Early Outpatient Treatment of COVID-19: A Retrospective Analysis of 392 Cases in Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Procedures
2.3. Questionnaire for Participating Doctors
2.4. Statistical Analysis
3. Results
3.1. Characteristics of COVID-19 Patients
3.2. Drug Prescriptions
3.3. Outcomes
3.4. Doctors’ Attitudes towards the Use of Drugs in COVID-19
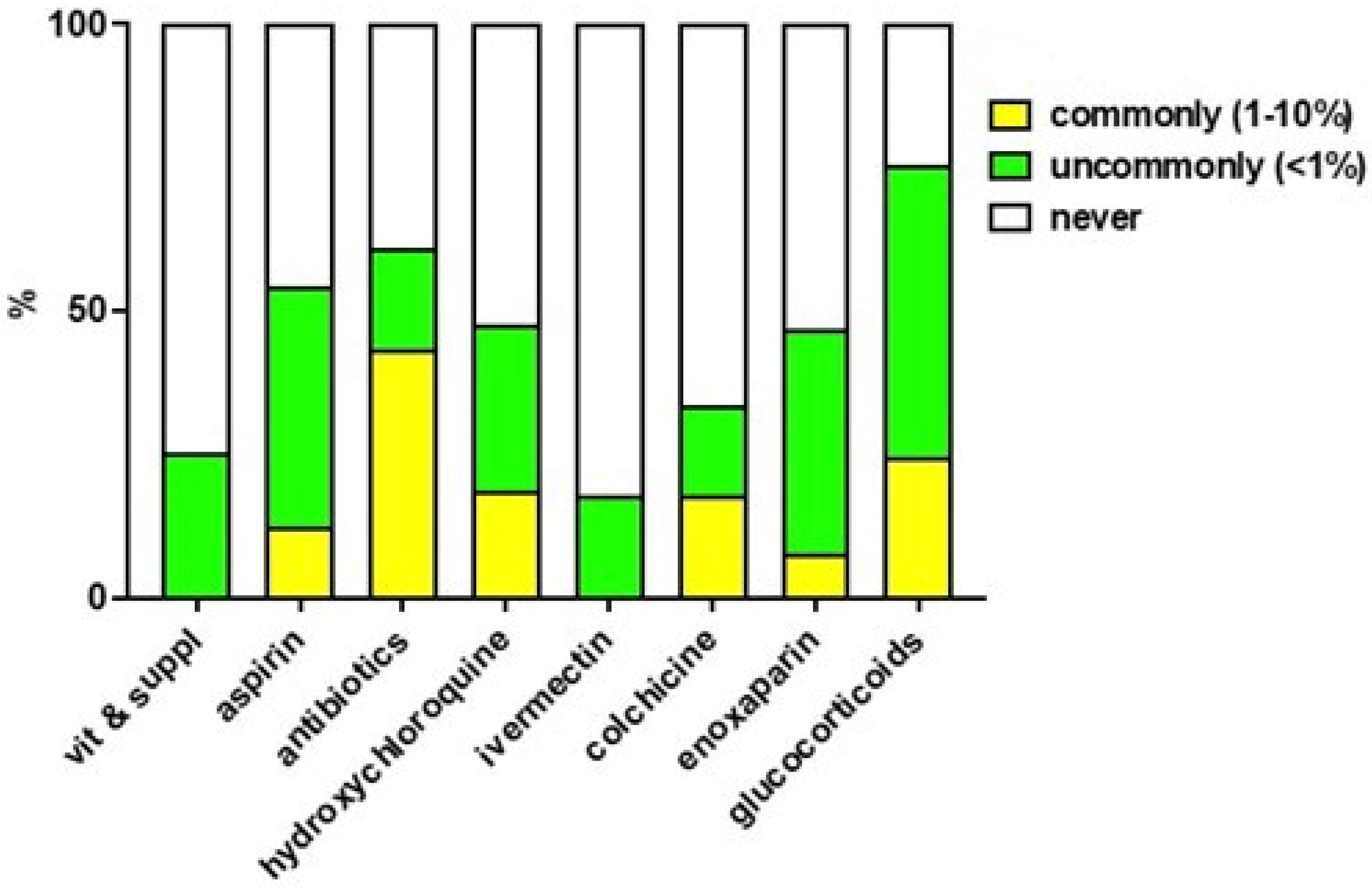
3.5. Perception and Reporting of Adverse Reactions to Drugs Used to Treat COVID-19
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- World Health Organization WHO Announces COVID-19 Outbreak a Pandemic. Available online: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic (accessed on 3 October 2022).
