; Liu, J.; Gao, Y.; Li, B.; Qiu, C.; Bao, W.; et al. Efficacy and tolerability of repetitive transcranial magnetic stimulation for the treatment of obsessive-compulsive disorder in adults: A systematic review and network meta-analysis. Transl. Psychiatry 2021, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, L.; Zalesky, A.; Nott, Z.; Whybird, G.; Fitzgerald, P.B.; Breakspear, M. Transcranial magnetic stimulation in obsessive-compulsive disorder: A focus on network mechanisms and state dependence. NeuroImage Clin. 2018, 19, 661–674. [Google Scholar] [CrossRef]

| Sample Size | 26 | ||
|---|---|---|---|
| Age (mean ± S.D.) | 35.8 (12) | ||
| Sex | Male | 14 | 54 |
| Female | 12 | 46 | |
| %patients on medication | 14 | 53.9% |
| Case | Medication Treatment | Duration of OCD (Years) | Duration of Medication (Years) | Comorbidity |
|---|---|---|---|---|
| 1 | Paroxetine CR 50 mg, Mirtazapine 15 mg, Clonazepam 2 mg, Cloxazolam 1 mg | 25 | Details unknown | - |
| 2 | Lexapro 10 mg | 4 | 4 | - |
| 3 | Maprotiline 100 mg, Vortioxetine 20 mg | 30 | 3 | MDD |
| 4 | - | 20 | 0 | MDD |
| 5 | - | 1 | 1 | - |
| 6 | Paroxetine CR 25 mg, Alprazolam 0.4 mg | 9 | 1 | - |
| 7 | - | 12 | 0 | MDD |
| 8 | - | 6 | 0 | - |
| 9 | Fluvoxamine 150 mg, Alprazolam 0.8 mg, Risperidone 1 mg, Lithium 1000 mg, Brotizolam 0.25 mg | Details unknown | Details unknown | BD |
| 10 | Paroxetine CR 12.5 mg, Aripiprazole 3 mg | 4 | 1 | - |
| 11 | Lamotrigine 150 mg, Valproate 100 mg, Bromazepam 5 mg | 1 | 1 | MDD |
| 12 | Paroxetine 25 mg | 9 | 1 | ADHD |
| 13 | - | 1 | 0 | - |
| 14 | - | 1 | 0 | - |
| 15 | - | 33 | Details unknown | - |
| 16 | Lexapro 10 mg, Brexpiprazole 0.5 mg, Mirtazapine 15 mg | 4 | 4 | - |
| 17 | - | 19 | 17 | SAD |
| 18 | Paroxetine 45 mg, Lithium 600 mg | Details unknown | Details unknown | BD |
| 19 | Fluvoxamine 150 mg | 13 | 1 | - |
| 20 | - | Details unknown | Details unknown | - |
| 21 | - | 11 | 3 | MDD |
| 22 | Fluvoxamine 300 mg, Risperidone1 mg | 1 | 1 | - |
| 23 | - | 1 | 0 | - |
| 24 | - | 10 | Details unknown | MDD |
| 25 | Paroxetine 50 mg, Zopiclone 10 mg | 47 | 10 | - |
| 26 | Clomipramine 75 mg, Fluvoxamine 100 mg, Bromazepam 15 mg | 27 | 27 | MDD |
| Mean (S.D.) | t-Value | p-Value | ||
|---|---|---|---|---|
| Pre-dTMS | Post-dTMS | |||
| Total score of the Y-BOCS | 28.5 (5.0) | 20. (6.67) | 7.12 | * p < 0.01 |
| Individual items on the Y-BOCS factors | ||||
| 1. Time occupied by obsession | 3.0 (1.0) | 2.2 (0.8) | 3.98 | * p < 0.01 |
| 2. Interference due to obsessive thoughts | 2.8 (0.8) | 2.1 (0.7) | 3.94 | * p < 0.01 |
| 3. Distress associated with obsessions | 3.3 (0.8) | 2.4 (0.8) | 5.12 | * p < 0.01 |
| 4. Resistance against obsessions | 2.5 (1.4) | 1.7 (1.3) | 2.27 | p = 0.32 |
| 5. Control over obsessions | 3.3 (0.9) | 2.4 (0.9) | 4.67 | * p < 0.01 |
| 6. Time spent on compulsions | 2.4 (0.8) | 1.8 (0.9) | 3.89 | * p < 0.01 |
| 7. Interference due to compulsions | 2.5 (0.7) | 1.9 (0.9) | 3.56 | p = 0.02 |
| 8. Distress associated with compulsions | 3.3 (0.9) | 2.5 (0.9) | 4.54 | * p < 0.01 |
| 9. Resistance against compulsions | 2.5 (1.3) | 1.4 (1.2) | 3.58 | * p < 0.01 |
| 10. Control over compulsions | 3.1 (0.8) | 2.3 (1.0) | 4.39 | * p < 0.01 |
| Responder (improvement of >30%) | 14/26 = 53.9% | |||
| Whole | Males | Females | ||
|---|---|---|---|---|
| % changes of the Y-BOCS score | mean (S.D.) | 29.8 (4.0) | 24.9 (24.6) | 35.5 (13.4) |
| p-value | - | p = 0.20 | ||
| On-medication | Off-medication | |||
| mean (S.D.) | 29.8 (4.0) | 26.5 (23.3) | 33.5 (17.1) | |
| p-value | - | p = 0.40 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikawa, H.; Osawa, R.; Sato, A.; Mizuno, H.; Noda, Y. A Case Series of Deep Transcranial Magnetic Stimulation Treatment for Patients with Obsessive-Compulsive Disorder in the Tokyo Metropolitan Area. J. Clin. Med. 2022, 11, 6133. https://doi.org/10.3390/jcm11206133
Ikawa H, Osawa R, Sato A, Mizuno H, Noda Y. A Case Series of Deep Transcranial Magnetic Stimulation Treatment for Patients with Obsessive-Compulsive Disorder in the Tokyo Metropolitan Area. Journal of Clinical Medicine. 2022; 11(20):6133. https://doi.org/10.3390/jcm11206133
Chicago/Turabian StyleIkawa, Haruki, Ryota Osawa, Akiko Sato, Hoshimi Mizuno, and Yoshihiro Noda. 2022. "A Case Series of Deep Transcranial Magnetic Stimulation Treatment for Patients with Obsessive-Compulsive Disorder in the Tokyo Metropolitan Area" Journal of Clinical Medicine 11, no. 20: 6133. https://doi.org/10.3390/jcm11206133
APA StyleIkawa, H., Osawa, R., Sato, A., Mizuno, H., & Noda, Y. (2022). A Case Series of Deep Transcranial Magnetic Stimulation Treatment for Patients with Obsessive-Compulsive Disorder in the Tokyo Metropolitan Area. Journal of Clinical Medicine, 11(20), 6133. https://doi.org/10.3390/jcm11206133