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What Next? Lancet 2020, 395, 1225–1228. [Google Scholar]
- Ministero Della Salute CIRCOLARE Del 08/04/2020 Indicazioni Emergenziali Connesse Ad Epidemia COVID-19 Riguardanti Il Settore Funebre, Cimiteriale e Di Cremazione. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2020&codLeg=73832&parte=1%20&serie=null (accessed on 3 October 2022).
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary Post-Mortem Findings in a Series of COVID-19 Cases from Northern Italy: A Two-Centre Descriptive Study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar]
- Ministero della Salute CIRCOLARE Del 26/04/2021. Gestione Domiciliare Dei Pazienti Con Infezione Da SARS-CoV2 Aggiornata al 26 Aprile 2021. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=80056&parte=1%20&serie=null (accessed on 3 October 2022).
- AIFA Idrossiclorochina Nella Terapia dei Pazienti Adulti con COVID-19—Update del 22 Luglio 2020. Available online: https://www.aifa.gov.it/documents/20142/1123276/idrossiclorochina_22.07.2020.pdf/764add8f-f08f-0e26-df75-952986e54b8b (accessed on 21 September 2021).
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J. Heart Lung Transpl. 2020, 39, 405–407. [Google Scholar]
- Turk, C.; Turk, S.; Malkan, U.Y.; Haznedaroglu, I.C. Three Critical Clinicobiological Phases of the Human SARS-Associated Coronavirus Infections. Eur. Rev. Med. Pharm. Sci. 2020, 24, 8606–8620. [Google Scholar]
- Cordon-Cardo, C.; Pujadas, E.; Wajnberg, A.; Sebra, R.; Patel, G.; Firpo-Betancourt, A.; Fowkes, M.; Sordillo, E.; Paniz-Mondolfi, A.; Gregory, J.; et al. COVID-19: Staging of a New Disease. Cancer Cell 2020, 38, 594–597. [Google Scholar]
- Rango, M. Guarire Il COVID-19 a Casa: Manuale per Terapia Domiciliare Personalizzata; Independently published; 2021; ISBN 979-8717253413. [Google Scholar]
- Derwand, R.; Scholz, M.; Zelenko, V. COVID-19 outpatients: Early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: A retrospective case series study. Int. J. Antimicrob. Agents 2020, 56, 106214. [Google Scholar] [CrossRef]
- McCullough, P.A.; Alexander, P.E.; Armstrong, R.; Arvinte, C.; Bain, A.F.; Bartlett, R.P.; Berkowitz, R.L.; Berry, A.C.; Borody, T.J.; Brewer, J.H.; et al. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19). Rev. Cardiovasc. Med. 2020, 21, 517–530. [Google Scholar]
- Schwartz, I.; Boesen, M.E.; Cerchiaro, G.; Doram, C.; Edwards, B.D.; Ganesh, A.; Greenfield, J.; Jamieson, S.; Karnik, V.; Kenney, C.; et al. Assessing the efficacy and safety of hydroxychloroquine as outpatient treatment of COVID-19: A randomized controlled trial. CMAJ Open 2021, 9, E693–E702. [Google Scholar]
- Reis, G.; Moreira Silva, E.A.D.S.; Medeiros Silva, D.C.; Thabane, L.; Singh, G.; Park, J.J.H.; Forrest, J.I.; Harari, O.; Quirino Dos Santos, C.V.; Almeida, A.P.F.; et al. Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19: The TOGETHER Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e216468. [Google Scholar]
- Tardif, J.-C.; Bouabdallaoui, N.; L’Allier, P.L.; Gaudet, D.; Shah, B.; Pillinger, M.H.; Lopez-Sendon, J.; da Luz, P.; Verret, L.; Audet, S.; et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): A phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir. Med. 2021, 9, 924–932. [Google Scholar] [CrossRef]
- Oldenburg, C.E.; Pinsky, B.A.; Brogdon, J.; Chen, C.; Ruder, K.; Zhong, L.; Nyatigo, F.; Cook, C.A.; Hinterwirth, A.; Lebas, E.; et al. Effect of Oral Azithromycin vs Placebo on COVID-19 Symptoms in Outpatients with SARS-CoV-2 Infection: A Randomized Clinical Trial. JAMA 2021, 326, 490–498. [Google Scholar]
- Hinks, T.S.C.; Cureton, L.; Knight, R.; Wang, A.; Cane, J.L.; Barber, V.S.; Black, J.; Dutton, S.J.; Melhorn, J.; Jabeen, M.; et al. Azithromycin versus standard care in patients with mild-to-moderate COVID-19 (ATOMIC2): An open-label, randomised trial. Lancet Respir. Med. 2021, 9, 1130–1140. [Google Scholar]
- Guzman-Esquivel, J.; Galvan-Salazar, H.R.; Guzman-Solorzano, H.P.; Cuevas-Velazquez, A.C.; Guzman-Solorzano, J.A.; Mokay-Ramirez, K.A.; Paz-Michel, B.A.; Murillo-Zamora, E.; Delgado-Enciso, J.; Melnikov, V.; et al. Efficacy of the Use of Mefenamic Acid Combined with Standard Medical Care vs. Standard Medical Care Alone for the Treatment of COVID-19: A Randomized Double-Blind Placebo-Controlled Trial. J. Mol. Med. 2022, 49, 1–9. [Google Scholar]
- Clemency, B.M.; Varughese, R.; Gonzalez-Rojas, Y.; Morse, C.G.; Phipatanakul, W.; Koster, D.J.; Blaiss, M.S. Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 42–49. [Google Scholar]
- Ezer, N.; Belga, S.; Daneman, N.; Chan, A.; Smith, B.M.; Daniels, S.A.; Moran, K.; Besson, C.; Smyth, L.Y.; Bartlett, S.J.; et al. Inhaled and intranasal ciclesonide for the treatment of covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial. BMJ 2021, 375, e068060. [Google Scholar]
- Ramakrishnan, S.; Nicolau, D.V.; Langford, B.; Mahdi, M.; Jeffers, H.; Mwasuku, C.; Krassowska, K.; Fox, R.; Binnian, I.; Glover, V.; et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): A phase 2, open-label, randomised controlled trial. Lancet Respir. Med. 2021, 9, 763–772. [Google Scholar]
- Roozbeh, F.; Saeedi, M.; Alizadeh-Navaei, R.; Hedayatizadeh-Omran, A.; Merat, S.; Wentzel, H.; Levi, J.; Hill, A.; Shamshirian, A. Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: A double-blind, randomized controlled trial. J. Antimicrob. Chemother. 2020, 76, 753–757. [Google Scholar]
- Connors, J.M.; Brooks, M.M.; Sciurba, F.C.; Krishnan, J.A.; Bledsoe, J.R.; Kindzelski, A.; Baucom, A.L.; Kirwan, B.-A.; Eng, H.; Martin, D.; et al. Effect of Antithrombotic Therapy on Clinical Outcomes in Outpatients With Clinically Stable Symptomatic COVID-19: The ACTIV-4B Randomized Clinical Trial. JAMA 2021, 326, 1703–1712. [Google Scholar] [CrossRef]
- Korley, F.K.; Durkalski-Mauldin, V.; Yeatts, S.D.; Schulman, K.; Davenport, R.D.; Dumont, L.J.; El Kassar, N.; Foster, L.D.; Hah, J.M.; Jaiswal, S.; et al. Early Convalescent Plasma for High-Risk Outpatients with Covid-19. N. Engl. J. Med. 2021, 385, 1951–1960. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Feld, J.J.; Kandel, C.; Biondi, M.J.; Kozak, R.A.; Zahoor, M.A.; Lemieux, C.; Borgia, S.M.; Boggild, A.K.; Powis, J.; McCready, J.; et al. Peginterferon lambda for the treatment of outpatients with COVID-19: A phase 2, placebo-controlled randomised trial. Lancet Respir. Med. 2021, 9, 498–510. [Google Scholar]
- Ochoa, A.J.G.; Raffetto, J.D.; Hernández, A.G.; Zavala, N.; Gutiérrez, O.; Vargas, A.; Loustaunau, J. Sulodexide in the Treatment of Patients with Early Stages of COVID-19: A Randomized Controlled Trial. Thromb. Haemost. 2021, 121, 944–954. [Google Scholar] [CrossRef]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e210369. [Google Scholar]
- Rodrigues, C.; Freitas-Santos, R.S.; Levi, J.E.; Senerchia, A.A.; Lopes, A.T.A.; Santos, S.R.; Siciliano, R.F.; Pierrotti, L.C. Hydroxychloroquine plus azithromycin early treatment of mild COVID-19 in an outpatient setting: A randomized, double-blinded, placebo-controlled clinical trial evaluating viral clearance. Int. J. Antimicrob. Agents 2021, 58, 106428. [Google Scholar]
- Fazio, S.; Bellavite, P.; Zanolin, E.; McCullough, P.A.; Pandolfi, S.; Affuso, F. Retrospective Study of Outcomes and Hospitalization Rates of Patients in Italy with a Confirmed Diagnosis of Early COVID-19 and Treated at Home within 3 Days or after 3 Days of Symptom Onset with Prescribed and Non-Prescribed Treatments Between November 2020 and August 2021. Med. Sci. Monit. 2021, 28, e935379. [Google Scholar]
- Suter, F.; Consolaro, E.; Pedroni, S.; Moroni, C.; Pastò, E.; Paganini, M.V.; Pravettoni, G.; Cantarelli, U.; Rubis, N.; Perico, N.; et al. A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: A retrospective observational matched-cohort study. eClinicalMedicine 2021, 37, 100941. [Google Scholar]
- McCullough, P.A.; Kelly, R.J.; Ruocco, G.; Lerma, E.; Tumlin, J.; Wheelan, K.R.; Katz, N.; Lepor, N.E.; Vijay, K.; Carter, H.; et al. Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection. Am. J. Med. 2021, 134, 16–22. [Google Scholar]
- Alliance, F.L.C.C.C. I-MASK+—Prevention & Early Outpatient Treatment Protocol for COVID-19. Version 2022, 19, 1–4. [Google Scholar]
- The Frohman Foundation. Outpatient COVID-19 Treatment to Prevent Severe Disease. Available online: https://thefrohmanfoundation.org/research/covid-19-treatment/outpatient-covid-19-treatment-to-prevent-severe-disease/ (accessed on 3 October 2022).
- Fabiani, M.; Onder, G.; Boros, S.; Spuri, M.; Minelli, G.; Urdiales, A.M.; Andrianou, X.; Riccardo, F.; Del Manso, M.; Petrone, D.; et al. Il case fatality rate dell’infezione SARS-CoV-2 a livello regionale e attraverso le differenti fasi dell’epidemia in Italia. Versione del 20 gennaio 2021. Ist. Super. Sanità 2021, 51, 15. [Google Scholar]
- Task Force COVID-19 del Dipartimento Malattie Infettive e Servizio di Informatica, Istituto Superiore di Sanità Epidemia COVID-19. Aggiornamento Nazionale: 28 Aprile 2021. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_28-aprile-2021.pdf (accessed on 3 October 2022).
- Million, M.; Lagier, J.C.; Tissot-Dupont, H.; Ravaux, I.; Dhiver, C.; Tomei, C.; Cassir, N.; Delorme, L.; Cortaredona, S.; Amrane, S.; et al. Early combination therapy with hydroxychloroquine and azithromycin reduces mortality in 10,429 COVID-19 outpatients. Rev. Cardiovasc. Med. 2021, 22, 1063–1072. [Google Scholar]
- Bae, M.; Kim, H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence That Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Xu, Y.; Baylink, D.J.; Chen, C.-S.; Reeves, M.E.; Xiao, J.; Lacy, C.; Lau, E.; Cao, H. The Importance of Vitamin d Metabolism as a Potential Prophylactic, Immunoregulatory and Neuroprotective Treatment for COVID-19. J. Transl. Med. 2020, 18, 322. [Google Scholar] [CrossRef]
- Vitamin D for COVID-19: Real-Time Analysis of All 315 Studies. Available online: https://c19vitamind.com/ (accessed on 17 October 2022).
- Holford, P.; Carr, A.C.; Jovic, T.H.; Ali, S.R.; Whitaker, I.S.; Marik, P.E.; Smith, A.D. Vitamin C—An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients 2020, 12, 3760. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Vitamin C for COVID-19: Real-Time Analysis of All 86 Studies. Available online: https://c19early.com/ (accessed on 17 October 2022).
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef]
- Skalny, A.V.; Rink, L.; Ajsuvakova, O.P.; Aschner, M.; Gritsenko, V.A.; Alekseenko, S.I.; Svistunov, A.A.; Petrakis, D.; Spandidos, D.A.; Aaseth, J.; et al. Zinc and Respiratory Tract Infections: Perspectives for COVID-19 (Review). Int. J. Mol. Med. 2020, 46, 17–26. [Google Scholar] [CrossRef]
- Zinc for COVID-19: Real-Time Analysis of All 90 Studies. Available online: https://c19zinc.com/ (accessed on 17 October 2022).
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New Light on the Healthy Function of Citrus Fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential Roles of Medicinal Plants for the Treatment of Viral Diseases Focusing on COVID-19: A Review. Phytother. Res. 2021, 35, 1298–1312. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Di Pierro, F. A Role for Quercetin in Coronavirus Disease 2019 (COVID-19). Phytother. Res. 2021, 35, 1230–1236. [Google Scholar] [CrossRef]
- Quercetin for COVID-19: Real-Time Analysis of All 22 Studies. Available online: https://c19quercetin.com/ (accessed on 17 October 2022).
- Wang, Y.; Wang, P.; Wang, H.; Luo, Y.; Wan, L.; Jiang, M.; Chu, Y. Lactoferrin for the Treatment of COVID-19 (Review). Exp. Ther. Med. 2020, 20, 272. [Google Scholar] [CrossRef]
- Chang, R.; Ng, T.B.; Sun, W.-Z. Lactoferrin as Potential Preventative and Adjunct Treatment for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106118. [Google Scholar] [CrossRef]
- Depfenhart, M.; de Villiers, D.; Lemperle, G.; Meyer, M.; Di Somma, S. Potential New Treatment Strategies for COVID-19: Is There a Role for Bromhexine as Add-on Therapy? Intern. Emerg. Med. 2020, 15, 801–812. [Google Scholar] [CrossRef]
- Brendler, T.; Al-Harrasi, A.; Bauer, R.; Gafner, S.; Hardy, M.L.; Heinrich, M.; Hosseinzadeh, H.; Izzo, A.A.; Michaelis, M.; Nassiri-Asl, M.; et al. Botanical Drugs and Supplements Affecting the Immune Response in the Time of COVID-19: Implications for Research and Clinical Practice. Phytother. Res. 2021, 35, 3013–3031. [Google Scholar] [CrossRef]
- Kiani, A.K.; Dhuli, K.; Anpilogov, K.; Bressan, S.; Dautaj, A.; Dundar, M.; Beccari, T.; Ergoren, M.C.; Bertelli, M. Natural Compounds as Inhibitors of SARS-CoV-2 Endocytosis: A Promising Approach against COVID-19. Acta Biomed. 2020, 91, e2020008. [Google Scholar] [CrossRef]
- Carrouel, F.; Gonçalves, L.S.; Conte, M.P.; Campus, G.; Fisher, J.; Fraticelli, L.; Gadea-Deschamps, E.; Ottolenghi, L.; Bourgeois, D. Antiviral Activity of Reagents in Mouth Rinses against SARS-CoV-2. J. Dent. Res. 2021, 100, 124–132. [Google Scholar] [CrossRef]
- Trasino, S.E. A Role for Retinoids in the Treatment of COVID-19? Clin. Exp. Pharmacol. Physiol. 2020, 47, 1765–1767. [Google Scholar] [CrossRef]
- Midha, I.K.; Kumar, N.; Kumar, A.; Madan, T. Mega Doses of Retinol: A Possible Immunomodulation in Covid-19 Illness in Resource-Limited Settings. Rev. Med. Virol. 2021, 31, 1–14. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic Potential of Resveratrol against Emerging Respiratory Viral Infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef]
- Bianconi, V.; Violi, F.; Fallarino, F.; Pignatelli, P.; Sahebkar, A.; Pirro, M. Is Acetylsalicylic Acid a Safe and Potentially Useful Choice for Adult Patients with COVID-19? Drugs 2020, 80, 1383–1396. [Google Scholar] [CrossRef]
- Cacciapuoti, F.; Cacciapuoti, F. Could Low Doses Acetylsalicylic Acid Prevent Thrombotic Complications in COVID-19 Patients? Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211014592. [Google Scholar] [CrossRef]
- Aspirin for COVID-19: Real-Time Analysis of All 61 Studies. Available online: https://c19aspirin.com/ (accessed on 17 October 2022).
- Kory, P.; Meduri, G.U.; Varon, J.; Iglesias, J.; Marik, P.E. Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19. Am. J. Ther. 2021, 28, e299–e318. [Google Scholar] [CrossRef]
- Marik, P.E.; Kory, P. Ivermectin, A Reanalysis of the Data. Am. J. Ther. 2021, 28, e579–e580. [Google Scholar] [CrossRef]
- Ivermectin for COVID-19: Real-Time Analysis of All 185 Studies. Available online: https://c19ivermectin.com/ (accessed on 17 October 2022).
- Million, M.; Roussel, Y.; Gautret, P.; Raoult, D. Effect of Hydroxychloroquine and Azithromycin on SARS-CoV-2 Clearance in COVID-19 Patients, a Meta-Analysis. Int. J. Antimicrob. Agents 2021, 57, 106240. [Google Scholar] [CrossRef]
- Nina, P.B.; Dash, A.P. Hydroxychloroquine as Prophylaxis or Treatment for COVID-19: What Does the Evidence Say? Indian J. Public Health 2020, 64, S125–S127. [Google Scholar] [CrossRef]
- Sinha, N.; Balayla, G. Hydroxychloroquine and COVID-19. Postgrad. Med. J. 2020, 96, 550–555. [Google Scholar] [CrossRef]
- HCQ for COVID-19: Real-Time Analysis of All 444 Studies. Available online: https://c19hcq.com/ (accessed on 17 October 2022).
- Echeverría-Esnal, D.; Martin-Ontiyuelo, C.; Navarrete-Rouco, M.E.; De-Antonio Cuscó, M.; Ferrández, O.; Horcajada, J.P.; Grau, S. Azithromycin in the Treatment of COVID-19: A Review. Expert Rev. Anti. Infect. Ther. 2021, 19, 147–163. [Google Scholar] [CrossRef]
- Yates, P.A.; Newman, S.A.; Oshry, L.J.; Glassman, R.H.; Leone, A.M.; Reichel, E. Doxycycline Treatment of High-Risk COVID-19-Positive Patients with Comorbid Pulmonary Disease. Ther. Adv. Respir. Dis. 2020, 14, 1753466620951053. [Google Scholar] [CrossRef]
- Reyes, A.Z.; Hu, K.A.; Teperman, J.; Wampler Muskardin, T.L.; Tardif, J.-C.; Shah, B.; Pillinger, M.H. Anti-Inflammatory Therapy for COVID-19 Infection: The Case for Colchicine. Ann. Rheum. Dis. 2021, 80, 550–557. [Google Scholar] [CrossRef]
- Colchicine for COVID-19: Real-Time Analysis of All 39 Studies. Available online: https://c19colchicine.com/ (accessed on 17 October 2022).
- Drago, F.; Gozzo, L.; Li, L.; Stella, A.; Cosmi, B. Use of Enoxaparin to Counteract COVID-19 Infection and Reduce Thromboembolic Venous Complications: A Review of the Current Evidence. Front. Pharmacol. 2020, 11, 579886. [Google Scholar] [CrossRef]
- Susen, S.; Tacquard, C.A.; Godon, A.; Mansour, A.; Garrigue, D.; Nguyen, P.; Godier, A.; Testa, S.; Levy, J.H.; Albaladejo, P.; et al. Prevention of Thrombotic Risk in Hospitalized Patients with COVID-19 and Hemostasis Monitoring. Crit. Care 2020, 24, 364. [Google Scholar] [CrossRef]
- Shi, Z.; Puyo, C.A. N-Acetylcysteine to Combat COVID-19: An Evidence Review. Ther. Clin. Risk Manag. 2020, 16, 1047–1055. [Google Scholar] [CrossRef]
- Aigner, L.; Pietrantonio, F.; Bessa de Sousa, D.M.; Michael, J.; Schuster, D.; Reitsamer, H.A.; Zerbe, H.; Studnicka, M. The Leukotriene Receptor Antagonist Montelukast as a Potential COVID-19 Therapeutic. Front. Mol. Biosci. 2020, 7, 610132. [Google Scholar] [CrossRef]
- Alexaki, V.I.; Henneicke, H. The Role of Glucocorticoids in the Management of COVID-19. Horm. Metab. Res. 2021, 53, 9–15. [Google Scholar] [CrossRef]

| COVID-19 Stage | Total | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2a | 2b | ||
| n (% of total) | 61 (15.6) | 196 (50.0) | 113 (28.8) | 22 (5.6) | 392 (100) |
| days since symptoms began mean (min-max) | 3.3 (0–7) | 4 (0–20) | 5.5 (1–20) *,# | 5 (1–10) * | 4.4 (0–20) |
| missing (n, % of total) | 0 (0) | 3 (1.5) | 0 (0) | 0 (0) | 3 (0.8) |
| F/M (% F) | 27/34 (44.3) | 110/86 (56.1) | 58/55 (51.3) | 6/16 (27.3) # | 201/191 (51.3) |
| age (years) mean (min-max) | 39.2 (0.5–89) | 47.7 (1.4–97) * | 51.3 (13–85) * | 66.5 (44–90) *,#,§ | 48.5 (0.5–97) |
| age (n, % of total) | |||||
| <18 | 12 (19.7) | 10 (5.1) | 3 (2.7) | 0 (0) | 25 (6.4) |
| 18–29 | 6 (9.8) | 13 (6.6) | 8 (7.1) | 0 (0) | 27 (6.9) |
| 30–39 | 10 (16.4) | 26 (13.3) | 13 (11.5) | 0 (0) | 49 (12.5) |
| 40–49 | 15 (24.6) | 55 (28.1) | 20 (17.7) | 3 (13.6) | 93 (23.7) |
| 50–59 | 9 (14.8) | 43 (21.9) | 33 (29.2) | 5 (22.7) | 90 (23) |
| 60–69 | 4 (6.6) | 27 (13.8) | 20 (17.7) | 4 (18.2) | 55 (14) |
| 70–79 | 1 (1.6) | 12 (6.1) | 7 (6.2) | 3 (13.6) | 23 (5.9) |
| 80–89 | 3 (4.9) | 3 (1.5) | 5 (4.4) | 6 (27.3) | 17 (4.3) |
| >89 | 0 (0) | 1 (0.5) | 0 (0) | 1 (4.5) | 2 (0.5) |
| missing | 1 (1.6) | 6 (3.1) | 4 (3.5) | 0 (0) | 11 (2.8) |
| weight status n (% of total) | |||||
| Underw. (BMI <18.5) | 3 (4.9) | 7 (3.6) | 4 (3.5) | 0 (0) | 14 (3.6) |
| Normal w. (BMI = 18.5–24.9) | 21 (34.4) | 77 (39.3) | 37 (32.7) | 5 (22.7) | 140 (35.7) |
| Overw. (BMI = 25–29.9) | 10 (16.4) | 55 (28.1) | 28 (24.8) | 9 (40.9) | 102 (26) |
| Obese (BMI > 30) | 5 (8.2) | 17 (8.6) | 20 (17.7) | 3 (13.7) | 45 (11.5) |
| Underw. or Normal w./Overw. or Obese | 24/15 | 84/72 | 41/48 | 5/12 * | 154/147 |
| missing | 22 (36.1) | 40 (20.4) | 24 (21.3) | 5 (22.7) | 91 (23.2) |
| chronic comorbidities n (% of total) | 12 (19.7) | 62 (31.6) | 47 (41.6) *,# | 16 (72.7) *,#,§ | 137 (34.9) |
| cardiovascular | 5 (8.2) | 38 (19.4) | 35 (31) | 12 (54.5) | 90 (23) |
| metabolic | 5 (8.2) | 25 (12.7) | 14 (12.4) | 8 (36.4) | 52 (13.3) |
| respiratory | 0 (0) | 3 (1.5) | 3 (2.6) | 3 (13.7) | 9 (2.3) |
| oncologic | 2 (3.3) | 1 (0.5) | 4 (3.5) | 3 (13.7) | 10 (2.5) |
| neurologic | 4 (6.6) | 8 (4.1) | 5 (4.4) | 1 (4.5) | 18 (4.6) |
| autoimmune | 2 (3.3) | 13 (6.6) | 7 (6.2) | 1 (4.5) | 23 (5.9) |
| chronic comorbidities n/patient (mean ± SD) | 1.3 ± 0.5 | 1.4 ± 0.7 | 1.4 ± 0.7 | 1.8 ± 0.8 | 0.5 ± 0.8 |
| chronic comorbidities n (% of total) | |||||
| 0 | 49 (80.3) | 134 (68.4) | 66 (58.4) | 6 (27.3) | 255 (66.1) |
| 1 | 7 (11.5) | 43 (21.9) | 30 (26.5) | 7 (31.8) | 87 (22.2) |
| 2 | 4 (6.6) | 13 (6.6) | 14 (12.4) | 6 (27.3) | 37 (9.4) |
| 3 | 1 (1.6) | 5 (2.5) | 2 (1.8) | 3 (13.7) | 11 (2.8) |
| 4 | 0 (0) | 1 (0.5) | 1 (0.9) | 0 (0) | 2 (0.5) |
| nasal swab n (% of total) | |||||
| positive | 56 (91.8) | 175 (89.2) | 110 (97.4) | 21 (95.5) | 362 (92.3) |
| negative | 0 (0) | 7 (3.6) | 0 (0) | 0 (0) | 7 (1.8) |
| not done | 1 (1.6) | 7 (3.6) | 3 (2.6) | 1 (4.5) | 12 (3.1) |
| missing | 4 (6.6) | 7 (3.6) | 0 (0) | 0 (0) | 11 (2.8) |
| COVID-19 Stage | Total | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2a | 2b | ||
| n (% of total) | 61 (15.6) | 196 (50.0) | 113 (28.8) | 22 (5.6) | 392 (100) |
| recommended since stage 0 | |||||
| vitamins and supplements | 61 (100) | 196 (100) | 108 (95.6) | 22 (100) | 387 (98.7) |
| recommended since stage 1 | |||||
| Aspirin | 16 (26.2) | 139 (70.9) | 89 (78.8) | 15 (68.2) | 259 (66.1) |
| Antibiotics | 17 (27.9) | 104 (53.1) | 100 (88.5) | 22 (100) | 243 (62) |
| Hydroxychloroquine | 4 (6.6) | 65 (33.2) | 38 (33.6) | 9 (40.9) | 116 (29.6) |
| Ivermectin | 0 (0) | 3 (1.5) | 6 (5.3) | 2 (9.1) | 11 (2.8) |
| Colchicine | 1 (1.6) | 20 (10.2) | 12 (10.6) | 2 (9.1) | 35 (8.9) |
| ≥1 Stage 1 drug | 26 (42.6) | 174 (88.8) | 111 (98.2) | 22 (100) | 323 (82.4) |
| recommended since stage 2a | |||||
| Enoxaparin | 3 (4.9) | 41 (20.9) | 52 (46) | 16 (72.7) | 112 (28.6) |
| recommended since stage 2b | |||||
| Glucocorticoids | 7 (11.5) | 50 (25.5) | 85 (75.2) | 22 (100) | 164 (41.8) |
| oxygen therapy | 0 (0) | 5 (2.5) | 11 (9.7) | 11 (50) | 27 (6.9) |
| COVID-19 Stage | Total | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2a | 2b | ||
| n (% of total) | 61 (15.6) | 196 (50.0) | 113 (28.8) | 22 (5.6) | 392 (100) |
| hospitalized n (% of total) | 1 (1.6) | 9 (4.6) | 9 (8) | 6 (27.3) *,#,§ | 25 (5.8) |
| recovered n (% of total) | 61 (100) | 196 (100) | 111 (98.2) | 22 (100) | 390 (99.6) |
| without sequelae | 50 (82) | 173 (88.3) | 100 (90.1) | 19 (86.4) | 342 (88.7) |
| with sequelae | 11 (18) | 23 (11.7) | 11 (9.9) | 3 (13.6) | 48 (12.3) |
| deceased n (% of total) | 0 (0) | 0 (0) | 1 (0.9) | 0 (0) | 1 (0.2) |
| missing n (% of total) | 0 (0) | 0 (0) | 1 (0.9) | 0 (0) | 1 (0.2) |
| COVID-19 Risk Group 1 | Total | ||||
|---|---|---|---|---|---|
| Low | High A | High B | High C | ||
| n (% of total) | 154 (40.4) | 97 (25.5) | 68 (17.8) | 62 (16.3) | 381 (100) |
| hospitalized n (% of total) | 2 (1.3) | 12 (12.4) * | 8 (11.8) * | 3 (4.8) | 25 (100) |
| recovered n (% of total) | 154 (100) | 96 (99) | 67 (98.5) | 62 (100) | 379 (100) |
| without sequelae | 138 (89.6) | 84 (86.6) | 49 (72.1) | 60 (96.8) | 331 (100) |
| with sequelae | 16 (10.4) | 12 (12.4) | 18 (26.5) | 2 (3.2) | 48 (100) |
| deceased n (% of total) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 (100) |
| missing n (% of total) | 0 (0) | 0 (0) | 1 (1.5) | 0 (0) | 1 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosentino, M.; Vernocchi, V.; Martini, S.; Marino, F.; Allasino, B.; Bàlzola, M.A.; Burigana, F.; Dallari, A.; Pagano, C.S.F.; Palma, A.; et al. Early Outpatient Treatment of COVID-19: A Retrospective Analysis of 392 Cases in Italy. J. Clin. Med. 2022, 11, 6138. https://doi.org/10.3390/jcm11206138
Cosentino M, Vernocchi V, Martini S, Marino F, Allasino B, Bàlzola MA, Burigana F, Dallari A, Pagano CSF, Palma A, et al. Early Outpatient Treatment of COVID-19: A Retrospective Analysis of 392 Cases in Italy. Journal of Clinical Medicine. 2022; 11(20):6138. https://doi.org/10.3390/jcm11206138
Chicago/Turabian StyleCosentino, Marco, Veronica Vernocchi, Stefano Martini, Franca Marino, Barbara Allasino, Maria Antonietta Bàlzola, Fabio Burigana, Alberto Dallari, Carlo Servo Florio Pagano, Antonio Palma, and et al. 2022. "Early Outpatient Treatment of COVID-19: A Retrospective Analysis of 392 Cases in Italy" Journal of Clinical Medicine 11, no. 20: 6138. https://doi.org/10.3390/jcm11206138
APA StyleCosentino, M., Vernocchi, V., Martini, S., Marino, F., Allasino, B., Bàlzola, M. A., Burigana, F., Dallari, A., Pagano, C. S. F., Palma, A., Rango, M., & on behalf of IppocrateOrg Association Working Group for the Early Outpatient Treatment of COVID-19. (2022). Early Outpatient Treatment of COVID-19: A Retrospective Analysis of 392 Cases in Italy. Journal of Clinical Medicine, 11(20), 6138. https://doi.org/10.3390/jcm11206138







